Abstract
Endothelin-converting enzyme-2 (ECE-2) is a membrane-bound zinc-dependent metalloprotease that shares a high degree of sequence homology with ECE-1, but displays an acidic pH optimum characteristic of maturing enzymes acting late in the secretory pathway. Although ECE-2, like ECE-1, can cleave the big endothelin intermediate to produce the vasoconstrictive endothelin peptide, its true physiological function remains to be elucidated, a task that is hampered by the lack of specific tools to study and discriminate ECE-2 from ECE-1, i.e. specific substrates and/or specific inhibitors. To fill this gap, we searched for novel ECE-specific peptide substrates. To this end, peptides derived from the big endothelin intermediate were tested using ECE-1 and ECE-2, leading to the identification of an ECE-1-specific substrate. Moreover, screening of our proprietary fluorigenic peptide Fluofast® libraries using ECE-1 and ECE-2 allowed the identification of Ac-SKG-Pya-F-W-Nop-GGK-NH2 (PL405), as a specific and high affinity ECE-2 substrate. Indeed, ECE-2 cleaved PL405 at the Pya-F amide bond with a specificity constant (kcat/Km) of 8.1 ± 0.9 × 103 m−1 s−1. Using this novel substrate, we also characterized the first potent (Ki = 7.7 ± 0.3 nm) and relatively selective ECE-2 inhibitor and developed a quantitative fluorigenic ECE-2 assay. The assay was used to study the ex vivo ECE-2 activity in wild type and ECE-2 knock-out tissues and was found to truly reflect ECE-2 expression patterns. The PL405 assay is thus the first tool to study ECE-2 inhibition using high throughput screening or for ex vivo ECE-2 quantification.
Keywords: Enzymes, Fluorescence, Metalloprotease, Peptide Arrays, Peptide Biosynthesis
Introduction
Endothelin-converting enzyme-2 (ECE-2)2 is a zinc-dependant metalloprotease belonging to the M13 family, in which it shares the highest degree of sequence homology with ECE-1 (EC 3.4.24.71) (1, 2). The family is also constituted by neprilysin (NEP, EC 3.4.24.11) (3); damage-induced neuronal endopeptidase, also known as endothelin converting enzyme like (ECEL) (4, 5); the Kell blood group antigen (6); phosphate-regulating neutral endopeptidase on the X chromosome (7); and neprilysin 2 (NEP2) (8–11). These peptidases are type II integral membrane glycoproteins composed of a small cytosolic tail followed by a transmembrane region and a large C-terminal core that is typically exposed at the cell surface. The active site of the enzyme is located within this last domain and is characterized by two highly conserved zinc-binding consensus motifs HEXXH and EXXXD (where X is any amino acid) (12). The structure of the human ECE-1 enzyme co-crystallized with phosphoramidon, a generic inhibitor of the family, was recently reported (13) showing the conserved “thermolysin-like” arrangement of its catalytic site (14, 15).
The endothelin-converting enzymes were first characterized as cleaving the big endothelin inactive intermediate at its Trp21–Val22 bond to produce the highly vasoconstrictive endothelin peptide (1, 2). It was thereafter found that ECE-1 can also hydrolyze smaller peptides in vitro such as big endothelin 18–34 (16) as well as neurotensin, substance P, and bradykinin with increased efficiency as compared with big endothelin (17). These latter results not only suggest that ECE-1 may possess other physiological functions but have also prompted the development of fluorigenic model substrates for ECE-1 quantification: one based on the bradykinin sequence (18) and the other based on the big endothelin 19–38 sequence (19). Although ECE-2 was also initially characterized as an endothelin-producing metalloprotease, it displays an acidic optimal pH, characteristic of processing enzymes acting within the secretory pathway (2). In a subsequent study, more detailed analysis of its substrate specificity revealed that ECE-2 could indeed be involved in the maturation of peptide intermediates to produce bioactive peptides, because it also produces bovine adrenal medulla 12, a κ opioid receptor activator, by cleaving the bovine adrenal medulla 22 intermediate (20).
More recently, it has also been suggested that some members of the M13 family, including NEP and the ECEs, are involved in the clearance of β-amyloid peptides (21–24). These three metalloproteases have been shown to influence endogenous Aβ levels in the brains of their respective knock-out mice (22, 23), and a human polymorphism of the human ECE-1 gene has been linked to Alzheimer disease (25). Lastly, the expression of the ECE-2 gene was recently found to be the most down-regulated in an unbiased microarray screen of the gene expression in the inferior parietal lobe of late onset Alzheimer disease patients compared with non-Alzheimer disease-associated dementia and nondemented controls (26).
Thus, although the physiological functions of the ECE-2 metalloprotease still remain unclear, it could constitute a novel target in human neurodegenerative disease treatment and/or prevention. Specific tools to study its enzymatic activity and to differentiate it from ECE-1 in vitro and in vivo are, however, lacking. Indeed, to date, the enzymatic activities of both ECE proteases can be followed either by HPLC, through the generation of the endothelin-1 peptide from the big endothelin 1–38 intermediate, or by the nonspecific cleavage of a bradykinin-based fluorigenic peptide, McabK2, which is also cleaved by neprilysin and cathepsins (18, 20).
The goal of the present study was to develop specific fluorigenic substrates for ECE-2 and ECE-1. With this aim, we have explored the specific enzymatic activity of ECE-2 and compared it with that of ECE-1. Study of the cleavage of big endothelin 19–38 and of the previously published fluorigenic peptide substrate PL400 (19) by both ECEs revealed that ECE-2 did not process either peptide, thus identifying PL400 as an ECE-1-specific fluorigenic substrate. Small fluorigenic peptide libraries were then screened using ECE-1 and ECE-2. Using this technique, we present here the identification of the first specific ECE-2 substrate, which can be used to monitor its enzymatic activity, both in vitro and ex vivo. Indeed, combined with the identification of selective ECE-2/ECE-1 inhibitors, the novel substrate serves as a much needed tool to assess ECE-2 activity within tissues. Thus, using PL405, ECE-2 could be quantified in tissues of wild type mice, whereas no ECE-2 activity could be detected in tissues from knock-out mice.
EXPERIMENTAL PROCEDURES
Materials
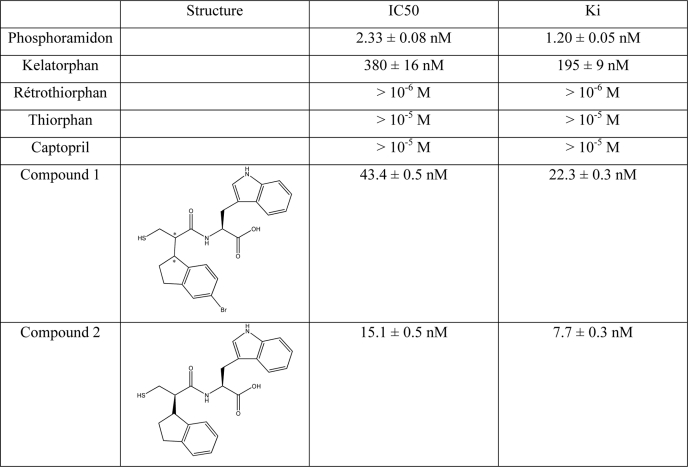
N-Methylpyrrolidone, dichloromethane, 4-dimethylaminopyridine, dicyclohexylcarbodiimide, hydroxybenzotriazole, HBTU, and Fmoc-protected amino acids were purchased from Applied Biosystems (Courtaboeuf, France). Fmoc-p-nitro-l-phenylalanine and Fmoc-l-pyrenylalanine were from Polypeptide Laboratories France SAS (Strasbourg, France). The McabK2 fluorigenic substrate was purchased from R & D Systems Europe (Lille, France). Big endothelin 22–38 was purchased from Bachem (Weil am Rhein, Germany), and big endothelin 19–38 was from American Peptide (Sunnyvale, CA). Compound 1 (2S)-2-(2-(5-bromo-2,3-dihydro-1H-inden-1-yl)-3-mercaptopropanamido)-3-(1H-indol-3-yl)propanoicacid and compound 2 ((S)-2-((R)-2-((R)-2,3-dihydro-1H-inden-1-yl)-3-mercaptopropanamido)-3-(1H-indol-3-yl)propanoic acid were synthesized as described (27, 28).
