Abstract
PiT1/SLC20A1 is a sodium-dependent Pi transporter expressed by most mammalian cells. Interestingly, PiT1 transcription has been shown to be up-regulated by the tumor necrosis factor α (TNF), and we have now investigated the possible involvement of PiT1 in TNF-induced apoptosis. We show that PiT1-depleted cells are more sensitive to the proapoptotic activity of TNF (i.e. when the antiapoptotic NFκB pathway is inactivated). These observations were made in the human HeLa cancer cell line either transiently or stably depleted in PiT1 by RNA interference and in immortalized mouse embryonic fibroblasts isolated from PiT1 knock-out embryos. Depletion of the closely related family member PiT2 had no effect on TNF-induced apoptosis, showing that this effect was specific to PiT1. The increased sensitivity of PiT1-depleted cells was evident regardless of the presence or absence of extracellular Pi, suggesting that a defect in Pi uptake was not involved in the observed phenotype. Importantly, we show that the re-expression of a Pi uptake mutant of PiT1 in PiT1−/− mouse embryonic fibroblasts delays apoptosis as efficiently as the WT protein, showing that this function of PiT1 is unrelated to its transport activity. Caspase-8 is more activated in PiT1-depleted cells, and our data reveal that the sustained activation of the MAPK JNK is up-regulated in response to TNF. JNK activity is actually involved in PiT1-depleted cell death because specific JNK inhibitors delay apoptosis.
Keywords: Apoptosis, Carcinogenesis, JNK, Multifunctional Protein, Tumor Necrosis Factor (TNF), Inorganic Phosphate, RIP1, Transporter, Caspase-8
Introduction
PiT1/SLC20A1 and PiT2/SLC20A2 are the unique members of the mammalian Pi transporter family SLC20A (1). Both were first identified as retrovirus receptors (2, 3) before being recognized as sodium-dependent Pi transporters (PiTs)2 (4). They also belong to the large family of transporters 2A.20 (Transport Classification Database (5)), which comprises symporters that use either a proton or sodium gradient to import Pi and are found throughout all kingdoms of life. The broad mRNA distribution of PiT1 and PiT2 in mammalian tissues and organs has led to the proposal that PiT1 and PiT2 could serve a housekeeping role in Pi homeostasis (4, 6) and provide cells with their basic Pi needs. However, the knock-out of PiT1 in mice (by two different groups, including ours (7, 8)) revealed an unexpected phenotype. Homozygous deletion of PiT1 resulted in embryonic lethality at embryonic day 12.5 (7). Further investigations showed that PiT1 is an essential gene for liver development (7). PiT1-deficient embryos displayed hypoplastic fetal livers and subsequent reduced hematopoiesis, resulting in severe anemia and death. Embryonic day 12.5 liver cells were reduced in number and showed signs of massive apoptosis (7). In another study published recently, we have also revealed the involvement of PiT1 in the regulation of cancer cell proliferation (9). In vitro depletion of PiT1 in HeLa or HepG2 cells impairs their proliferation. Importantly, we showed that this property was not shared with PiT2, whose depletion had no effect on proliferation. Finally, we provided direct evidence that the modulation of cell proliferation by PiT1 is independent of its transport function because the proliferation of PiT1-depleted cells could be rescued by non-transporting PiT1 mutants (9).
Although not formally demonstrated, several studies have suggested that PiT1 expression could be regulated by the induced or basal activity of the transcription factor NFκB (nuclear factor κB) (10–12). The NFκB pathway is a well described antiapoptotic pathway that is induced by various cytokines or chemicals, such as tumor necrosis factor α (TNF), interleukin-1α, or phorbol 12-myristate 13-acetate for example. Interestingly, these agents also increase the expression of PiT1 in diverse cell types (10, 12). The up-regulation of PiT1 expression induced by the NFκB pathway prompted us to investigate whether PiT1 could be involved in providing some protection against cell apoptosis. We chose to investigate the TNF-induced apoptosis model because of the physiological importance of TNF (13, 14) and because TNF up-regulates PiT1 mRNA (12).
TNF is a pleiotropic proinflammatory cytokine that has important roles in diverse cellular events, such as cell proliferation, differentiation, and apoptosis (14). TNF can bind to two different receptors (TNFRs), TNFR1 and TNFR2. TNFR1 is broadly expressed in most tissues, whereas the expression of TNFR2 is highly regulated and is typically found in cells of the immune system. TNF binding to TNFR1 promotes its clustering and the formation of several sequential intracellular complexes (15, 16). The first complex (termed complex I) (15) is proposed to be mainly involved in signaling pathways, inducing the activation of several kinases, such as IκB kinase, JNK, p38, ERK, and others. Early signaling through JNK (17, 18) and especially through IκB kinase and the NFκB pathway (14, 19, 20) constitutes the main antiapoptotic signals triggered in response to TNF. Subsequently, complex I dissociates from the membrane-bound TNFR1 and relocates to the cytoplasm to form several distinct proapoptotic complexes, all of them containing caspase-8 as the initiator caspase (15, 16). Apoptosis occurrence is mainly regulated by the interplay between the prosurvival NFκB pathway mentioned above and the proapoptotic sustained phase of JNK activation (21–24). NFκB induces the synthesis of important antiapoptotic proteins regulating caspase-8 activation and also limits the duration of JNK activity via several mechanisms (21, 25–28). JNK is a stress-activated MAPK, and there are three mammalian JNK genes with splicing variants p46 and p54 (29). JNK1 and -2 are ubiquitously expressed, whereas JNK3 is restricted to specific tissues. Both JNK1 and JNK2 have been shown to be involved in TNF-induced apoptosis in different cell types, and the sustained phase of JNK signaling is emerging as a central activator of apoptosis (18). JNK induces the accelerated degradation of the antiapoptotic protein cFLIP (cellular FLICE-interacting protein) (30, 31) and also mediates the release of Smac (small mitochondrial activator of caspase) from the mitochondria, which is essential for caspase-8 activation (32).
Our results demonstrate that PiT1 depletion (but not PiT2) sensitizes both human and mouse immortalized cells to the proapoptotic activity of TNF. This phenotype is independent of the presence or absence of Pi in the extracellular medium. The re-expression of PiT1 in PiT1 knock-out mouse fibroblasts delays TNF-induced apoptosis. Importantly, the same protection is provided by the expression of a transport-incompetent mutant of PiT1, showing that the involvement of PiT1 in TNF-induced apoptosis is independent of its Pi transport function. Finally, we show that the sustained phase of JNK activity is enhanced in PiT1-depleted cells and that JNK activity is instrumental in their death.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
Human TNF was purchased from Gentaur (Brussels, Belgium), JNKi from Merck. DNase-free RNase, cycloheximide, propidium iodide, BI-78D3, and SP600125 were from Sigma. Lipofectamine LTX was obtained from Invitrogen. The following antibodies were purchased from Cell Signaling Technology: anti-phospho-SAPK/JNK (Thr183–Tyr185) (catalog no. 9251), IκBα (catalog no. 4814), RIP1 (receptor-interacting protein 1) (catalog no. 3493), cleaved caspase-3 (catalog no. 9661), poly(ADP-ribose) polymerase (PARP) (catalog no. 9532), caspase-8 (catalog no. 9746), AMPKα (catalog no. 2532), and phospho-AMPKα Thr172 (catalog no. 2535). The human PiT1 antibody was produced in the laboratory and described previously (9). Monoclonal anti-β-actin clone AC-74 (catalog no. A5316) and anti-β-tubulin clone TUB2.1 (catalog no. T4026) were from Sigma.
Cells
HeLa cells and immortalized fibroblasts were grown in Dulbecco's minimum essential medium (Invitrogen) supplemented with 5% fetal bovine serum (Hyclone) and gentamicin (Invitrogen). Isolation of embryonic day 12.5 mouse embryonic fibroblasts (MEFs) was performed using established procedures (33). They were immortalized at passage 2 by the transfection (with Lipofectamine LTX) of a plasmid encoding the thermosensitive SV40 large T antigen under the human vimentin promoter (34) and then grown at 33 °C. HeLa cell clones stably expressing shRNAs were described (9).
