Abstract
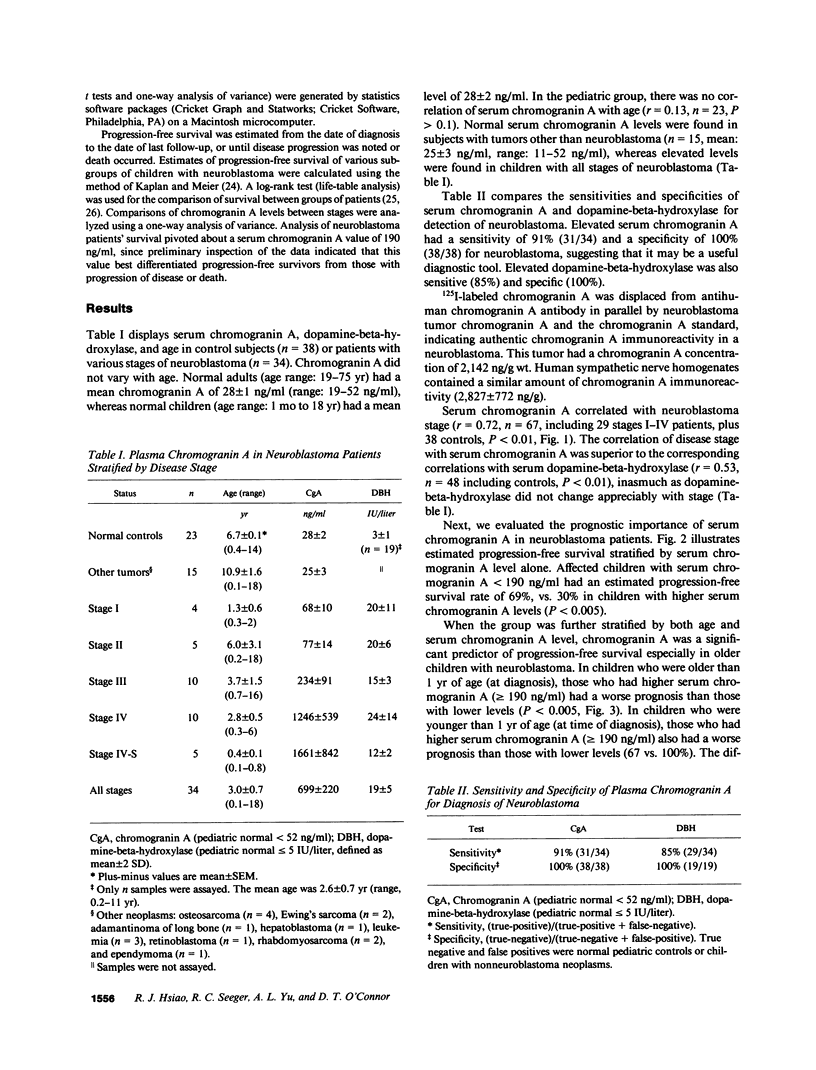
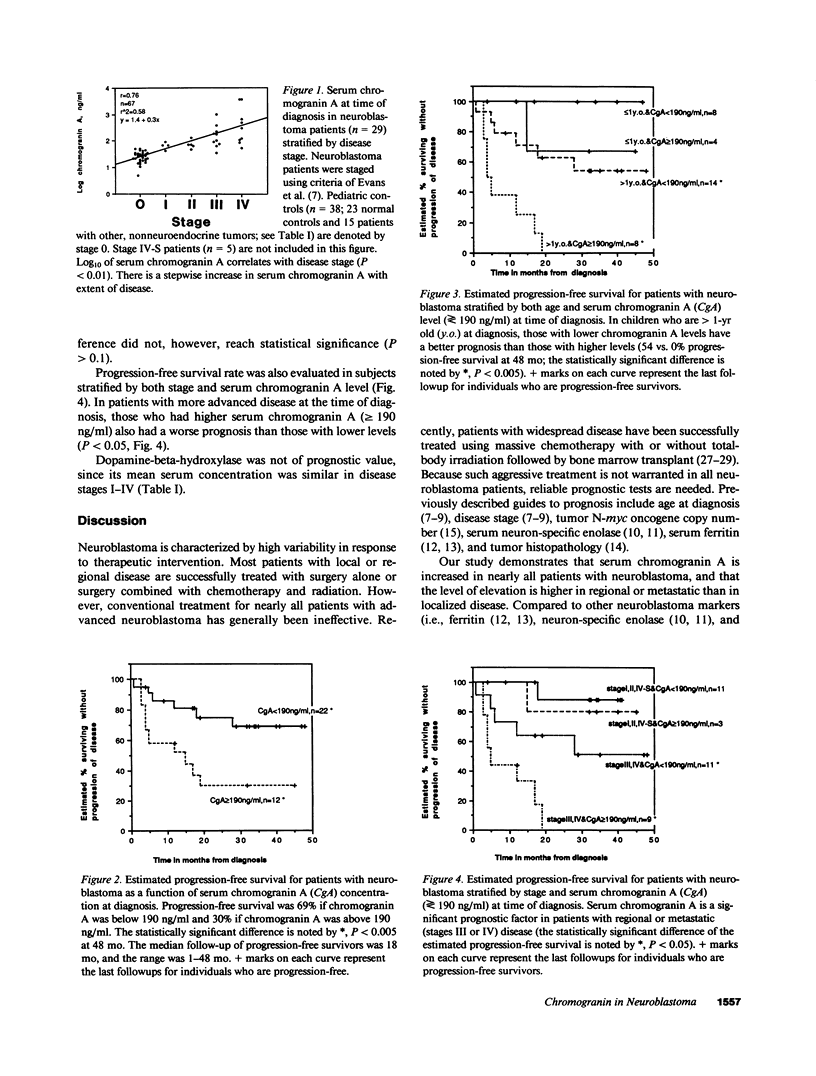
Chromogranin A is an acidic protein costored and coreleased with catecholamines from storage vesicles. Its serum concentration is elevated in patients with peptide-producing endocrine neoplasia. We measured serum chromogranin A at the time of diagnosis in 34 children with all stages of neuroblastoma. With a sensitivity of 91% and specificity of 100%, serum chromogranin A emerged as a useful diagnostic tool for neuroblastoma, comparable to or better than other measurements such as neuron-specific enolase, ferritin, or dopamine-beta-hydroxylase. Mean serum chromogranin A correlated with disease stage (r = 0.76, P less than 0.01). The relationship of prognosis (progression-free survival) to baseline serum chromogranin A, age, and disease stage was determined in 34 patients at risk for relapse, with a median followup period of 18 mo (range, 1-48 mo). The survival rate for patients with lower serum chromogranin A levels (less than 190 ng/ml at the time of diagnosis) was 69%, whereas it was 30% for those with higher chromogranin A levels (P less than 0.05). Furthermore, when subjects were additionally stratified by either age or stage, chromogranin A was an effective prognostic tool in patients who either were older than 1 yr (P less than 0.005) or had more advanced disease (stage III or IV; P less than 0.05). We conclude that serum chromogranin A in neuroblastoma is (a) a valuable (sensitive and specific) diagnostic tool, (b) a correlate of tumor burden, and (c) a useful predictor of survival.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August C. S., Serota F. T., Koch P. A., Burkey E., Schlesinger H., Elkins W. L., Evans A. E., D'Angio G. J. Treatment of advanced neuroblastoma with supralethal chemotherapy, radiation, and allogeneic or autologous marrow reconstitution. J Clin Oncol. 1984 Jun;2(6):609–616. doi: 10.1200/JCO.1984.2.6.609. [DOI] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Cooper M. J., Helman L. J., Evans A. E., Swamy S., O'Connor D. T., Helson L., Israel M. A. Chromogranin A expression in childhood peripheral neuroectodermal tumors. Prog Clin Biol Res. 1988;271:175–184. [PubMed] [Google Scholar]
- D'Angio G. J., Evans A. E., Koop C. E. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet. 1971 May 22;1(7708):1046–1049. doi: 10.1016/s0140-6736(71)91606-0. [DOI] [PubMed] [Google Scholar]