Enzyme Preparations
Recombinant human ECE-2 was produced as previously described (29). Briefly, the construct codes for a secreted form of ECE-2 by removal of the N-terminal transmembrane sequence and its replacement with the signal peptide from rat CPE followed by a polyhistidine tag. The recombinant secreted, soluble ECE-2 (corresponding to amino acids 81–766) was produced in Sf9 insect cells using a baculovirus expression system. Culture medium from the cells expressing recombinant protein was concentrated using Amicon column, and ECE-2 was further purified using DEAE ion exchange and Talon metal affinity beads. Purified enzyme was stored in 50% glycerol to preserve the activity. The purified preparation was electrophoresed on a 12% acrylamide denaturing gel, stained, and quantified by densitometry using a BSA scale and the Quantity One program of Bio-Rad. The concentration of the pure ECE-2 preparation was thus estimated to be 6.25 μg/ml (supplemental Fig. S1).
Soluble recombinant human ECE-1 (Gln90–Trp770; R & D Systems, Minneapolis, MN) was reconstituted in 25 mm Tris, 100 mm, pH 8.0, 150 mm NaCl at 0.1 mg/ml. NEP was purified to homogeneity from rabbit kidney as previously described (30). The ACE metalloprotease was purified from rat testis as described by Piquilloud et al. (31). Purity of the preparations was verified by SDS-PAGE and Coomassie staining, and the enzyme concentrations were determined by densitometry as described above. The NEP stock solution was at a concentration of 750 μg/ml, whereas ACE was at 25.3 μg/ml. Human recombinant MMP7 and 9 were purchased from Calbiochem (France), pepsin was from Roche Applied Science, and trypsin and subtilisin were from Sigma.
Tissue Preparation
Brain, liver, and heart were dissected out from wild type and ECE-2 knock-out mice (32, 33), snap frozen, and stored at −80 °C until use. The tissues were quickly thawed in homogenization buffer (10 mm HEPES, pH 6.8, 320 mm sucrose, 100 μm phenylmethylsulfonyl fluoride, and 10 μm leupeptin) and disrupted using a polytron. Homogenized tissues were first cleared for 10 min at 2500 rpm at 4 °C, and the supernatant was centrifuged for 40 min at 18,000 rpm at 4 °C. The membrane pellet was resuspended in 10 mm HEPES, pH 6.8, supplemented with 100 μm phenylmethylsulfonyl fluoride and 10 μm leupeptin and solubilized overnight using 1% Triton X-100. A solubilized membrane fraction was obtained after a 1-h centrifugation at 5,000 rpm. Total protein concentration in the solubilized preparations was quantified using the Bradford method.
Peptide Synthesis and Purification
The peptides were synthesized using Fmoc chemistry on methylbenzhydrylamine resin on an Applied Biosystems ABI 433 synthesizer, using a classical HBTU/1-hydroxybenzotriazole/diisopropylethylamine protocol.
The syntheses proceeded in N-methylpyrrolidone with 10 equivalents of amino acids. The l-pyrenylalanine amino acid was introduced by mixing the resin in a syringe using (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate as a coupling reagent in the presence of diisopropylethylamine. Deprotection of the lateral chains of the amino acids was accomplished by mixing the resin in presence of TFA, tri-isopropylsilane and water (95/2.5/2.5) for 2 h.
Peptides were purified by semi-preparative HPLC on a Waters 600 device equipped with a 2487 dual absorbance detector using a Kromasil C18 100 Å, 5-μm 250 × 20 mm column using a 10–90% CH3CN (0.1% TFA)/H2O (0.1% TFA) gradient in 30 min.
Synthesis of the Ac-SKG-Pya-N-X-Nop-GGK-NH2 Peptide Libraries
The peptides were synthesized as described above. The 10 amino acids were mixed together in equivalent concentrations to yield equimolar amounts of the 10 peptides after synthesis on the ABI433 device. These peptides were used as a mixture without further purification.3
Deconvolution of Ac-SKG-Pya-F-X-Nop-GGK-NH2 Peptide Library
The 10 peptides were synthesized simultaneously on a Protein Technology Symphony device equipped with 12 separate channels using HBTU, 1-hydroxybenzotriazole chemistry with NMP as solvent: Ac-SKG-Pya-F-A-Nop-GGK-NH2, Rt = 15.29 min, ESI (+) [M + H]+ = 1255.7; Ac-SKG-Pya-F-I-Nop-GGK-NH2, Rt = 15.99 min, ESI (+) [M + H]+ = 1297.7; Ac-SKG-Pya-F-L-Nop-GGK-NH2, Rt = 15.98 min, ESI (+) [M + H]+ = 1297.8; Ac-SKG-Pya-F-K-Nop-GGK-NH2, Rt = 14.25 min, ESI (+) [M + H]+ = 1313.6; Ac-SKG-Pya-F-F-Nop-GGK-NH2, Rt = 16.24 min, ESI (+) [M + H]+ = 1332.8; Ac-SKG-Pya-F-W-Nop-GGK-NH2 (PL405), Rt = 16.33 min, ESI (+) [M + H]+ = 1372.0; Ac-SKG-Pya-F-E-Nop-GGK-NH2, Rt = 15.15 min, ESI (+) [M + H]+ = 1313.6; Ac-SKG-Pya-F-Q-Nop-GGK-NH2, Rt = 15.14 min, ESI (+) [M + H]+ = 1312.6; Ac-SKG-Pya-F-T-Nop-GGK-NH2, Rt = 16.78 min, ESI (+) [M + H]+ = 1157.6; and Ac-SKG-Pya-F-P-Nop-GGK-NH2, Rt = 15.15 min, ESI (+) [M + H]+ = 1281.7. The Ac-SKG-Pya (PL415) cleavage product was purified using the same column but using an isocratic elution protocol (35% CH3CN (0.1% TFA), 75% H2O (0.1% TFA), Rt = 7.22 min, ESI (+) [M + H]+ = 604.4.
Spectrometric Measurements
The fluorimetric properties of the PL405 substrate and of the PL415 cleavage product were determined using a PerkinElmer Life Sciences LS50B spectrofluorimeter. To this end, 1 μm solutions (200 mm sodium acetate, pH 5.5) of PL405 and PL415 were used. Ultraviolet absorption spectra of the substrate and its ECE-2 cleavage product (50 μm in assay buffer with 0.5% Me2SO) were recorded on a Shimadzu spectrophotometer UV mini 1240.
HPLC and Mass Spectrometry
HPLC analysis was performed on a Shimadzu Prominence LC-20AB equipped with a diode array display and a Kromasil C18 column (5 μm, 100 Å, 4.6 × 250 mm) using A (H2O with 0.1% TFA) and B (CH3CN with 0.1% TFA) elution systems as mobile phase.
The reaction products formed by the hydrolysis of human big endothelin 19–38 (50 μm) after a 3-h incubation at 37 °C in either 100 mm HEPES, pH 6.8, alone or with 1 μg/ml of ECE-1 were separated on a Kromasil C18 column (5 μm, 100 Å, 4.6 × 250 mm) using a linear gradient of 0–60% of B over 30 min with a flow rate of 1 ml/min. The detection was performed by UV at 210 nm. The same experiment was performed using 1 μg/ml of ECE-2 in 200 mm sodium acetate, pH 5.5. Hydrolysis products were identified using synthetic peptides and electrospray mass spectrometry.
The reaction products formed by the hydrolysis of PL405 by ECE-2 were characterized by HPLC. A total of 100 μl of the reaction mixture was injected onto the Kromasil C18 column (5 μm, 100 Å, 4.6 × 250 mm) as described above but using a linear gradient of 10–90% of B in 60 min with a flow rate of 1 ml/min. The detection was performed by UV at 343 nm. Hydrolysis products were collected, and their molecular weights were confirmed by deconvolution of the mass spectra obtained with an electrospray mass spectrometer (Thermo Finnigan) using Xcalibur software.
Enzymatic Assays
The differential screening of the Ac-SKG-Pya-N-X-Nop-GGK-NH2 peptide libraries (100 μm) was performed using 1 μg/ml of either ECE-1 or ECE-2 in 100 mm HEPES buffer, pH 6.8, or in 200 mm sodium acetate buffer, pH 5.5, respectively, in a final reaction volume of 100 μl. Positive controls using the commercial McabK2 fluorigenic peptide (10 μm) were included in each experiment. Individual peptides from the deconvoluted library were tested in a similar fashion but using a 10 μm concentration of substrate.