Lentiviral Vector Stocks
The introduction of the S621A mutation into PiT1 was described previously (9). The DNA constructs containing either WT PiT1 or PiT1 S621A were inserted in the lentiviral vector pHAGE-CMV-MCS-IZsGreenW. Two independent populations of cells were obtained for each construct by infecting cells with two distinct viral stocks. GFP-positive cells were enriched by fluorescent cell sorting.
Pi Uptake
The transport of phosphate was measured as described previously (9).
Apoptosis Induction on Human and Mouse Cells
HeLa cells were transfected with small interfering RNA as described (9) 48–72 h before treatment. MEFs were split 48 h before treatment. Apoptosis was induced by the treatment of cells with 50 ng/ml human TNF and 10–100 μg/ml cycloheximide in complete medium. When JNK inhibitors were used, the cells were pretreated for 30 min with the drug and then treated with TNF plus cycloheximide (TNF+C) together with the drug. When experiments in defined Pi medium were performed, the cells were prepared as usual and washed three times with 0.9% NaCl before treatment to remove all traces of Pi. Pi-free/pyruvate-free Dulbecco's minimum essential medium (Invitrogen catalog no. 11971) (supplemented with 1 mm sodium pyruvate, Invitrogen catalog no. 11360) or the corresponding regular medium (Invitrogen catalog no. 41966) (containing 0.9 mm Pi and 1 mm sodium pyruvate) were used without any serum added because serum contains Pi.
Propidium Iodide Staining for DNA Analysis by Flow Cytometry
Cells were transfected with the corresponding siRNAs 72 h before treatment. All cells were collected (floating and attached), resuspended in PBS, and fixed in cold 70% ethanol overnight (or longer) at 4 °C. They were then washed in PBS and resuspended in a solution containing 0.02 mg/ml propidium iodide in PBS containing 0.1% Triton X-100, 0.2 mg/ml DNase-free RNase for 30 min at room temperature before FACS analysis. The DNA content was analyzed by flow cytometry on a BD Biosciences FACSCalibur flow cytometer with CELLQuest software.
Immunoblot Analysis
Cells were placed on ice, and floating cells were collected. Attached cells were rinsed once with 0.9% NaCl, and this solution was used to wash the pellet of detached cells. Cells were then lysed by the addition of ice-cold lysis buffer (150 mm NaCl, 10 mm Tris-HCl, 5 mm EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, and complete protease inhibitor mix (Roche Applied Science), 2 mm Na3VO4, 2 mm NaF, 5 mm sodium pyrophosphate, 20 mm N-ethylmaleimide) on ice and detached with cell scrapers. The lysate was used to resuspend the pellet of detached cells, and the combined lysate was left on ice for 30 min. Lysates were then centrifuged for 15 min at 16,000 × g (4 °C) to remove insoluble materials, and the supernatants were used for Western blot analysis, which was performed as described (9). The numbers to the left of all Western blot figures represent the molecular weight of proteins from the Full-Range Rainbow marker (catalog no. RPN800E, Amersham Biosciences), and numbers to the right are the theoretical sizes of the proteins recognized by the specific antibodies.
Statistics
Blots were quantified with the ImageJ software. All graphs are plotted as mean ± S.E. Statistics for dual comparison were generated using Student's t tests (not significant (ns), p ≥ 0.05; *, p < 0.05; **, p < 0.005; ***, p < 0.0005).
RESULTS
Acute or Stable PiT1 Depletion Sensitizes Immortalized Human and Mouse Cells to TNF-induced Apoptosis
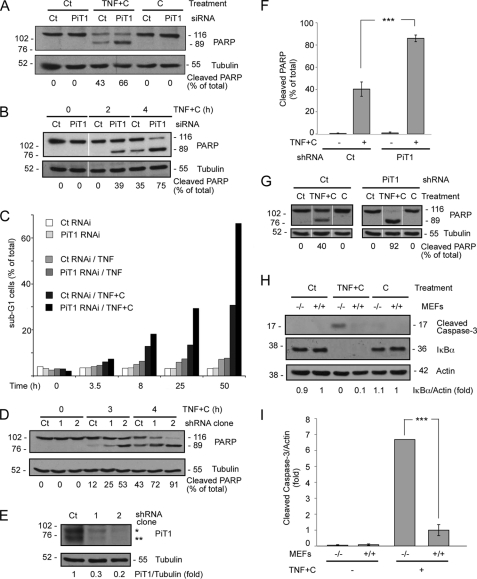
PiT1 was transiently depleted in the human carcinoma HeLa cell line with an siRNA oligonucleotide. The efficiency of the siRNAs used in this study at down-regulating the expression of the endogenous PiT1 in HeLa cells has been demonstrated previously (9). When treated with the proinflammatory cytokine TNF alone, most cells resist its proapoptotic activity, unless they are sensitized with the addition of the protein synthesis inhibitor cycloheximide. TNF is a powerful inducer of the antiapoptotic NFκB pathway, and the production of NFκB-dependent proteins needs to be inhibited for apoptosis to proceed (19). HeLa cells were treated with a combination of TNF and cycloheximide for 4 h. Apoptosis progression was followed by detection of the specific cleavage of PARP, a protein whose processing is under the control of the “terminal caspase” caspase 3. Fig. 1A shows that PiT1-depleted HeLa cells were more sensitive to the proapoptotic activity of TNF than the control cells (66% of cleaved PARP versus 43%). The depletion of PiT1 alone or the addition of cycloheximide alone could not induce apoptosis in these cells, showing the specific activity of TNF in apoptosis induction. PARP processing can be detected earlier (at 2 h post-treatment) and progresses faster (at 4 h post-treatment) in PiT1-depleted cells than in WT cells (Fig. 1B).
FIGURE 1.
PiT1-depleted cells are more sensitive to TNF-induced apoptosis. A–C, HeLa cells were transiently transfected with an siRNA control (Ct) or an siRNA directed against PiT1. 48 h after transfection, they were either left untreated or treated with 10 μg/ml cycloheximide (C) alone, 50 ng/ml TNF alone (TNF), or 50 ng/ml TNF plus 10 μg/ml cycloheximide (TNF+C) for different times. A, cells were treated for 4 h and then analyzed by Western blot against PARP and tubulin as a loading control. The bands corresponding to full-length PARP (p116) and cleaved PARP (p89) were quantified, and the percentage of cleaved PARP is given below the panels of blots. B, cells were treated with TNF+C for 0, 2, or 4 h and analyzed by Western blot against PARP and tubulin. The bands corresponding to full-length PARP (p116) and cleaved PARP (p89) were quantified, and the percentage of cleaved PARP is given under the panels of blots. C, cells were stained with propidium iodide for the analysis of their DNA content, and the percentage of cells with a sub-G1 staining was calculated. D, HeLa cell clones stably expressing either a control (Ct) or a PiT1 shRNA (clones 1 and 2) were treated with 50 ng/ml TNF and 10 μg/ml cycloheximide (TNF+C) for 0, 3, or 4 h. Apoptosis induction was detected by Western blot showing the cleavage of PARP. The detection of tubulin was used as a loading control. The percentage of cleaved PARP was quantified and is given below. E, Western blot showing the total amount of PiT1 protein expressed by the different clones. The PiT1 antibody (9) is specific for the human protein and detects mainly two forms of the protein in HeLa cells indicated by an asterisk and a double asterisk, which may differ by the glycosylation status. The numbers given below are the ratio of the intensity of PiT1 bands over tubulin, used as a loading control. F, cells (shCt clone or shPiT1 clone 2) were treated with TNF+C for 4 h and analyzed for PARP cleavage by Western blot as in D. Values are the mean ± S.E. (error bars) of six independent experiments. ***, p < 0.0005. G, shRNA control or PiT1 cells were either left untreated (Ct) or treated with 10 μg/ml cycloheximide alone (C) or 50 ng/ml TNF plus 10 μg/ml cycloheximide (TNF+C) for 4 h. Apoptosis was detected by the cleavage of PARP, and tubulin was used as a loading control. The intensity of the bands was quantified, and the percentage of cleaved PARP is given below the Western blot panels. H, T SV40 immortalized MEFs from a knock-out PiT1 embryo (−/−) or a WT littermate (+/+) were incubated with 50 ng/ml human TNF and 100 μg/ml cycloheximide (TNF+C) or 100 μg/ml cycloheximide alone (C) for 7.5 h. Cells were lysed and analyzed by Western blot for the appearance of the cleaved form of caspase-3 and tubulin as a loading control as well as the amount of IκBα. The numbers given below are the ratio of the intensity of IκBα signal over actin, used as a loading control. I, quantification of the apoptosis induction for four independent experiments performed as in H. Values are the mean ± S.E. ***, p < 0.0005.