- Evans A. E., D'Angio G. J., Propert K., Anderson J., Hann H. W. Prognostic factor in neuroblastoma. Cancer. 1987 Jun 1;59(11):1853–1859. doi: 10.1002/1097-0142(19870601)59:11<1853::aid-cncr2820591102>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Evans A. E., D'Angio G. J., Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer. 1971 Feb;27(2):374–378. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Freedman L. S., Ohuchi T., Goldstein M., Axelrod F., Fish I., Dancis J. Changes in human serum dopamine- -hydroxylase activity with age. Nature. 1972 Apr 7;236(5345):310–311. doi: 10.1038/236310a0. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Freedman L. S., Bohuon A. C., Guerinot F. Serum dopamine-beta-hydroxylase activity in neuroblastoma. N Engl J Med. 1972 May 25;286(21):1123–1125. doi: 10.1056/NEJM197205252862102. [DOI] [PubMed] [Google Scholar]
- Hann H. W., Evans A. E., Siegel S. E., Wong K. Y., Sather H., Dalton A., Hammond D., Seeger R. C. Prognostic importance of serum ferritin in patients with Stages III and IV neuroblastoma: the Childrens Cancer Study Group experience. Cancer Res. 1985 Jun;45(6):2843–2848. [PubMed] [Google Scholar]
- Hann H. W., Levy H. M., Evans A. E. Serum ferritin as a guide to therapy in neuroblastoma. Cancer Res. 1980 May;40(5):1411–1413. [PubMed] [Google Scholar]
- Helman L. J., Thiele C. J., Linehan W. M., Nelkin B. D., Baylin S. B., Israel M. A. Molecular markers of neuroendocrine development and evidence of environmental regulation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2336–2339. doi: 10.1073/pnas.84.8.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T., Udenfriend S. Photometric assay of dopamine- -hydroxylase activity in human blood. Clin Chem. 1972 Sep;18(9):980–983. [PubMed] [Google Scholar]
- O'Connor D. T., Bernstein K. N. Radioimmunoassay of chromogranin A in plasma as a measure of exocytotic sympathoadrenal activity in normal subjects and patients with pheochromocytoma. N Engl J Med. 1984 Sep 20;311(12):764–770. doi: 10.1056/NEJM198409203111204. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Deftos L. J. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986 May 1;314(18):1145–1151. doi: 10.1056/NEJM198605013141803. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Frigon R. P., Sokoloff R. L. Human chromogranin A. Purification and characterization from catecholamine storage vesicles of human pheochromocytoma. Hypertension. 1984 Jan-Feb;6(1):2–12. doi: 10.1161/01.hyp.6.1.2. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Levine G. L., Frigon R. P. Homologous radio-immunoassay of human plasma dopamine-beta-hydroxylase: analysis of homospecific activity, circulating plasma pool and intergroup differences based on race, blood pressure and cardiac function. J Hypertens. 1983 Oct;1(3):227–233. doi: 10.1097/00004872-198310000-00006. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Pandlan M. R., Carlton E., Cervenka J. H., Hslao R. J. Rapid radioimmunoassay of circulating chromogranin A: in vitro stability, exploration of the neuroendocrine character of neoplasia, and assessment of the effects of organ failure. Clin Chem. 1989 Aug;35(8):1631–1637. [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip T., Ghalie R., Pinkerton R., Zucker J. M., Bernard J. L., Leverger G., Hartmann O. A phase II study of high-dose cisplatin and VP-16 in neuroblastoma: a report from the Société Française d'Oncologie Pédiatrique. J Clin Oncol. 1987 Jun;5(6):941–950. doi: 10.1200/JCO.1987.5.6.941. [DOI] [PubMed] [Google Scholar]
- Sage H. J., Smith W. J., Kirshner N. Mechanism of secretion from the adrenal medulla. I. A microquantitative immunologic assay for bovine adrenal catecholamine storage vesicle protein and its application to studies of the secretory process. Mol Pharmacol. 1967 Jan;3(1):81–89. [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Shimada H., Chatten J., Newton W. A., Jr, Sachs N., Hamoudi A. B., Chiba T., Marsden H. B., Misugi K. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984 Aug;73(2):405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J. 1967 May;103(2):483–492. doi: 10.1042/bj1030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. J., Kirshner N. A specific soluble protein from the catecholamine storage vesicles of bovine adrenal medulla. I. Purification and chemical characterization. Mol Pharmacol. 1967 Jan;3(1):52–62. [PubMed] [Google Scholar]
- Zeltzer P. M., Marangos P. J., Evans A. E., Schneider S. L. Serum neuron-specific enolase in children with neuroblastoma. Relationship to stage and disease course. Cancer. 1986 Mar 15;57(6):1230–1234. doi: 10.1002/1097-0142(19860315)57:6<1230::aid-cncr2820570628>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Zeltzer P. M., Marangos P. J., Parma A. M., Sather H., Dalton A., Hammond D., Siegel S. E., Seeger R. C. Raised neuron-specific enolase in serum of children with metastatic neuroblastoma. A report from the Children's Cancer Study Group. Lancet. 1983 Aug 13;2(8346):361–363. doi: 10.1016/s0140-6736(83)90342-2. [DOI] [PubMed] [Google Scholar]