For cleavage site determination of isolated peptides, substrate (20 μm) was incubated with 500 ng/ml of ECE-2 in 200 mm sodium acetate, pH 5.5, at 37 °C for 180 min, after which time the reaction was stopped with 0.2 n HCl, separated by HPLC, and analyzed by LC/MS.
The specificity of the enzymatic assay using PL405 was compared with that using McabK2 by investigating their respective cleavage by ECE-2 and comparing it with a series of metalloproteases, i.e. ECE-1, NEP, and ACE, as well as the matrix metalloproteases MMP7 and MMP9. These enzymatic assays were all performed in the ECE-2 buffer conditions, i.e. 200 mm sodium acetate buffer, pH 5.5, with either 20 μm of PL405 or 10 μm of McabK2 substrate and 250 ng/ml of each enzyme. Positive controls were performed in parallel in the optimal conditions of each enzyme, i.e. 100 mm HEPES buffer, pH 6.8, for ECE-1 and 50 mm Tris, pH 7.4, for NEP, whereas ACE was incubated in 50 mm Tris, pH 8.0, 1% NaCl, and MMPs in 25 mm Tris, pH 7.5, 10 mm CaCl2, 0.2 m NaCl (not shown). Linearity of the reaction was demonstrated by incubating increasing concentrations of ECE-2 ranging from 10 to 500 ng/ml, for 180 min, using 20 μm of PL405 substrate.
Ex vivo enzymatic activities were measured using 10 μg of solubilized membrane preparations from wild type and ECE-2 knock-out mice. ECE-2 activity was monitored using PL405 (20 μm) diluted in 200 mm sodium acetate buffer, pH 5.5, supplemented with 0.05% protease inhibitor mixture (without EDTA; Sigma) to inhibit any nonmetalloprotease nonspecific activity. The ECE-2 nonspecific activity was monitored in parallel using the same buffer with 500 nm of compound 2. The ECE-2 enzymatic activity was also measured in the same conditions but with the commercial McabK2 substrate (10 μm). The ex vivo enzymatic activity of ECE-1 was measured in 100 mm HEPES, pH 6.8, supplemented with 0.05% protease inhibitor mixture using PL400 (100 μm). ECE-1 nonspecific activity was measured in the same conditions but with 500 nm of compound 1. The specific activity is obtained by subtracting the nonspecific activity result from the total activity measured in assay buffer.
Substrate hydrolysis was monitored on a multiwell plate reader fluorimeter (Berthold Series TwinkleTM LB 970 coupled to Mikrowin 2000 software) with excitation at 340 nm, emission at 405 nm, lamp energy at 10,000, and temperature at 37 °C. Every experiment was performed at least twice independently, in duplicate.
Kinetic Parameters of PL405
Determinations of Km values were performed in initial rate conditions (supplemental Fig. S2), and PL405 at five different concentrations ranging from 1 to 100 μm was incubated with 250 ng/ml of ECE-2 in 200 mm sodium acetate, pH 5.5, for 60 min at 37 °C. For each substrate concentration, a standard calibration was established for 5, 10, 15, and 20% cleavage by using the corresponding mixture of substrate and cleavage product (PL415). The obtained fluorescence was then reported to each standard calibration to quantify the amount of PL415 formed. The Km constant was also evaluated using UV analysis of the cleavage products separated by HPLC and a standard curve of PL415. The apparent Km values (mean of at least two independent assays in duplicate) were calculated using the Graphpad Prism 4 program, and the kcat was determined using the equation kcat = Vmax/[E] with an enzyme molar mass of 85 kDa.
Inhibitory Potencies
A series of known metalloprotease competitive inhibitors were tested for their specific affinities toward ECE-2 using our novel substrate. In an initial set of experiments, the inhibitory potencies obtained with this substrate were compared with those obtained with the McabK2 substrate, using 10 μm of peptide in the same conditions. ECE-2 at 100 ng/ml was preincubated for 10 min at 37 °C in 200 mm sodium acetate, pH 5.5, with increasing concentrations of inhibitors (from 10−9 to 10−5 m). The reaction was initiated by the addition of 20 μm PL405 followed by an incubation of 30 min at 37 °C. The same protocol was followed for ECE-1 using the McabK2 substrate (10 μm) and 100 mm HEPES pH 6.8 reaction buffer. The fluorescence was measured with a Twinkle fluorimeter (Berthold). The samples with 0% hydrolysis were obtained by adding the substrate to the buffer, and the samples with 100% relative activity were prepared without the inhibitor. The percentage of cleavage was evaluated and compared with 100% relative activity, and the IC50 values were determined accordingly. The Ki values of the inhibitors (mean of at least two independent assays in duplicate) were calculated using the Cheng and Prussoff equation Ki = IC50/(1 + [S]/Km).
RESULTS
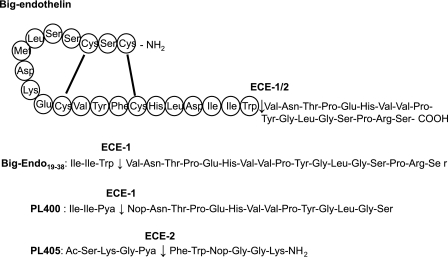
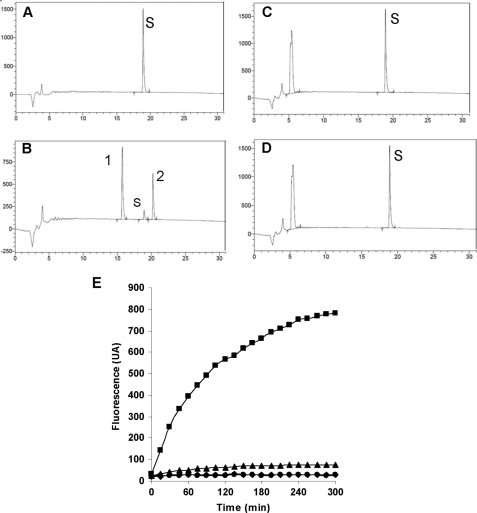
Both ECE-1 and ECE-2 cleave the inactive big endothelin 1–38 intermediate at the Trp21–Val22 bond to produce the 21-amino acid active endothelin peptide (Fig. 1) (1, 2). To reduce the size of this substrate, we compared the ability of both ECEs to cleave the smaller synthetic big endothelin 19–34 peptide, in which the same Trp-Val amide bond is not preceded by the cyclic endothelin-1 part of the native peptide (Fig. 1). Surprisingly, HPLC analysis of the reaction products revealed that although ECE-1 cleaved this peptide, ECE-2 did not (Fig. 2). PL400 is a previously published model substrate for ECE-1 (Fig. 1) (19). Its amino acid sequence is based on that of 19–34 big endothelin, in which the scissile bond was replaced by the fluorophore/repressor pair Pya/Nop. Comparative analysis of the cleavage of this quenched fluorogenic substrate by ECE-1 and ECE-2 unravels the unsuspected specificity of PL400, the peptide being efficiently cleaved by ECE-1 but not by ECE-2 (Fig. 2).
FIGURE 1.
Top sequence, amino acid sequence of the native big endothelin 1–38 intermediate containing the cyclic 21-amino acid sequence of endothelin 1, which is released after cleavage of the Trp21–Val22 bond by ECE-1 and ECE-2. Second sequence, amino acid sequence of the synthetic big endothelin 19–38 peptide containing the Trp-Val scissile bond in positions 3 and 4 of the peptide, which is cleaved by ECE-1 only. Third sequence, amino acid sequence of the selective ECE-1 peptide substrate, PL400 (19), based on the big endothelin 19–35, in which the scissile bond is formed by the fluorophore and repressor pair Pya and Nop. Fourth sequence, the PL405 peptide substrate cleaved exclusively by ECE-2 at the Pya-Phe amide bond.
FIGURE 2.