We also followed later events in apoptosis by detecting the appearance of a sub-G1 DNA peak by propidium iodide staining of the cells and FACS analysis (Fig. 1C). Wild-type (WT) cells started showing some apoptotic staining by 8 h, reaching 30% at 50 h post-treatment. PiT1-depleted cells were more sensitive to apoptosis, and the percentage of apoptotic cells was double that of WT cells at 25 and 50 h post-treatment. When treated with TNF alone, HeLa cells (with or without PiT1) did not show any massive apoptosis, confirming that cycloheximide was required to reveal the proapoptotic activity of TNF.
We have previously generated HeLa cell lines with a stable reduced expression of PiT1 by using an shRNA transfection (9). Fig. 1D shows that two different shRNA PiT1 clones were more sensitive to apoptosis than HeLa cells expressing a control shRNA, as shown by their increased PARP processing at 3 and 4 h post-TNF+C treatment. This shows that TNF-mediated apoptosis was affected by either transient or stable PiT1 depletion. Moreover, this effect may be dependent on the amount of PiT1 still expressed by the cells because clone 2, which expresses a lesser amount of PiT1 than clone 1 (Fig. 1E), was more sensitive to apoptosis than clone 1. Clone 2 was chosen for further experiments, and Fig. 1F represents the results gathered from six independent experiments performed with this cell clone. On average, 86 ± 3% of PARP was cleaved in PiT1-depleted HeLa cells after 4 h of TNF+C treatment compared with 40 ± 6% in control cells. Consistent with results obtained in HeLa cells transiently depleted in PiT1 by siRNA, this clone was not sensitive to cycloheximide alone, shown as a control in Fig. 1G.
In order to strengthen our results, we also tested whether we could reproduce them in a different cellular system. We immortalized MEFs isolated from WT and PiT1 knock-out embryonic day 12.5 embryos (7) with SV40 large T antigen expression. As with HeLa cells, we treated these cells with TNF and 10 μg/ml cycloheximide in preliminary experiments (not shown). We chose to use the human TNF cytokine because it exclusively binds to mouse TNFR1 (and not TNFR2) (35). The amount of cycloheximide used so far (10 μg/ml) was actually not enough for a complete inhibition of NFκB dependent protein synthesis in response to TNF (36). Because MEFs (as opposed to HeLa cells) can withstand an increased amount of cycloheximide without adverse short term effects, we increased the concentration of cycloheximide to 100 μg/ml in subsequent experiments. Cycloheximide alone did not induce apoptosis (Fig. 1H) but was indeed able to fully block protein synthesis induced by the activation of the NFκB pathway in response to TNF, as shown by the complete lack of IκBα de novo synthesis several h after TNF stimulation (Fig. 1H). IκBα is known to be rapidly degraded in response to TNF stimulation, and its resynthesis normally starts within 1 h, strictly under the control of the NFκB transcription factor (36). Fig. 1, H and I, shows that PiT1 gene knock-out increased the sensitivity to apoptosis of immortalized mouse fibroblasts, as detected by the increased cleavage of caspase 3. There was an averaged 6.5-fold increase in caspase-3 cleavage in PiT1−/− MEFs compared with PiT1+/+ MEFs after 7 h of TNF+C treatment (Fig. 1I).
Taken together, these data showed that PiT1 depletion (either by transient or stable RNA interference or gene knock-out) increased the sensitivity to TNF-mediated apoptosis in both human and mouse immortalized cells.
The Role of PiT1 in TNF-induced Apoptosis Is Not Shared with PiT2 and Does Not Depend on the Presence of Extracellular Phosphate
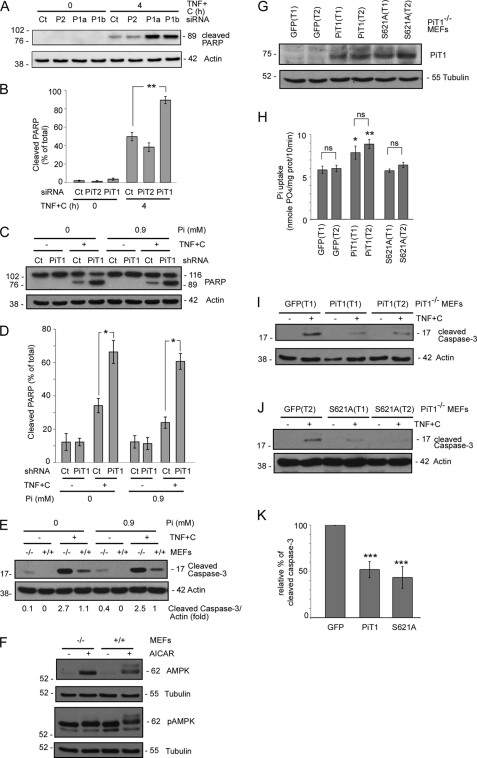
HeLa cells also express PiT2/SLC20A2, the second Pi transporter encoded by the PiT/SLC20A family (9). PiT1 and PiT2 depletion reduce Pi uptake in HeLa cells (9). However, as opposed to PiT1 depletion, siRNA-mediated PiT2 depletion (9) had no effect on the sensitivity of HeLa cells to TNF-induced apoptosis, showing that this effect is specific to PiT1 depletion (Fig. 2A). This specificity was further ensured by the use of two different siRNA oligonucleotides targeting distinct regions of the PiT1 mRNA in the experiment shown in Fig. 2A. Both were equally efficient at reducing the amount of endogenous PiT1 expressed by HeLa cells (9). Cells transfected with either PiT1 siRNA presented an enhanced cleavage of PARP as compared with WT cells, strengthening the involvement of PiT1 in TNF-induced apoptosis. As opposed to cells transfected with PiT1 siRNAs, cells transfected with a PiT2 siRNA did not show any increase in PARP cleavage and apoptosis, showing that this effect is specific to PiT1 depletion. Fig. 2B represents the averaged data gathered from three independent experiments and shows that the amount of apoptosis in cells treated with siRNAs directed against PiT1 was almost twice the amount shown by cells treated either with a control (89 ± 4% of cleaved PARP versus 50 ± 5%) or a PiT2 (38 ± 5% of cleaved PARP) siRNA after 4 h of TNF+C treatment.
FIGURE 2.