Cleavage of big endothelin 19–38 (human) and of PL400 by ECE-1 and ECE-2. A–D, big endothelin 19–38 (S, 50 μm) was incubated for 3 h at 37 °C either in 100 mm HEPES pH 6.8 buffer alone (A) or in buffer with 1 μg/ml ECE-1 (B), and the products were separated by HPLC on a Kromasil C18 column (5 μm, 100 Å, 4.6 × 150 mm). The same experiment was performed with ECE-2 by incubating the big endothelin 19–38 peptide (50 μm) alone in 200 mm sodium acetate, pH 5.5, for 3 h at 37 °C (C) or in buffer with 1 μg/ml of ECE-2 (D). The reaction products were separated according to a 0–60% gradient of solvent B in 30 min. Solvent A was 0.1% trifluoroacetic acid in H2O. Solvent B was 0.1% trifluoroacetic acid in CH3CN. The flow rate was 1 ml/min. Absorbance detection (UV) was 210 nm. Hydrolysis products 1 and 2 were found to correspond to big endothelin 22–38 and to the Ile-Ile-Trp tri-peptide, respectively. E, the PL400 peptide (20 μm) was incubated for 5 h at 37 °C either alone in ECE-1 reaction buffer (♦) or in the same buffer with 1 μg/ml of ECE-1 (■). For ECE-2, 20 μm PL400 was incubated either in ECE-2 reaction buffer (●) or in buffer with 1 μg/ml of ECE-2 (▴).
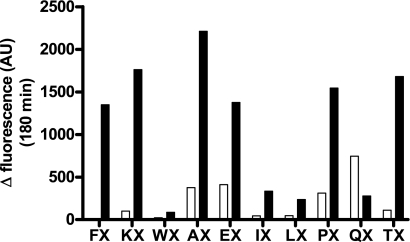
Using our FluoFast® Ac-SKG-Pya-N-X-Nop-GGK-NH2 peptide libraries for differential screening of ECE-1 and ECE-2 activity, we identified a peptide specifically cleaved by the latter enzyme. Briefly, the water-soluble libraries are constituted of internally quenched fluorigenic peptides of various lengths, all based on the following sequence: Ac-SKG-Pya-N(0–2)-X-Nop-GGK-NH2, where N is any amino acid chosen from Ala, Ile, Leu, Lys, Phe, Trp, Glu, Gln, Thr, and Pro, and X is a mixture of these 10. Within these peptide sequences, the Pya amino acid represents the highly fluorigenic l-pyrenylalanine moiety that is physically separated from its Nop quencher upon protease cleavage. For the present purpose, we screened the 10 Ac-SKG-Pya-N1-X-Nop-GGK-NH2 peptide libraries, representing the 100 possible amino acid combinations between the fluorigenic and quencher moieties. The results of this initial screening process revealed that ECE-2 efficiently cleaved six peptide libraries, i.e. Ac-SKG-Pya-F-X-Nop-GGK-NH2, Ac-SKG-Pya-K-X-Nop-GGK-NH2, Ac-SKG-Pya-A-X-Nop-GGK-NH2, Ac-SKG-Pya-E-X-Nop-GGK-NH2, Ac-SKG-Pya-P-X-Nop-GGK-NH2, andAc-SKG-Pya-T-X-Nop-GGK-NH2. Of these six peptide libraries, although Ac-SKG-Pya-A-X-Nop-GGK-NH2 yielded the highest fluorescent signal, only Ac-SKG-Pya-F-X-Nop-GGK-NH2 was specifically cleaved by ECE-2, producing only background fluorescent responses with ECE-1 (Fig. 3).
FIGURE 3.
Comparative screening of the Ac-SKG-Pya-N1-X-Nop-GGK-CONH2 peptide libraries. Either ECE-1 (white) or ECE-2 (black) at 1 μg/ml was incubated at 37 °C for 3 h with 100 μm of each peptide library in a final volume of 100 μl of 100 mm HEPES, pH 6.8, or 200 mm sodium acetate, pH 5.5, respectively. Control base fluorescence was measured by incubating the peptide library alone in 100 μl of its reaction buffer. The histogram shows the deltas of fluorescence, which correspond to the fluorescence of the assay minus that of the base fluorescence of the library at 180 min.
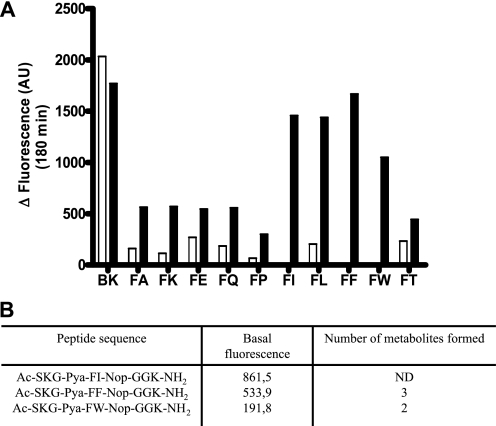
Based on these first results, which suggested the existence of an ECE-2-specific substrate within the Ac-SKG-Pya-F-X-Nop-GGK-NH2 peptide library, we proceeded to test each peptide independently. From the deconvoluted peptide family, three potentially specific ECE-2 versus ECE-1 substrates were identified, i.e. those where X was either Ile, Phe, or Trp (Fig. 4A). Indeed, whereas the commercial substrate McabK2 was efficiently cleaved by both ECE-1 and ECE-2, peptides where X was either isoleucine, phenylalanine, or tryptophan were exclusively cleaved by ECE-2 with fluorescent signals comparable with those obtained with the nonspecific McabK2 substrate (Fig. 4A). Of these candidate peptide substrates, the compound containing an Ile residue was discarded because of its relatively high basal fluorescence resulting from incomplete quenching (Fig. 4B). Study of the cleavage profile by high pressure liquid chromatography of the Ac-SKG-Pya-F-F-Nop-GGK-NH2 and Ac-SKG-Pya-F-W-Nop-GGK-NH2 peptides and analysis of the hydrolysis products by mass spectrometry revealed that although the first peptide was cleaved at two sites, the latter was exclusively cleaved at the Pya–F amide bond (Figs. 4B and 5 and supplemental Fig. S3). Altogether, these results prompted us to choose the latter peptide for further characterization as a candidate model substrate specific for ECE-2, to which we hereafter refer as PL405.
FIGURE 4.
A, comparative ECE-1 and ECE-2 enzymatic activities of the deconvoluted Ac-SKG-Pya-FX-Nop-GGK-CONH2 peptide library. Either ECE-1 (white) or ECE-2 (black) at 1 μg/ml was incubated for 3 h at 37 °C with each peptide (10 μm) in a final volume of 100 μl of 100 mm HEPES, pH 6.8, or 200 mm sodium acetate, pH 5.5, respectively. Positive control incubation was performed in parallel using the McabK2 substrate (BK) at 10 μm with both enzymes, in the same conditions. B, peptide sequence of the three best peptide substrates. The base fluorescence of these three peptides (10 μm) in the ECE-2 reaction buffer was measured. The number of hydrolysis products was observed by HPLC after incubation with 1 μg/ml of ECE-2, and they were subsequently analyzed by mass spectrometry.
FIGURE 5.
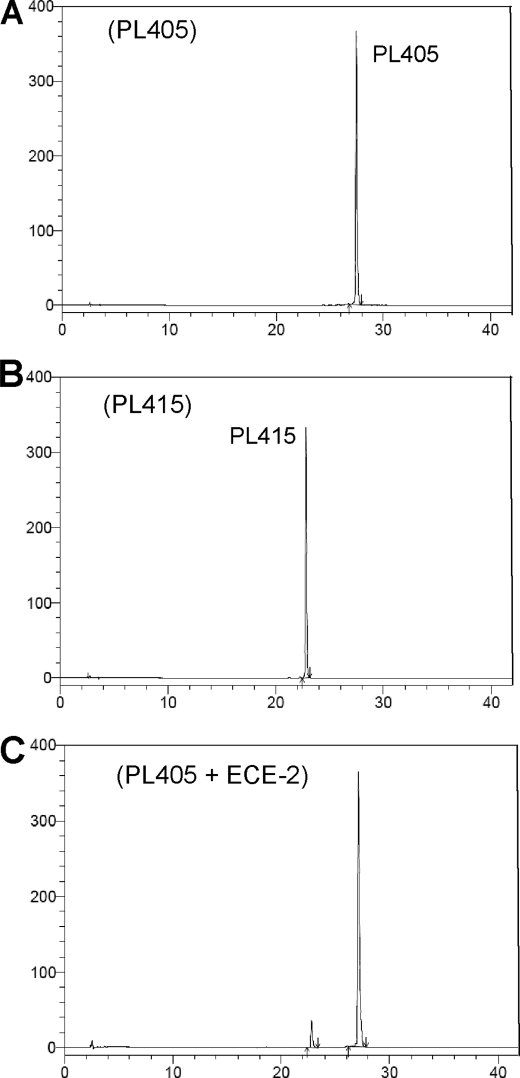
HPLC analysis of the synthetic substrate PL405 cleavage by ECE-2. The HPLC conditions were Kromasil C18 column (5 μm, 100 Å, 4.6 × 150 mm); gradient 10–90% solvent B in 60 min. Solvent A was 0.1% trifluoroacetic acid in H2O. Solvent B was 0.1% trifluoroacetic acid in CH3CN. The flow rate was 1 ml/min. Absorbance detection (UV) was 343 nm. A, injection of 100 μl of 10 μm of PL405 substrate in 200 mm sodium acetate, pH 5.5, 0.2 n HCl. The peak of the substrate has a retention time of 27.8 min. B, injection of 100 μl of 10 μm of Ac-SKG-Pya cleavage product PL415 in the same buffer. The retention time of the peak was 23.1 min. C, the PL405 substrate (20 μm) was incubated 3 h at 37 °C in 200 mm sodium acetate pH 5.5 with 500 ng/ml of ECE-2. The reaction was stopped with 0.2 n HCl, and 100 μl of the reaction were separated by HPLC.