PiT1 involvement in TNF-induced apoptosis is (i) not shared with PiT2, (ii) independent of the presence of extracellular Pi and (iii) unrelated to its transport activity. A, HeLa cells were transiently transfected with a siRNA control (Ct), two different siRNAs against PiT1 (P1a and P1b), or an siRNA against PiT2 (P2). 48 h post-transfection, cells were either mock-treated (0) or treated with 50 ng/ml TNF and 10 μg/ml cycloheximide for 4 h. Cells were lysed and analyzed by Western blot with antibodies directed against PARP and actin (as a loading control). B, values are the mean ± S.E. of three independent experiments performed as in A. **, p < 0.005. C, shCt or shPiT1 HeLa cells were incubated with or without a combination of 50 ng/ml TNF and 10 μg/ml cycloheximide (TNF+C) in medium containing either 0 or 0.9 mm Pi for 4 h. Apoptosis was detected by Western blot with antibodies against PARP and actin as loading control. D, three independent experiments were performed as in C and quantified. Values are the mean ± S.E. (error bars). *, p < 0.05. E, PiT1+/+ and PiT1−/− MEFs were incubated with or without 50 ng/ml TNF plus 100 μg/ml cycloheximide (TNF+C) for 7 h in medium containing either 0 or 0.9 mm Pi. Apoptosis was detected by the appearance of cleaved caspase-3, and actin was used as a loading control. This representative blot was quantified, and the corresponding values are given below the Western blot panel. F, PiT1+/+ and PiT1−/− MEFs were either left untreated (−) or treated (+) with 2 mm AICAR for 7 h. Cell lysates were analyzed by Western blot for the phosphorylation of AMPK (pAMPK) and the total amount of AMPK. The detection of tubulin was used as a loading control. G–K, PiT1−/− MEFs were transduced with lentiviral vectors expressing either the human WT PiT1 protein or the S621A mutant and the GFP protein from an internal ribosome entry site. Two independent infections (with two different virus stocks) were performed to obtain two independent populations in each case (T1 and T2). GFP-positive cells were enriched by fluorescent cell sorting. G, PiT1 proteins were detected with the anti-PiT1 antibody. H, sodium-dependent Pi uptake was measured in the different transduced populations. *, p < 0.05; **, p < 0.005; ns, nonsignificant. I and J, the different populations were treated (+) or not (−) with 50 ng/ml TNF and 100 μg/ml cycloheximide for 3.5 h. Cell lysates were analyzed for the appearance of cleaved caspase-3, and the detection of actin was used as a loading control. K, quantification of the blots shown in I and J. Three independent experiments (with the two different cell populations for each constructs) were analyzed. The ratio of the intensities of the cleaved caspase-3 band/actin band was quantified and pooled for each construct and is presented as the relative percentage of the intensity of caspase-3 cleavage quantified in GFP expressing control PiT1−/− MEFs. ***, p < 0.0005.
These data also suggested that a deficiency in Pi transport was not likely to be responsible for the observed phenotype. In order to investigate further the impact of Pi on TNF-induced apoptosis, we performed experiments in the presence or absence of Pi in the incubation medium. As shown in Fig. 2, C and D, the impact of PiT1 depletion on TNF-induced apoptosis in HeLa cells was observed regardless of the presence or absence of Pi in the medium. Moreover, the sensitivity of WT HeLa and MEF cells (therefore expressing PiT1) to TNF-induced apoptosis was not different whether cells were incubated with or without Pi (Fig. 2, C–E), which strongly suggests that a defect in Pi uptake via PiT1 was not responsible for the increased sensitivity of PiT1-depleted cells to TNF-induced apoptosis. Finally, because Pi is a structural component of ribonucleotides, we reasoned that PiT1 depletion could affect the cellular ATP availability. The AMP-activated protein kinase (AMPK) is an extremely sensitive indicator of cell metabolic stress and becomes phosphorylated when the AMP/ATP ratio is increased (37). We therefore determined the basal phosphorylation status of AMPK in PiT1-depleted cells. Fig. 2F shows that this kinase was phosphorylated neither in WT nor in PiT1−/− MEFs, despite the presence of a functional enzyme, as demonstrated by its phosphorylation following the addition of the drug 5-amino-4-imidazolecarboxamide riboside (AICAR), a specific activator (37). Therefore, this observation suggested that PiT1 depletion did not create any basal energetic stress within the cell.
PiT1 Involvement in TNF-mediated Apoptosis Is Uncoupled from Its Pi Transport Activity
To test more directly whether PiT1 involvement in TNF-mediated apoptosis was linked to its Pi uptake activity, we generated a transport-incompetent mutant of PiT1 in which the serine at position 621 was mutated to an alanine (9). The V5-tagged construct, encoding either WT PiT1 or the S621A-mutated protein, was inserted into a lentiviral vector and stably transduced into PiT1−/− MEFs. MEF cells were chosen over HeLa cells because they provide the “cleanest” background for a rescue experiment, having no expression at all of endogenous PiT1 due to gene deletion. For each construct, we characterized two independently transduced populations (T1 and T2) of PiT1−/− MEFs expressing the recombinant proteins, detected with the anti-PiT1 antibody (Fig. 2G). As expected, PiT1−/− MEFs re-expressing the WT PiT1 protein displayed a significantly increased Pi uptake compared with the parental cells (Fig. 2H), whereas Pi uptake was unaffected by the expression of the transport-incompetent mutant PiT1 S621A. Fig. 2I showed that the re-expression of WT PiT1 in PiT1−/− cells delayed the appearance of the cleaved product of caspase-3, showing that PiT1 depletion was indeed involved in the increased sensitivity of the cells to apoptosis. Importantly, the expression of the PiT1 S621A mutant protein also delayed apoptosis, as shown by the reduced cleavage of caspase-3 (Fig. 2J). Both proteins were equally efficient at reducing the appearance of caspase-3 to 50% of that displayed by the control PiT1−/− MEFs (Fig. 2K). This demonstrates that PiT1 involvement in TNF-induced apoptosis can therefore be uncoupled from its Pi uptake function.
Caspase-8 Activation Is Increased by PiT1 Depletion in TNF-induced Apoptosis
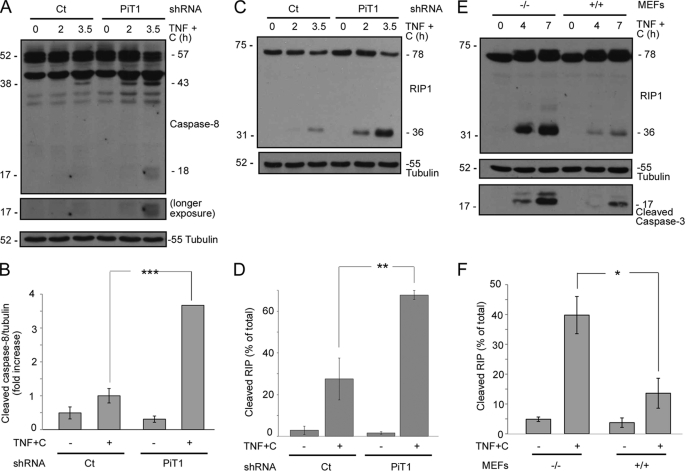
We next wished to characterize the molecular mechanism underlying the increased sensitivity of PiT1-depleted cells to the proapoptotic activity of TNF. One of the first caspases to be activated during TNF-induced apoptosis is the initiator caspase caspase-8 (15). We therefore investigated whether caspase-8 was more activated in PiT1-depleted cells. Western blot analysis could detect the cleavage of caspase-8 in its first processed form (p43) 3 h 30 min after TNF treatment in HeLa cells expressing a control shRNA (Fig. 3A). However, caspase-8 processing was evident as early as 2 h post-TNF+C treatment in shRNA PiT1 HeLa cells, and the fully activated form (p18) could be detected by 3 h 30 min in these cells. On average, caspase-8 cleavage was 3.5 more intense in PiT1-depleted cells compared with control cells (Fig. 3B). Consistent with this, we could also detect an important cleavage of RIP1 (receptor interacting protein 1), a caspase-8 substrate (38), at early time points in HeLa cells depleted of PiT1 (Fig. 3C). The amount of RIP1 cleavage was more than doubled in PiT1-depleted cells compared with control cells after a 3 h 30 min treatment with TNF+C (Fig. 3D). Similar results were obtained with mouse cells. RIP1 was cleaved earlier in PiT1−/− MEFs than in WT cells, showing that caspase-8 activation was, as in human cells, increased by PiT1 depletion (Fig. 3, E and F). PiT1 depletion therefore impairs early events associated with TNF-triggered apoptosis both in human and mouse immortalized cells.
FIGURE 3.
The initiator caspase, caspase-8, is activated early in PiT1-depleted cells. A–D, HeLa cell clones stably expressing either a control (Ct) or a PiT1 shRNA (PiT1) were treated with 50 ng/ml TNF and 10 μg/ml cycloheximide (TNF+C) for 0, 2, or 3.5 h. Cells were lysed and analyzed by Western blot with an antibody recognizing all forms of caspase-8 (full-length (p57) and cleaved (p43 and p18)) (A) and RIP1 (full-length p78 and cleaved p36) (C). Tubulin was used as a loading control. B and D, cells were incubated with TNF+C for 3.5 h and analyzed as described in A and C, respectively. Values are the mean ± S.E. (error bars) of three independent experiments. ***, p < 0.0005; **, p < 0.005. E and F, immortalized PiT1−/− (−/−) and WT (+/+) MEFs were incubated with 50 ng/ml TNF and 100 μg/ml cycloheximide (TNF+C) for 0, 4, or 7 h. Cells were lysed, and samples were analyzed by Western blot with an antibody against RIP1, cleaved caspase-3, or tubulin as a loading control. F, MEFs were treated with TNF+C for 7 h and analyzed as described in E. Values are the mean ± S.E. of five independent experiments. *, p < 0.05.
Sustained JNK Activity Is Increased in PiT1-depleted Cells in Response to TNF+C
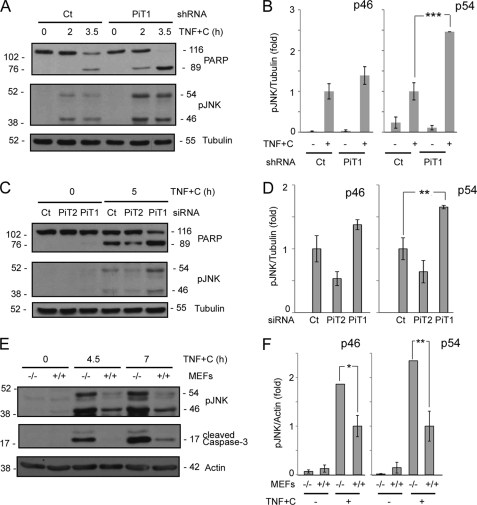
It has become increasingly clear that the MAPK JNK is a major proapoptotic effector of TNF-induced apoptosis. TNFR1 stimulation induces a very rapid and transient activation of JNK (30 min) in viable cells, whereas in NFκB-inhibited cells, which are primed to undergo cell death, JNK is persistently activated and remains so for several h (18). Sustained JNK activation (>1 h) is required for caspase 8 activation (30, 32), and we therefore investigated whether sustained JNK activation could be modulated by PiT1 depletion. Fig. 4A shows that both p46 and p54 isoforms of JNK were more phosphorylated in PiT1 shRNA HeLa cells than in control shRNA cells in response to TNF+C at 2 or 3.5 h post-treatment. This correlates well with an increase in apoptosis, detected by PARP cleavage. The quantification of six independent experiments suggested that although both isoforms of JNK were overphosphorylated in shRNA PiT1 cells, only p54 pJNK was significantly increased (Fig. 4B).
FIGURE 4.
JNK sustained activity is increased in PiT1-depleted cells. A and B, HeLa cell clones stably expressing either a control (Ct) or a PiT1 shRNA (PiT1) were treated with 50 ng/ml TNF and 10 μg/ml cycloheximide (TNF+C) for 0, 2, or 3.5 h. Cells were lysed and analyzed by Western blot with an antibody recognizing the phosphorylated forms (pJNK) of all JNK isoforms and genes (p46 and p54). The apoptosis progression was followed by the appearance of the cleaved form of PARP. B, cells were treated or not with TNF+C for 3.5 h and analyzed as described in A. Values are the mean ± S.E. (error bars) of six independent experiments. ***, p < 0.0005. C and D, HeLa cells were either transiently transfected with an siRNA control or directed against PiT1 or PiT2. 48 h post-transfection, the cells were either left untreated (0) or treated with 50 ng/ml TNF and 10 μg/ml cycloheximide (TNF+C) for 5 h. Apoptosis progression was followed by the cleavage of PARP, and phosphorylated JNK was detected by Western blot (pJNK). D, values are the mean ± S.E. of four independent experiments performed as described in C. **, p < 0.005. E and F, PiT1−/− and PiT1+/+ MEFs were incubated with 50 ng/ml TNF and 100 μg/ml cycloheximide (TNF+C) for 0, 3.5, or 7 h. Cell lysates were analyzed with antibodies against phosphorylated JNK and cleaved caspase-3. Tubulin or actin was used as a loading control. F, cells were treated with TNF+C for 7 h and analyzed as described in E. Values are the mean ± S.E. of four independent experiments. *, p < 0.05; **, p < 0.005.
We confirmed these results by transiently knocking down PiT1 in HeLa cells. The transient depletion of PiT1 also resulted in an increased sensitivity to TNF-mediated apoptosis (as shown by PARP cleavage), which correlated with an increased phosphorylation of p46 and p54 JNK (Fig. 4, C and D). This shows that JNK overactivation was not due to an adaptation of the cell clones to the long term depletion of PiT1. Once again, p54 JNK was significantly overactivated (Fig. 4D). Moreover, siRNA-mediated PiT2 depletion did not increase apoptosis and did not enhance sustained JNK phosphorylation as compared with siRNA control-treated cells, showing the specificity of the effect mediated by PiT1 depletion (Fig. 4, C and D). Finally, Fig. 4, E and F, shows that these observations are also valid in mouse cells. Immortalized PiT1−/− MEFs displayed an enhanced phosphorylation of JNK compared with PiT1+/+ cells, which correlated well with the increased apoptosis detected by the appearance of cleaved caspase-3. Both JNK isoforms were significantly overactivated in this cell type (Fig. 4F). Importantly, the enhanced activation of JNK therefore occurred in PiT1-depleted cells whether the NFκB pathway was partly (HeLa cells) or fully (MEFs) inactivated.
JNK Activity Is Instrumental in TNF-induced Apoptosis in PiT1-depleted Cells
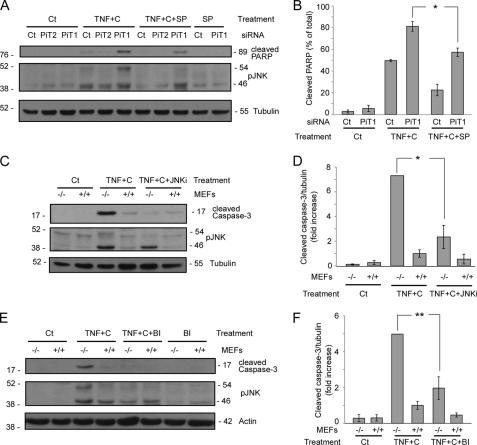
To test whether JNK activity could be responsible for the observed increased sensitivity to apoptosis in PiT1-depleted cells, we treated HeLa cells with the JNK inhibitor SP600125, which acts as an ATP competitive inhibitor (39). Figs. 5, A and B, shows that SP600125 significantly reduced the appearance of cleaved PARP in HeLa cells transiently transfected with a PiT1 siRNA (from 81 ± 5 to 57 ± 4%). In order to rule out any off target effect of the drug used, we next tested whether two other JNK inhibitors, JNKi (40) and BI-78D3 (41) could also prevent TNF-induced apoptosis on MEFs. These inhibitors act as substrate competitive inhibitors. Fig. 5 (C–F) shows that both JNK inhibitors greatly reduced the cleavage of caspase-3 in PiT1−/− MEFs (Fig. 5D, more than 3 times for JNKi; Fig. 5F, 2.5 times for BI-78D3). These results indicate that the hyperactivation of JNK is indeed involved in the increased sensitivity to apoptosis of PiT1-depleted cells.