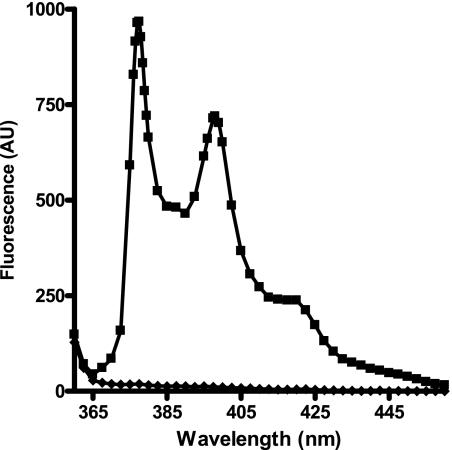
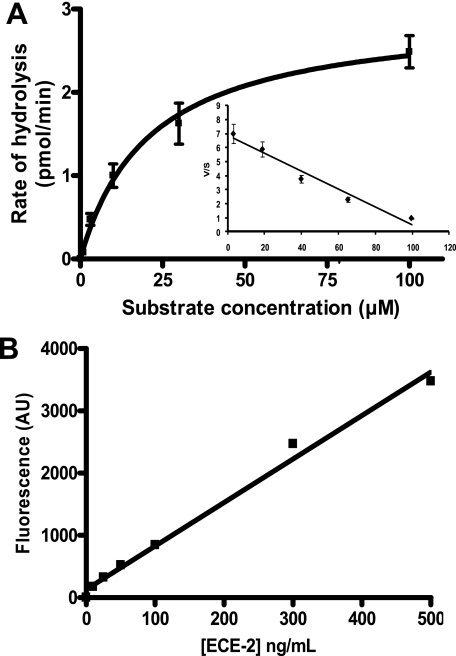
The UV spectrum of the PL405 substrate revealed a maximum absorption (λmax) of 342.5 nm (ϵ = 19,780 liters· mol−1·cm−1), whereas that of its fluorescent cleavage product (PL415) was of 342 nm (ϵ = 24,940 liters·mol−1·cm−1). Their fluorescent spectra obtained after excitation at 343 nm show that although they both display two emission maxima (λem), one at 277 and the other at 397 nm, the PL405 substrate barely emits any signal (Fig. 6). Determination of the kinetic parameters of PL405 was performed using either fluorescence or UV analysis (Fig. 7A and supplemental Fig. S4). The results obtained by fluorescence are confirmed by those obtained by UV quantification of the fluorescent metabolite and show that PL405 is cleaved by ECE-2 with an apparent Michaelis constant of 21 ± 4 μm, a value not very different from the previously published affinity (9 μm) of the McabK2 substrate toward ECE-2 (20). The catalytic rate of PL405 cleavage by ECE-2 is estimated at 0.17 s−1, which, although better than that of its natural substrate, big endothelin-1 (0.0002 s−1), remains slower than that of bradykinin or of its derived McabK2 peptide (5.8 s−1) (20). Detection of ECE-2 using 20 μm of the PL405 substrate was linear, with a regression coefficient of 0.99, and allowed the detection of concentrations as low as 5 ng/ml of enzyme (Fig. 7B).
FIGURE 6.
Emission spectra (λex = 343 nm) of 1 μm of the PL405 substrate (♦) and of 1 μm of the PL415 cleavage product (■) in 200 mm sodium acetate, pH 5.5.
FIGURE 7.
A, kinetic parameters of the ECE-2 enzymatic activity using PL405. Increasing concentrations of PL405 were incubated 60 min at 37 °C in 200 mm sodium acetate, pH 5.5, containing 250 ng/ml of ECE-2. The mean values of two independent experiments performed in duplicate are represented. The Km of the substrate is 21 ± 4 μm, the catalytic constant (kcat) is 0.17 ± 0.02 s−1, and the kcat/Km is 8.1 × 103 ± 0.9 × 103 m−1·s−1. B, cleavage of the PL405 (20 μm) by ECE-2 as a function of enzyme concentrations in vitro (incubation 3 h at 37 °C).
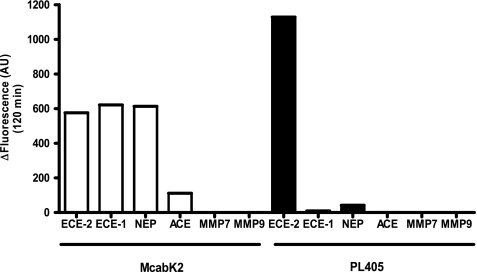
Enzymatic assays comparing the fluorescent signals of the two substrates in parallel, using standard conditions for McabK2 (6 μm) and 20 μm of PL405 with 250 ng/ml of ECE-2 diluted in ECE-2 buffer and incubated at 37 °C for 3 h, show that the fluorescent signal using the PL405 substrate is higher than that of the McabK2 commercial substrate (Fig. 8). Moreover, comparison of the enzymatic specificities of the two substrates reveals that although McabK2 is cleaved by ECE-1 and 2, it is also efficiently cleaved by NEP, as well as, to some extent, by ACE. In contrast, the PL405 fluorigenic peptide substrate was shown to be resistant not only to ECE-1 but also to the actions of NEP and ACE at pH 5.5, the optimal pH for ECE-2 enzymatic activity (Fig. 8). Testing of other, nonrelated metalloproteases of the matrix metalloprotease family (e.g. MMP7 and 9) also reveals that they do not cleave the PL405 substrate (Fig. 8). Serine proteases like trypsin and subtilisin were also found to be inactive in the degradation of PL405, whereas the aspartic protease pepsin produced a fluorescent signal. Cleavage of PL405 by pepsin was, however, completely inhibited in the presence of a protease inhibitor mixture (not shown), which was thereafter included in the assay.
FIGURE 8.
McabK2versus PL405 enzymatic assay: specificity of the PL405 substrate. Histogram showing the specific fluorescent signal measured (total fluorescence minus fluorescence of substrate alone in buffer) with either substrate in the presence of 250 ng/ml of ECE-2, ECE-1, NEP, ACE, MMP7, or MMP9 after a 120-min incubation at 37 °C in 200 mm sodium acetate, pH 5.5. In these conditions, whereas McabK2 is cleaved by ECE-1 and NEP as efficiently as by ECE-2, the novel PL405 substrate is exclusively metabolized by ECE-2.