FIGURE 5.
JNK activity is instrumental in the apoptosis of PiT1-depleted cells. A and B, HeLa cells were transiently transfected with a control siRNA (Ct) or an siRNA against PiT1 (PiT1) or PiT2 (PiT2). 48 h post-transfection, cells were either mock-treated (0) or treated with 50 ng/ml TNF and 10 μg/ml cycloheximide (TNF+C) for 5 h. Cells were preincubated (+SP) (or not) for 30 min with the JNK inhibitor SP600125 (30 μm), and the inhibitor was kept in the medium for the whole time course of the experiment. A, cells were lysed and analyzed by Western blot with antibodies directed against PARP and the phosphorylated forms of JNK (pJNK) and tubulin (as loading controls). B, values are the mean ± S.E. (error bars) of three independent experiments performed as described in A. *, p < 0.05. C and D, PiT1 knock-out (−/−), or WT MEFs (+/+) were preincubated (TNF+C+JNKi) or not (TNF+C) with the specific JNK inhibitor JNKi (5 μm) for 30 min. 50 ng/ml TNF and 100 μg/ml cycloheximide were then added to the medium for 6 h. C, total cell lysates were analyzed by Western blot with antibodies against cleaved caspase-3, phosphorylated JNK, and tubulin as a loading control. D, values are the mean ± S.E. of three independent experiments performed as described in C. *, p < 0.05. E and F, PiT1−/− and PiT1+/+ MEFs were preincubated (TNF+C+BI), or not (TNF+C), for 30 min with the JNK inhibitor BI-78D3 (5 μm) and then treated (or not; BI) with 50 ng/ml TNF and 100 μg/ml cycloheximide for 9 h. E, cell lysates were analyzed with antibodies against phosphorylated JNK and cleaved caspase-3. Actin was used as a loading control. F, values are the mean ± S.E. of four independent experiments performed as described in E. **, p < 0.005.
DISCUSSION
The data presented here show that PiT1 depletion sensitizes cells to TNF+C-mediated apoptosis. This can be reproduced either by acute or stable RNA interference in human cancer cells or by gene knock-out in mouse immortalized fibroblasts. We show that this effect is specific to the depletion of PiT1 because PiT2 depletion has no effect on this type of apoptosis. Because PiT1 functions as a sodium-Pi symporter (4), PiT1 depletion reduces Pi uptake in cells, both in HeLa cells and immortalized MEFs. However, PiT1−/− mouse fibroblasts do not show any sign of major energetic stress, as shown by the absence of phosphorylation of the kinase AMPK, which rapidly reacts to fluctuations in the AMP/ATP ratio (37). Furthermore, PiT2 depletion in HeLa cells, which also results in a decrease in Pi uptake, does not have any effect on apoptosis, suggesting that the increased sensitivity to TNF-induced apoptosis revealed in PiT1-deficient cells is independent of Pi uptake. This hypothesis is strengthened by the fact that the increased sensitivity to TNF-induced apoptosis that is mediated by PiT1 depletion is conserved regardless of the presence or absence of Pi in the medium, arguing for a Pi-independent role of PiT1 in these effects. Finally, we show that the expression of a PiT1 mutant that cannot transport Pi is equally efficient at delaying apoptosis in PiT1−/− cells as the wild type transporter. This demonstrates that PiT1 involvement in TNF-induced apoptosis is uncoupled from its Pi transport activity. In accordance with this hypothesis, we have previously shown that PiT1 had another transport-independent function in the regulation of cancer and normal cell proliferation (7, 9).
We have initiated the dissection of the molecular mechanism underlying the increased sensitivity of PiT1-depleted cells to TNF-induced apoptosis. The treatment of PiT1-depleted cells with TNF in proapoptotic conditions leads to the early activation of the initiator caspase caspase-8. The absence of PiT1 therefore impacts on early events induced by TNF. The activation of caspase-8 appears to occur through the formation of two distinct complexes in response to TNF (16, 42). Complex IIA is formed via the association of TNFR-associated death domain, Fas-associated death domain, and caspase-8, whereas complex IIB contains Fas-associated death domain, RIP1, and caspase-8. Our results suggest that complex IIB may constitute the main apoptotic complex that arises from TNF stimulation in our conditions. In agreement with this hypothesis, Fig. 3 shows that RIP1 is cleaved during the course of apoptosis in PiT1-depleted cells, suggesting that complex IIB indeed forms and thereby allows for the close association of caspase-8 with RIP1 that is necessary for RIP1 processing (38). Regardless of the type of proapoptotic complex that may form in PiT1-depleted cells, we have revealed an increased phosphorylation of JNK, which could be directly involved in caspase-8 activation. JNK has previously been shown to be necessary for caspase-8 activation (30, 32), probably within both complexes (16). Our data also revealed that although both p46 and p54 JNK isoforms were overphosphorylated in PiT1-depleted cells, p54 increase was more pronounced (Fig. 4). There are conflicting results regarding which gene and/or isoform is more important for TNF-induced apoptosis (43, 44). Importantly, we show that JNK activity is instrumental in the apoptosis of PiT1-depleted cells, and three different JNK inhibitors delay apoptosis in PiT1-depleted human and mouse cells. The regulation of JNK sustained activity in response to TNF is still incompletely understood. The production of antiapoptotic proteins under the control of NFκB is the main protective mechanism that is induced by TNF in most cells (45), and NFκB controls the duration of JNK activity (21, 25–28). We show here that PiT1-depleted cells display an increase in sustained JNK activation and that this is true whether the NFκB pathway is only partly (in HeLa cells) or fully inhibited (in MEFs) by increasing concentrations of cycloheximide. This therefore suggests that PiT1 absence deregulates an antiapoptotic pathway involved in the dampening of sustained JNK activity. This pathway would then be additional to the main NFκB pathway because it protects WT MEFs when cycloheximide fully inhibits NFκB-dependent protein synthesis. Alternatively, a stronger proapoptotic signal (resulting in an enhanced activation of JNK) may be triggered by TNF in cells depleted for PiT1. We are currently investigating the upstream kinases that are involved in JNK activation in PiT1-depleted cells, and preliminary data show that both MKK4 and MKK7 (JNK direct upstream kinases) may be overactivated in response to proapoptotic TNF (data not shown). It is conceivable that PiT1 could bind to some intracellular proteins (via its large central hydrophilic domain (9)) involved in the composition of one of the multiple signaling complexes formed in response to TNF, and its absence would therefore affect the response of the cell.
There are only sparse examples in the literature of transporters or channels that have been shown to possess additional functions independently of their transport function. A first class would be constituted by the so-called “transceptors” that are acting as external sensors of the compounds they are transporting and then signal to the cell, independently of the actual transport activity (reviewed in Ref. 46). The yeast Pho84 phosphate transporter is one such example (46), and binding of Pi to Pho84 induces a conformational change that mediates the rapid activation of the protein kinase A pathway. The complete transport cycle is not required for signaling (46). Human PiT2 has been shown to modify its oligomerization independently of Pi uptake in response to changes in the extracellular Pi concentration, suggesting a possible role of PiT2 in Pi sensing (47, 48). However, it is not known whether PiT2 (or PiT1) has any direct signaling activity in response to Pi. Nevertheless, experiments performed in the present study show that the presence of Pi is not required for TNF-induced apoptosis to proceed. This argues against a role of PiT1 (or PiT2) in Pi sensing and of extracellular Pi itself during TNF-induced apoptosis. A potential role of PiT proteins in Pi sensing may be important for other cellular functions, such as proliferation, and this interesting hypothesis requires further investigation.