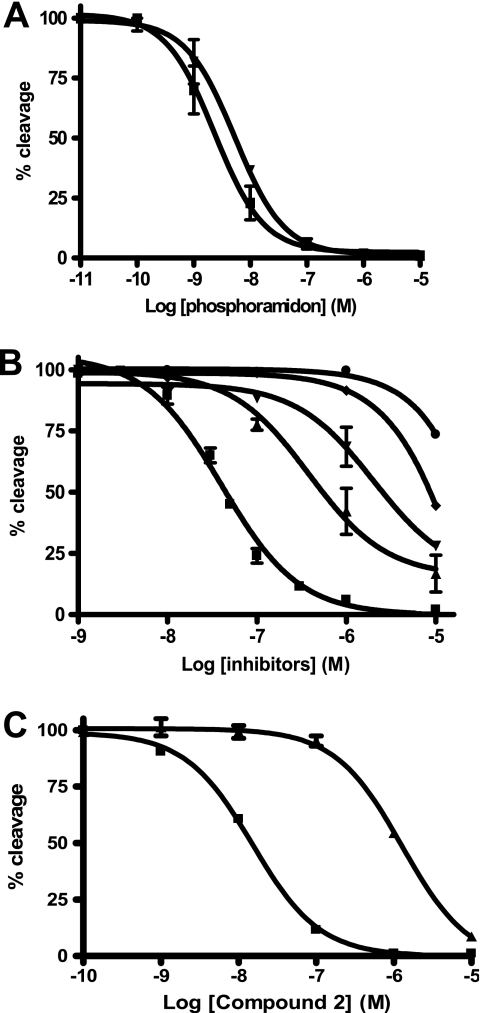
The PL405 substrate was also used to test a variety of inhibitors for their potency against ECE-2. To date, the most potent inhibitor of this metalloprotease is the generic inhibitor of the M13 family, phosphoramidon, with an inhibitory potency in the low nanomolar range (2, 29). Comparison of the inhibitory potency of phosphoramidon against ECE-2 using either McabK2 or PL405 yielded comparable results, in line with those previously published in the literature. Indeed, using either substrate, the Ki of phosphoramidon against ECE-2 was found to be between 1 and 2 nm (Fig. 9A and Table 1). The inhibitory potencies of various compounds known to inhibit zinc-dependent metalloproteases were tested against ECE-2 using the novel PL405 assay. The results of these experiments show that neither thiorphan, retrothiorphan, or kelatorphan, known inhibitors of NEP, nor captopril, an ACE inhibitor, possess inhibitory potency against ECE-2 (Fig. 9B and Table 1). Compounds with nanomolar affinity toward NEP, ACE, and ECE-1 have previously been obtained through rational design (27, 28), but their affinities toward ECE-2 have never been investigated. While testing such inhibitors using PL405, compounds 1 and 2 were found to bind ECE-2 with high affinity. Indeed, whereas compound 1 displayed an inhibitory potency of 22.3 nm (Fig. 9B and Table 1), compound 2 not only bound ECE-2 with even higher affinity, displaying a Ki of 7.7 ± 0.3 nm, but it was also discriminative of the ECEs because it displayed a Ki toward ECE-1 of 480 ± 15 nm (Fig. 9C and Table 1).
FIGURE 9.
A, inhibition of ECE-2 enzymatic activity by phosphoramidon using either McabK2 (▾) or PL405 (■) fluorogenic substrates. Increasing concentrations of phosphoramidon were incubated 10 min at 37 °C with 100 ng/ml of ECE-2 in 200 mm sodium acetate, pH 5.5. The reactions were initiated by the addition of either 10 μm McabK2 or 20 μm PL405 followed by a 30- or 60-min incubation at 37 °C, respectively. B, inhibition of ECE-2 by various metalloprotease inhibitory compounds. Different concentrations of either compound 1 (■), kelatorphan (▴), retrothiorphan (▾), thiorphan (♦), or captopril (●) were incubated 10 min at 37 °C with 100 ng/ml of ECE-2 in 200 mm sodium acetate, pH 5.5. The reactions were initiated by the addition of 20 μm PL405 followed by a 60-min incubation at 37 °C. C, inhibitory potency of compound 2 against ECE-2 using the PL405 assay (■) compared with that against ECE-1 using the McabK2 substrate (▴), revealing a discriminating factor of 62. The obtained Ki values from the conversion of the obtained IC50 values are reported in Table 1.
TABLE 1.
Inhibitory potencies of various compounds against ECE-2
Inhibitory potencies of various metalloprotease inhibitors were evaluated using PL405 (20 μm) as described under “Experimental Procedures.” Ki values were determined from the data displayed in Fig. 9.
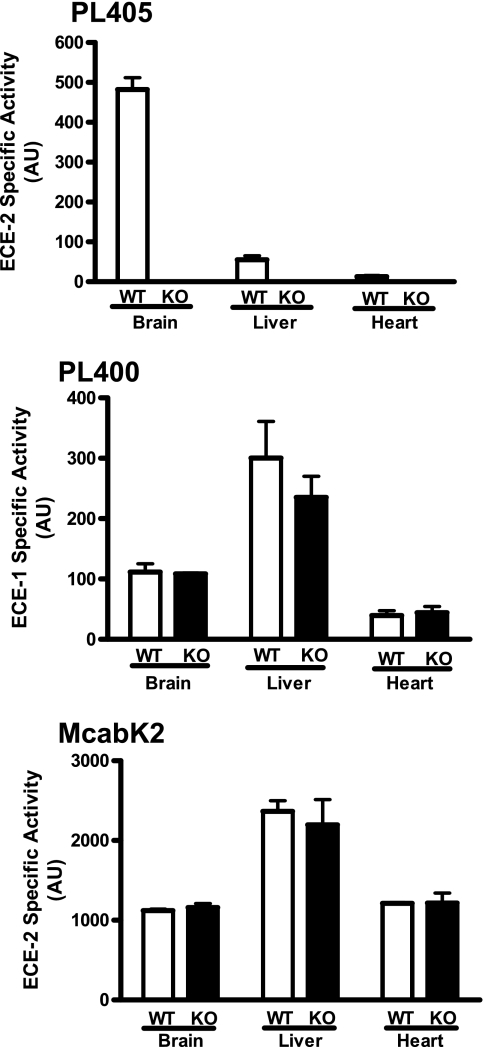
The PL405 substrate also allowed the detection of specific ECE-2 activity in a complex environment, that of membrane preparations from either brain, liver, or heart tissue (Fig. 10). Relevance of this novel assay was moreover illustrated using preparations from tissues dissected from ECE-2 knock-out mice, in which no enzymatic activity could be detected in the same conditions (Fig. 10). Furthermore, enzymatic activity measurements performed in the same conditions but using McabK2 were found to yield the same specific activity for ECE-2 using either wild type or knock-out preparations. The same preparations were also used to test their content in active ECE-1 using the specific PL400 substrate, revealing equal ECE-1 activity in wild type and ECE-2 knock-out tissues (Fig 10).
FIGURE 10.
Ex vivo ECE-2 enzymatic activity in WT and ECE-2 knock-out (KO) tissues. The specific ECE-2 enzymatic activity in solubilized membrane preparations from brain, liver, and heart tissue from either WT or KO animals was measured using 20 μm of the specific PL405 substrate (top panel). The same preparations were used to monitor specific ECE-1 activity (middle panel) using 100 μm PL400. The bottom panel shows the nondiscriminative results obtained using the McabK2 substrate (10 μm). The enzymatic reaction was monitored for 5 h at 37 °C. The results depicted are the means from two independent experiments performed using tissues from two WT and two KO animals.
The assay appears reliable because the PL405 basal fluorescence was stable in heat-denatured membrane preparations, showing that the proteinaceous environment did not affect its internal quenching (not shown). An ECE-2 calibration curve performed in the presence of 10 μg of heat-denatured brain membrane preparation yielded results comparable with those obtained in buffer alone and displayed in Fig. 7B. Taken together, these results allow us to estimate the quantity of active ECE-2 in our brain membrane preparation at 734 ng/mg of total membrane protein.
DISCUSSION
Metalloproteases are emerging as key players in messenger peptide regulation processes, with potential applications in human therapeutics (34). A great number of novel proteases have been identified by DNA sequencing during the genomic era, many of which still remain orphans as far as their true biological functions are concerned because of the lack of specific tools available to study their functions, i.e. specific model substrates and inhibitors.
The ECEs are members of the M13 family of metalloproteases whose prototypical member, NEP, has been well characterized, for which specific inhibitors have been developed and are used in human therapeutics in the gastroentorologic field (35). There has also been considerable interest in the development of “dual” or “triple” inhibitors of NEP, ACE, and ECE-1 for applications in the cardiovascular field (36, 37). Indeed, whereas ECE-1 and ACE are involved in the inactivation of the endothelin-1 and angiotensin II vasoconstrictive peptides, respectively, NEP regulates the half-life of the vasorelaxant natriuretic peptides. Thus, combining the inhibition of NEP with that of either ECE-1 or ACE or both could regulate the two opposing systems to provide a better therapeutic efficacy. The physiological function of ECE-2, however, remains unknown, and the high degree of homology shared between ECE-1 and ECE-2 (∼60%) suggests that specific ECE-1 versus ECE-2 inhibitory compounds may be difficult to identify, as evidenced by the recent modeling of the ECE-2 structure (29). Multiplying the targets for a single compound may, however, raise problems, inducing unwanted side effects.
To address these questions, proper tools to study the specific enzymatic activity of each therapeutic target are required. Thus, to identify a still lacking specific ECE-2 substrate, we screened a series of small fluorigenic peptide libraries. By screening the 10 Ac-SKG-Pya-N(1)-X-Nop-GGK-CONH2 libraries, where N is Ala, Ile, Leu, Lys, Phe, Trp, Glu, Gln, Thr, or Pro and X is a mixture of these 10 amino acids, we identified Ac-SKG-Pya-F-W-Nop-GGK-NH2 as a highly specific and sensitive substrate for ECE-2.
Along this screening process, the systematic change of amino acid in either the N or X position of the Ac-SKG-Pya-N-X-Nop-GGK-CONH2 peptide also allowed us to gain some insight into the active site structure of the enzyme. Indeed, during the initial screening of the libraries, only the peptides containing the AX, EX, TX, KX, PX, or FX amino acid combinations were significantly cleaved by ECE-2, with only the latter being cleaved exclusively by ECE-2. On the assumption that the N and X positions fill the S′1 and S′2 positions in the active site, respectively (positions defined according to the Schechter and Berger nomenclature), these results suggest that the S′1 subsite of ECE-2 can accommodate a charged amino acid but seems to prefer large aromatic or ramified side chains with a specificity toward a single aromatic side chain (Fig. 3). Moreover, the results using the deconvoluted Ac-SKG-Pya-F-X-Nop-GGK-CONH2 library suggest a marked preference for large hydrophobic amino acids in the S′2 position, peptides containing either isoleucine, leucine, phenylalanine, or tryptophan being cleaved at least twice as efficiently as peptides containing small hydrophobic (Ala and Pro) charged (Lys), polar (Gly and Thr), or acidic (Glu) residues (Fig. 4). These results are further illustrated by the observed affinities of phosphoramidon, which contains a large tryptophan-like structure binding in its P′2 position, whereas thiorphan, with its small glycine side chain in the same position, displays no affinity toward ECE-2 (Fig. 9 and Table 1).
The selection of PL405, Ac-SKGPya FWNopGGK-NH2, as the most appropriate ECE-2 substrate among the peptides reported in Fig. 4B was made by taking into account the number of hydrolysis products formed and the level of the basal fluorescence of the substrate. This last parameter is governed by conformational preferences of each peptide substrate because the fluorescence quenching results from internal collision-induced fluorescence quenching (38).
To date, the only fluorigenic peptide available to assess ECE-2 activity in vitro or in vivo is McabK2, which was initially developed as an ECE-1 model substrate by Johnson and Ahn (18). With this and other small substrates, the pH optimum of ECE-1 has been shown to be ∼6 (18, 39), whereas it is in the physiological range for big endothelin cleavage (40). At pH 5.5, corresponding to the ECE-2 pH optimum, we show here that McabK2 is efficiently cleaved not only by ECE-2, but also by ECE-1, as well as by NEP (Fig. 8B). This result is in accordance with the previously published results concerning the pH optimum of ECE-1, as well as that of NEP, which, although it possesses a physiological pH optimum in line with its cell surface localization, is a rather robust enzyme that retains much of its enzymatic activity over a wide range of pH (41).
In contrast, the PL405 substrate is not hydrolyzed by ACE, NEP, ECE-1, or MMPs, but it is efficiently cleaved by ECE-2 in the same conditions (Fig. 8), thus bringing to light, to our best knowledge, the first specific ECE-2 fluorigenic model substrate. The PL405 peptide substrate possesses good kinetic characteristics, with a specificity constant of 8.3 × 103 m−1·s−1. Although this value is 2 orders of magnitude lower than that previously determined for McabK2, it is over 15 times higher than that of its only identified physiological substrate, big endothelin-1 (17). Inhibitory potencies calculated using this substrate are identical to those obtained with the McabK2 substrate and are in accordance with the literature (Fig. 9A and Table 1) (2, 24). Taken together, these data make it a good candidate for high throughput screening endeavors.
Moreover, the previously characterized ECE-1 model substrate, PL400 (16), was shown here to be resistant to ECE-2 cleavage, also bringing to light the first ECE-1-specific versus ECE-2-specific substrate. These results suggest that conformational equilibria of the linear part of big endothelin are different in the presence and in the absence of the cyclic N-terminal part of the endogenous peptide. Thus, the 19–38 big endothelin as well as its modified analog PL400 are cleaved by ECE-1 but not by ECE-2, suggesting that none of the conformers present in these two linear peptides are able to bind the active site of ECE-2 efficiently enough to be cleaved by this enzyme. This unfavorable situation disappears when the cyclic part of big endothelin is present. Nevertheless this cyclic peptide is not indispensable to obtain a good ECE-2 substrate, as illustrated with the short linear selective ECE-2 substrate, PL405 (Figs. 1, 2, and 7).
The proposed role of ECEs in neurodegenerative disease brings to light the necessity of carefully assessing the selectivity of existing or novel inhibitory compounds used or under development for the treatment of other, non-neurodegenerative diseases (27, 28). Among the inhibitors with potential therapeutic applications in the cardiovascular field, it is interesting to note that compound 2, previously identified as a lead triple NEP/ECE-1/ACE inhibitor (27), displays a better ECE-2 recognition than ECE-1, identifying it as the first high affinity discriminating ECE-2/ECE-1 inhibitor, with a specificity factor of 62. It is also noteworthy that compound 1, identified as one of the best triple inhibitors (28) with nanomolar affinity toward the three metalloproteases, also displays an affinity toward ECE-2 in the same range and thus becomes a quadruple inhibitor.
Taken together, the identification of specific substrates not only for ECE-2 but also for ECE-1, combined with that of an inhibitor with some selectivity toward ECE-2, allows the design of specific, sensitive, and rapid detection and quantification methods that can now be used to study and discriminate the ECE-2 and ECE-1 enzymatic activities and in vivo functions. Indeed, as illustrated in Fig. 10, whereas the current ECE-2 enzymatic assay (using McabK2) does not reveal any difference in ECE-2 activity in wild type as compared with ECE-2 knock-out tissues, the PL405 assay provides the first available method to truly assess its ex vivo activity. Moreover, the specific activity of ECE-1 can also be measured using PL400 and reveals for instance that ECE-1 activity is not altered in ECE-2 knock-out animals.
Although the ECEs have been suggested to play a role in Alzheimer disease and β-amyloid clearance (23–25), their true physiological functions in these processes have been difficult to assess. ECE-2 has been identified as a possible specific marker of Alzheimer disease-type dementia in an unbiased screen where it was found to be the most down-regulated gene in human parietal lobe of Alzheimer patients (26). The results of this study have, however, recently been put into question by Palmer et al. (42), who find, using the same techniques but looking specifically at ECE-2, an increase in its expression in the temporal lobe of Alzheimer disease patients. The novel ECE-2 assay now provides a new and unique means to directly monitor and quantify ECE-2 enzymatic activity in healthy versus diseased tissue, which in the end is the only physiologically relevant parameter that should be considered. Moreover, PL405 could also be used to label active ECE-2 directly on brain sections (43), for example to assess the proximity of the enzyme to its putative substrate(s) in distinct cell populations.
In conclusion, by screening our proprietary peptide libraries, we identified the first ECE-2-specific fluorigenic peptide. The PL405 Ac-SKG-Pya-F-W-Nop-GGK-NH2 substrate is cleaved exclusively by ECE-2 at the Pya–F amide bond to yield the highly fluorigenic Ac-SKG-Pya cleavage product. Moreover, during this study, we have also identified the previously published PL400 fluorigenic peptide as an ECE-1-specific substrate. Use of these peptides in vitro as well as ex vivo should aid in the identification of the specific physiological functions of ECE-1 and ECE-2, as well as in high throughput screening endeavors.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants NS26880 and DA19521 (to L. A. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
H. Poras, S. V. Orng, E. Dange, T. Ouimet, M.-C. Fournié-Zaluski, and B. P. Roques, manuscript in preparation.
- ECE
- endothelin-converting enzyme
- NEP
- neprilysin
- ACE
- angiotensin-converting enzyme
- HBTU
- O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- Fmoc
- 9-fluorenylmethoxycarbonyl
- Nop
- para-nitro-l-phenylalanine
- MMP
- matrix metalloprotease
- Rt
- retention time.