Members of the Na,K-ATPase family could constitute a second class of transporters having functions in addition to the transport of their specific substrate. They are involved in morphogenesis and oncogenesis, and these functions are independent of their transport activity (49–51). In Caenorhabditis elegans, the cation-transporting ATPase CATP-1 interacts with the insulin/IGF and Ras-MAPK pathways to control several postembryonic events, independently of its transport function (52). A potential extracellular ligand has not been identified in this case. In contrast, it is now well known that mammalian Na,K-ATPases bind to cardiotonic steroids like ouabain and relay the extracellular signal to intracellular compartments via several kinase cascades, some mediated by a direct binding of the pump to Src (53, 54). It is possible that PiT1 has ligands in addition to its transported substrates, inorganic phosphate and sodium. In line with this, it is noteworthy to point out that PiT1 and PiT2 also operate as retrovirus receptors. It would therefore be possible that specific envelope proteins produced from endogenous retroviral elements could serve as additional ligands for both transporters. Interestingly, such a ligand (named FeLIX) has been found in the genome of cats (55). It presents a very high homology to the envelope of the feline leukemia virus and plays a crucial role in helping the infection of these cells (via PiT1) by the T cell-tropic feline leukemia virus, which is otherwise fusion-deficient on its own. The possible modulation of the feline PiT1 cellular functions by FeLIX has not yet been investigated, and the human genome does not encode a homolog of FeLIX. However, two human endogenous retroviral envelopes (syncytin 1/HERV-W and 2/HERV-FRD) have been found to perform important physiological functions (in the morphogenesis of the placenta, reviewed in Ref. 56) through their specific interaction with their cognate receptor (57, 58), whereas the expression of others may be involved in the development of several pathologies, including cancer (reviewed in Ref. 59). Sixteen sequences encoding full-length endogenous retroviral envelopes have now been identified in the human genome (60), and some of them may therefore potentially act as ligands for PiT proteins and modulate cellular functions.
A third family of receptors seem to have transport-independent and ligand-independent functions, such as some potassium chloride co-transporters. For example, the overexpression of NKCC1/SLC12A2 transporter impairs the embryogenesis of Xenopus laevis (61), whereas the neuronal KCC2 is associated with neural development in mammals (62, 63). KCC2 plays a structural role and interacts with the underlying cytoskeleton to promote dendritic spine development, independently of its Cl− transport activity (63). The interaction of transporters with the cytoskeleton beneath is thought to be another important property of these multitransmembrane molecules, in addition to or independent of their transport function (64). This anchoring can regulate cell migration, adhesion, and shape (64). A scaffolding role is also revealed by the Drosophila Na,K-ATPase, which seems to have evolved from having primarily a pumping function to a more specialized, pump-independent role as a scaffold protein for the formation and functioning of epithelial junctions (50). PiT1 has been shown to co-localize with the actin cytoskeleton in murine osteoclasts (65), and its absence could therefore affect the whole coupling of the plasma membrane to the cytoskeleton and indirectly modify the cell response to exogenous stimuli. PiT1 could also play a role as a scaffolding protein, via its large intracellular domain, and gather the proteins involved in signaling or the dampening of this signaling.
In conclusion, considering that PiT1 (but not PiT2) mRNA seems to be transcribed in response to an activation of the NFκB pathway (10–12),3 it is possible that PiT1 expression could be increased during cancer cell progression, which may rely on elevated NFκB activity (19, 66). Results presented here suggest that PiT1 may provide some protection to cancer cells against TNF-induced apoptosis. Taken together with our recent results revealing the impaired proliferation of PiT1-depleted cancer cells (9), this may suggest that PiT1 could play a role during cancer pathogenesis.
Acknowledgments
We are grateful to Dr. S. Fabrega (Plateforme “Transfert de Gènes à l'Aide de Vecteurs Viraux,” IFR94, Université Paris Descartes, Paris) for the generation of lentiviral vector stocks and to C. Cordier and J. Megret (Plateforme Tri Cellulaire, IFR94, Paris) for the cell sorting. We thank Prof. D. Paulin (Université Pierre et Marie Curie, Paris) for the gift of the T SV40 plasmid and Dr. F. Jaisser (Centre de Recherche des Cordeliers, Paris) for helpful discussion on the immortalization of MEFs. Finally, we thank Dr. S. Sullivan for helpful discussion and for editing of the manuscript.
This study was supported by grants from INSERM, the Université Paris Descartes, and the Association Laboratoires de Recherches Physiologiques.
N. Paris, A. Forand, C. Leroy, C. Salaün, L. Beck, and G. Friedlander, unpublished results.
- PiT
- inorganic phosphate transporter
- NFκB
- nuclear factor κB
- TNFR
- TNF receptor
- TNF+C
- TNF plus cycloheximide
- MEF
- mouse embryonic fibroblast
- AICAR
- 5-amino-4-imidazolecarboxamide riboside
- AMPK
- AMP-activated protein kinase
- PARP
- poly(ADP-ribose) polymerase
- JNKi
- JNK inhibitor.
REFERENCES
- 1.Collins J. F., Bai L., Ghishan F. K. (2004) Pflugers Arch. 447, 647–652 [DOI] [PubMed] [Google Scholar]
- 2.O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. (1990) Cell Growth Differ. 1, 119–127 [PubMed] [Google Scholar]
- 3.Miller D. G., Edwards R. H., Miller A. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavanaugh M. P., Miller D. G., Zhang W., Law W., Kozak S. L., Kabat D., Miller A. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 7071–7075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saier M. H., Jr. (2000) Microbiol. Mol. Biol. Rev. 64, 354–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uckert W., Willimsky G., Pedersen F. S., Blankenstein T., Pedersen L. (1998) Hum. Gene Ther. 9, 2619–2627 [DOI] [PubMed] [Google Scholar]
- 7.Beck L., Leroy C., Beck-Cormier S., Forand A., Salaün C., Paris N., Bernier A., Ureña-Torres P., Prié D., Ollero M., Coulombel L., Friedlander G. (2010) PLoS ONE 5, e9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Festing M. H., Speer M. Y., Yang H. Y., Giachelli C. M. (2009) Genesis 47, 858–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck L., Leroy C., Salaün C., Margall-Ducos G., Desdouets C., Friedlander G. (2009) J. Biol. Chem. 284, 31363–31374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabatino D. E., Do B. Q., Pyle L. C., Seidel N. E., Girard L. J., Spratt S. K., Orlic D., Bodine D. M. (1997) Blood Cells Mol. Dis. 23, 422–433 [DOI] [PubMed] [Google Scholar]
- 11.Yang H., Minamishima Y. A., Yan Q., Schlisio S., Ebert B. L., Zhang X., Zhang L., Kim W. Y., Olumi A. F., Kaelin W. G., Jr. (2007) Mol. Cell 28, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan H., Hacohen N., Golub T. R., Van Parijs L., Lodish H. F. (2002) Diabetes 51, 1319–1336 [DOI] [PubMed] [Google Scholar]
- 13.Chen G., Goeddel D. V. (2002) Science 296, 1634–1635 [DOI] [PubMed] [Google Scholar]
- 14.Wajant H., Pfizenmaier K., Scheurich P. (2003) Cell Death Differ. 10, 45–65 [DOI] [PubMed] [Google Scholar]
- 15.Micheau O., Tschopp J. (2003) Cell 114, 181–190 [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Du F., Wang X. (2008) Cell 133, 693–703 [DOI] [PubMed] [Google Scholar]
- 17.Lamb J. A., Ventura J. J., Hess P., Flavell R. A., Davis R. J. (2003) Mol. Cell 11, 1479–1489 [DOI] [PubMed] [Google Scholar]
- 18.Ventura J. J., Hübner A., Zhang C., Flavell R. A., Shokat K. M., Davis R. J. (2006) Mol. Cell 21, 701–710 [DOI] [PubMed] [Google Scholar]
- 19.Karin M. (2006) Nature 441, 431–436 [DOI] [PubMed] [Google Scholar]
- 20.Beg A. A., Baltimore D. (1996) Science 274, 782–784 [DOI] [PubMed] [Google Scholar]
- 21.Lin A. (2003) BioEssays 25, 17–24 [DOI] [PubMed] [Google Scholar]
- 22.Varfolomeev E. E., Ashkenazi A. (2004) Cell 116, 491–497 [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Lin A. (2005) Cell Res. 15, 36–42 [DOI] [PubMed] [Google Scholar]
- 24.Karin M., Lin A. (2002) Nat. Immunol. 3, 221–227 [DOI] [PubMed] [Google Scholar]
- 25.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. (2005) Cell 120, 649–661 [DOI] [PubMed] [Google Scholar]
- 26.De Smaele E., Zazzeroni F., Papa S., Nguyen D. U., Jin R., Jones J., Cong R., Franzoso G. (2001) Nature 414, 308–313 [DOI] [PubMed] [Google Scholar]
- 27.Tang G., Minemoto Y., Dibling B., Purcell N. H., Li Z., Karin M., Lin A. (2001) Nature 414, 313–317 [DOI] [PubMed] [Google Scholar]
- 28.Nakajima A., Komazawa-Sakon S., Takekawa M., Sasazuki T., Yeh W. C., Yagita H., Okumura K., Nakano H. (2006) EMBO J. 25, 5549–5559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bode A. M., Dong Z. (2007) Mol. Carcinog. 46, 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L., Kamata H., Solinas G., Luo J. L., Maeda S., Venuprasad K., Liu Y. C., Karin M. (2006) Cell 124, 601–613 [DOI] [PubMed] [Google Scholar]
- 31.Lin A. (2006) Dev. Cell 10, 277–278 [DOI] [PubMed] [Google Scholar]
- 32.Deng Y., Ren X., Yang L., Lin Y., Wu X. (2003) Cell 115, 61–70 [DOI] [PubMed] [Google Scholar]
- 33.Nagy A. (2003) in Manipulating the Mouse Embryo: A Laboratory Manual (Nagy A., Gertsenstein M., Vintersten K., Behringer R. eds) 3rd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 34.Pinçon-Raymond M., Vicart P., Bois P., Chassande O., Romey G., Varadi G., Li Z. L., Lazdunski M., Rieger F., Paulin D. (1991) Dev. Biol. 148, 517–528 [DOI] [PubMed] [Google Scholar]
- 35.Lewis M., Tartaglia L. A., Lee A., Bennett G. L., Rice G. C., Wong G. H., Chen E. Y., Goeddel D. V. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 2830–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang G., Yang J., Minemoto Y., Lin A. (2001) Mol. Cell 8, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 37.Towler M. C., Hardie D. G. (2007) Circ. Res. 100, 328–341 [DOI] [PubMed] [Google Scholar]
- 38.Lin Y., Devin A., Rodriguez Y., Liu Z. G. (1999) Genes Dev. 13, 2514–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett B. L., Sasaki D. T., Murray B. W., O'Leary E. C., Sakata S. T., Xu W., Leisten J. C., Motiwala A., Pierce S., Satoh Y., Bhagwat S. S., Manning A. M., Anderson D. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonny C., Oberson A., Negri S., Sauser C., Schorderet D. F. (2001) Diabetes 50, 77–82 [DOI] [PubMed] [Google Scholar]
- 41.Stebbins J. L., De S. K., Machleidt T., Becattini B., Vazquez J., Kuntzen C., Chen L. H., Cellitti J. F., Riel-Mehan M., Emdadi A., Solinas G., Karin M., Pellecchia M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 16809–16813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson N. S., Dixit V., Ashkenazi A. (2009) Nat. Immunol. 10, 348–355 [DOI] [PubMed] [Google Scholar]
- 43.Liu J., Minemoto Y., Lin A. (2004) Mol. Cell. Biol. 24, 10844–10856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich N., Thastrup J., Holmberg C., Gyrd-Hansen M., Fehrenbacher N., Lademann U., Lerdrup M., Herdegen T., Jäättelä M., Kallunki T. (2004) Cell Death Differ. 11, 301–313 [DOI] [PubMed] [Google Scholar]
- 45.Van Antwerp D. J., Martin S. J., Verma I. M., Green D. R. (1998) Trends Cell Biol. 8, 107–111 [DOI] [PubMed] [Google Scholar]
- 46.Popova Y., Thayumanavan P., Lonati E., Agrochão M., Thevelein J. M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 2890–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salaün C., Gyan E., Rodrigues P., Heard J. M. (2002) J. Virol. 76, 4304–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salaün C., Maréchal V., Heard J. M. (2004) J. Mol. Biol. 340, 39–47 [DOI] [PubMed] [Google Scholar]
- 49.Liang M., Cai T., Tian J., Qu W., Xie Z. J. (2006) J. Biol. Chem. 281, 19709–19719 [DOI] [PubMed] [Google Scholar]
- 50.Paul S. M., Palladino M. J., Beitel G. J. (2007) Development 134, 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang M., Tian J., Liu L., Pierre S., Liu J., Shapiro J., Xie Z. J. (2007) J. Biol. Chem. 282, 10585–10593 [DOI] [PubMed] [Google Scholar]
- 52.Ruaud A. F., Bessereau J. L. (2007) Development 134, 867–879 [DOI] [PubMed] [Google Scholar]
- 53.Tian J., Cai T., Yuan Z., Wang H., Liu L., Haas M., Maksimova E., Huang X. Y., Xie Z. J. (2006) Mol. Biol. Cell 17, 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z., Askari A. (2002) Eur. J. Biochem. 269, 2434–2439 [DOI] [PubMed] [Google Scholar]
- 55.Anderson M. M., Lauring A. S., Burns C. C., Overbaugh J. (2000) Science 287, 1828–1830 [DOI] [PubMed] [Google Scholar]
- 56.Blesa J. M., Tattersall I., Konner J. A., Candel V. A., Pulla M. P. (2008) Cancer Ther. 6, 923–930 [Google Scholar]
- 57.Lavillette D., Marin M., Ruggieri A., Mallet F., Cosset F. L., Kabat D. (2002) J. Virol. 76, 6442–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esnault C., Priet S., Ribet D., Vernochet C., Bruls T., Lavialle C., Weissenbach J., Heidmann T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17532–17537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voisset C., Weiss R. A., Griffiths D. J. (2008) Microbiol. Mol. Biol. Rev. 72, 157–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Parseval N., Lazar V., Casella J. F., Benit L., Heidmann T. (2003) J. Virol. 77, 10414–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walters Z. S., Haworth K. E., Latinkic B. V. (2009) J. Physiol. 587, 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horn Z., Ringstedt T., Blaesse P., Kaila K., Herlenius E. (2010) Eur. J. Neurosci. 31, 2142–2155 [DOI] [PubMed] [Google Scholar]
- 63.Li H., Khirug S., Cai C., Ludwig A., Blaesse P., Kolikova J., Afzalov R., Coleman S. K., Lauri S., Airaksinen M. S., Keinänen K., Khiroug L., Saarma M., Kaila K., Rivera C. (2007) Neuron 56, 1019–1033 [DOI] [PubMed] [Google Scholar]
- 64.Denker S. P., Barber D. L. (2002) Curr. Opin. Cell Biol. 14, 214–220 [DOI] [PubMed] [Google Scholar]
- 65.Khadeer M. A., Tang Z., Tenenhouse H. S., Eiden M. V., Murer H., Hernando N., Weinman E. J., Chellaiah M. A., Gupta A. (2003) Am. J. Physiol. Cell Physiol. 284, C1633–C1644 [DOI] [PubMed] [Google Scholar]
- 66.Karin M., Greten F. R. (2005) Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]