REFERENCES
- 1.Xu D., Emoto N., Giaid A., Slaughter C., Kaw S., deWit D., Yanagisawa M. (1994) Cell 78, 473–485 [DOI] [PubMed] [Google Scholar]
- 2.Emoto N., Yanagisawa M. (1995) J. Biol. Chem. 270, 15262–15268 [DOI] [PubMed] [Google Scholar]
- 3.Roques B. P., Noble F., Daugé V., Fournié-Zaluski M. C., Beaumont A. (1993) Pharmacol Rev. 45, 87–146 [PubMed] [Google Scholar]
- 4.Valdenaire O., Richards J. G., Faull R. L., Schweizer A. (1999) Brain Res. Mol. Brain Res. 64, 211–221 [DOI] [PubMed] [Google Scholar]
- 5.Kiryu-Seo S., Sasaki M., Yokohama H., Nakagomi S., Hirayama T., Aoki S., Wada K., Kiyama H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 4345–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S., Zambas E. D., Marsh W. L., Redman C. M. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 6353–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du L., Desbarats M., Viel J., Glorieux F. H., Cawthorn C., Ecarot B. (1996) Genomics 36, 22–28 [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K., Emoto N., Raharjo S. B., Nurhantari Y., Saiki K., Yokoyama M., Matsuo M. (1999) J. Biol. Chem. 274, 32469–32477 [DOI] [PubMed] [Google Scholar]
- 9.Ghaddar G., Ruchon A. F., Carpentier M., Marcinkiewicz M., Seidah N. G., Crine P., Desgroseillers L., Boileau G. (2000) Biochem. J. 347, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouimet T., Facchinetti P., Rose C., Bonhomme M. C., Gros C., Schwartz J. C., Tanja O. (2000) Biochem. Biophys Res. Commun. 271, 565–570 [DOI] [PubMed] [Google Scholar]
- 11.Rose C., Voisin S., Gros C., Schwartz J. C., Ouimet T. (2002) Biochem J. 363, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roques B. P. (1993) Biochem. Soc. Trans. 3, 678–685 [DOI] [PubMed] [Google Scholar]
- 13.Schulz H., Dale G. E., Karimi-Nejad Y., Oefner C. (2009) J. Mol. Biol. 385, 178–187 [DOI] [PubMed] [Google Scholar]
- 14.Oefner C., Roques B. P., Fournie-Zaluski M. C., Dale G. E. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 392–396 [DOI] [PubMed] [Google Scholar]
- 15.Roderick S. L., Fournie-Zaluski M. C., Roques B. P., Matthews B. W. (1989) Biochemistry 28, 1493–1497 [DOI] [PubMed] [Google Scholar]
- 16.Ohnaka K., Takayanagi R., Nishikawa M., Haji M., Nawata H. (1993) J. Biol. Chem. 268, 26759–26766 [PubMed] [Google Scholar]
- 17.Johnson G. D., Stevenson T., Ahn K. (1999) J. Biol. Chem. 274, 4053–4058 [DOI] [PubMed] [Google Scholar]
- 18.Johnson G. D., Ahn K. (2000) Anal. Biochem. 286, 112–118 [DOI] [PubMed] [Google Scholar]
- 19.Luciani N., de Rocquigny H., Turcaud S., Romieu A., Roques B. P. (2001) Biochem. J. 356, 813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mzhavia N., Pan H., Che F. Y., Fricker L. D., Devi L. A. (2003) J. Biol. Chem. 278, 14704–14711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selkoe D. J. (2001) Neuron 32, 177–180 [DOI] [PubMed] [Google Scholar]
- 22.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N. P., Gerard C., Hama E., Lee H. J., Saido T. C. (2001) Science 292, 1550–1552 [DOI] [PubMed] [Google Scholar]
- 23.Eckman E. A., Watson M., Marlow L., Sambamurti K., Eckman C. B. (2003) J. Biol. Chem. 278, 2081–2084 [DOI] [PubMed] [Google Scholar]
- 24.Eckman E. A., Adams S. K., Troendle F. J., Stodola B. A., Kahn M. A., Fauq A. H., Xiao H. D., Bernstein K. E., Eckman C. B. (2006) J. Biol. Chem. 281, 30471–30478 [DOI] [PubMed] [Google Scholar]
- 25.Funalot B., Ouimet T., Claperon A., Fallet C., Delacourte A., Epelbaum J., Subkowski T., Léonard N., Codron V., David J. P., Amouyel P., Schwartz J. C., Helbecque N. (2004) Mol. Psychiatry 9, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 26.Weeraratna A. T., Kalehua A., Deleon I., Bertak D., Maher G., Wade M. S., Lustig A., Becker K. G., Wood W., 3rd, Walker D. G., Beach T. G., Taub D. D. (2007) Exp. Cell. Res. 313, 450–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inguimbert N., Poras H., Teffo F., Beslot F., Selkti M., Tomas A., Scalbert E., Bennejean C., Renard P., Fournié-Zaluski M. C., Roques B. P. (2002) Bioorg. Med. Chem. Lett. 12, 2001–2005 [DOI] [PubMed] [Google Scholar]
- 28.Inguimbert N., Coric P., Poras H., Meudal H., Teffot F., Fournié-Zaluski M. C., Roques B. P. (2002) J. Med. Chem. 45, 1477–1486 [DOI] [PubMed] [Google Scholar]
- 29.Gagnidze K., Sachchidanand, Rozenfeld R., Mezei M., Zhou M. M., Devi L. A. (2008) J. Med. Chem. 51, 3378–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubry M., Berteloot A., Beaumont A., Roques B. P., Crine P. (1987) Biochem. Cell. Biol. 65, 398–404 [DOI] [PubMed] [Google Scholar]
- 31.Piquilloud Y., Reinharz A., Roth M. (1970) Biochim. Biophys. Acta 206, 136–142 [DOI] [PubMed] [Google Scholar]
- 32.Yanagisawa H., Hammer R. E., Richardson J. A., Emoto N., Williams S. C., Takeda S., Clouthier D. E., Yanagisawa M. (2000) J. Clin. Invest. 105, 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguiz R. M., Gadnidze K., Ragnauth A., Dorr N., Yanagisawa M., Wetsel W. C., Devi L. A. (2008) Genes Brain Behav. 7, 418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roques B. P. (2000) Trends Pharmacol. Sci. 21, 475–483 [DOI] [PubMed] [Google Scholar]
- 35.Roques B. P., Fournié-Zaluski M. C., Soroca E., Lecomte J. M., Malfroy B., Llorens C., Schwartz J. C. (1980) Nature 288, 286–288 [DOI] [PubMed] [Google Scholar]
- 36.Daull P., Benrezzak O., Arsenault D., Pheng L. H., Blouin A., Cayer J., Beaudoin M., Belleville K., Sirois P., Nantel F., Jeng A. Y., Battistini B. (2005) Am. J. Hypertens. 18, 1606–1613 [DOI] [PubMed] [Google Scholar]
- 37.Fournié-Zaluski M. C., Gonzalez W., Turcaud S., Pham I., Roques B. P., Michel J. B. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 4072–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valeur B. (2004) Invitation à la Fluorescence Moléculaire, De Boech Université, Brussels, Belgium [Google Scholar]
- 39.Ahn K., Herman S. B., Fahnoe D. C. (1998) Arch. Biochem. Biophys. 359, 258–268 [DOI] [PubMed] [Google Scholar]
- 40.Fahnoe D. C., Knapp J., Johnson G. D., Ahn K. (2000) J. Cardiovasc. Pharmacol. 36, S22–S25 [DOI] [PubMed] [Google Scholar]
- 41.Devault A., Nault C., Zollinger M., Fournie-Zaluski M. C., Roques B. P., Crine P., Boileau G. (1988) J. Biol. Chem. 263, 4033–4040 [PubMed] [Google Scholar]
- 42.Palmer J. C., Baig S., Kehoe P. G., Love S. (2009) Am. J. Pathol. 175, 262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Back S. A., Gorenstein C. (1989) J. Neurosci. 9, 4439–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.