Abstract
In eukaryotic organisms, hydrogen peroxide has a dual effect; it is potentially toxic for the cell but also has an important signaling activity. According to the previously proposed floodgate hypothesis, the signaling activity of hydrogen peroxide in eukaryotes requires a transient increase in its concentration, which is due to the inactivation by overoxidation of 2-Cys peroxiredoxin (2-Cys Prx). Sensitivity to overoxidation depends on the structural GGLG and YF motifs present in eukaryotic 2-Cys Prxs and is believed to be absent from prokaryotic enzymes, thus representing a paradoxical gain of function exclusive to eukaryotic organisms. Here we show that 2-Cys Prxs from several prokaryotic organisms, including cyanobacteria, contain the GG(L/V/I)G and YF motifs characteristic of sensitive enzymes. In search of the existence of overoxidation-sensitive 2-Cys Prxs in prokaryotes, we have analyzed the sensitivity to overoxidation of 2-Cys Prxs from two cyanobacterial strains, Anabaena sp. PCC7120 and Synechocystis sp. PCC6803. In vitro analysis of wild type and mutant variants of the Anabaena 2-Cys Prx showed that this enzyme is overoxidized at the peroxidatic cysteine residue, thus constituting an exception among prokaryotes. Moreover, the 2-Cys Prx from Anabaena is readily and reversibly overoxidized in vivo in response to high light and hydrogen peroxide, showing higher sensitivity to overoxidation than the Synechocystis enzyme. These cyanobacterial strains have different strategies to cope with hydrogen peroxide. While Synechocystis has low content of less sensitive 2-Cys Prx and high catalase activity, Anabaena contains abundant and sensitive 2-Cys Prx, but low catalase activity, which is remarkably similar to the chloroplast system.
Keywords: Antioxidant, Bacteria, Chloroplast, Oxidative Stress, Peroxidase, Cyanobacteria, Hydrogen Peroxide, Overoxidation, Peroxiredoxin
Introduction
Hydrogen peroxide, a byproduct of aerobic metabolism, has potential cytotoxic effects but is also an intracellular messenger in eukaryotes (1). To balance the toxic and signaling effects of hydrogen peroxide, its intracellular concentration needs to be tightly controlled. For that purpose, cells are equipped with antioxidant enzymes including peroxiredoxins (Prxs),2 which are thiol-based peroxidases that catalyze the reduction of hydrogen peroxide and alkyl hydroperoxides by the concerted action of two cysteine residues (2). Although Prxs have a moderate catalytic efficiency, this is compensated for by the generally high abundance of these proteins (3). During catalysis, the N-terminal cysteine, termed peroxidatic, attacks the peroxide and becomes transiently oxidized to sulfenic acid, which then reacts and forms a disulfide bridge with the second cysteine residue, termed resolving (4). Prx recycling is in most cases achieved by a two-component system formed by thioredoxin and thioredoxin reductase (NTR). However, the bacterial Prx, AhpC, is reduced by AhpF, a bimodular enzyme composed of an N-terminal double thioredoxin fold and a C-terminal NTR domain (5). Similarly, the plant homologue to AhpC, the chloroplast-localized 2-Cys Prx, is most efficiently reduced by the bimodular enzyme, NTRC, composed of an NTR domain at the N terminus and a thioredoxin domain at the C terminus (6–8).
The peroxidatic cysteine residue of 2-Cys Prxs may undergo overoxidation from sulfenic to sulfinic acid (9), which provokes the inactivation of the enzyme. Initially described as an irreversible process, it was later shown that overoxidation may be reversed in an ATP-dependent process catalyzed by sulfiredoxin (Srx) (10, 11). The susceptibility to overoxidation has been considered specific for eukaryotic 2-Cys Prxs and depends on the presence of two motifs, GGLG and YF, located at the C terminus of these proteins (12). These sequence motifs impose a 14 Å separation of the two catalytic cysteine residues, which slows down disulfide formation, and hence, the sulfenic acid intermediate is occasionally overoxidized to sulfinic acid (12). For this reason, eukaryotic 2-Cys Prxs have been termed “sensitive” in contrast to the prokaryotic enzymes, which lack the GGLG and YF motifs, are much less sensitive to overoxidation, and are thus termed “robust” (12).
The dual action of hydrogen peroxide, as cytotoxic agent and intracellular messenger, is well documented in eukaryotes (1). In prokaryotes, however, hydrogen peroxide has not been previously attributed an extensive signaling activity with few exceptions, such as that of the transcription factor OxyR (13, 14). With the aim of explaining this different action of hydrogen peroxide as messenger in prokaryotes and eukaryotes, the floodgate hypothesis (12) proposed that inactivation of Prxs by overoxidation represents a gain of function of eukaryotes, which allows a rapid increase in hydrogen peroxide levels and, thus, permits its signaling activity. This would not be possible in prokaryotes, which are equipped with robust Prxs. Consequently, eukaryotic Prxs must have evolved to become sensitive to overoxidation.
In plants, as in other eukaryotes, hydrogen peroxide has an important signaling activity (15–18). In photosynthetic plant cells, the chloroplasts are a major source of hydrogen peroxide (19) and contain 2-Cys Prxs susceptible to overoxidation (20–22). Moreover, 2-Cys Prxs are among the most abundant chloroplast proteins and are involved in hydrogen peroxide-dependent signaling (23). The degree of overoxidation of chloroplast 2-Cys Prx is strongly affected by two plastidial enzymes: NTRC, the most efficient reductant of disulfide-bonded 2-Cys Prx (21), and Srx, which catalyzes the conversion of the overoxidized into the reduced form of the enzyme (22, 24).
In a sequence analysis, we found that cyanobacterial 2-Cys Prxs harbor GG(L/V/I)G and YF motifs and, hence, would possibly undergo overoxidation, unlike all previously reported prokaryotic enzymes. Thus, in search of sensitive 2-Cys Prxs in prokaryotic organisms, we set out to study 2-Cys Prx sensitivity to overoxidation in cyanobacteria. To this end, we have analyzed two cyanobacterial strains: Anabaena sp. PCC 7120, which is equipped with NTRC and Srx, and Synechocystis sp. PCC 6803, which lacks both NTRC and Srx genes. Our results show the existence of overoxidation in 2-Cys Prx from these prokaryotes in vivo and in vitro. The enzyme from Anabaena is considerably more sensitive than that of Synechocystis in vivo. Furthermore, we uncovered two different strategies to cope with hydrogen peroxide in cyanobacteria, and found that the strategy from Anabaena is remarkably similar to the chloroplast system.
EXPERIMENTAL PROCEDURES
Cyanobacterial Strains and Growth Conditions
Synechocystis sp. PCC 6803 and Anabaena sp. PCC 7120 were grown as described previously (25) at a light intensity of 50 μmol photons m−2 s−1. For experiments under high light conditions, cultures at a density of 2.5 μg of chlorophyll/ml were illuminated with white light at an intensity of 800 μmol photons m−2 s−1, and the temperature was kept at 30 °C.
Cloning, Expression, and Purification of Recombinant Proteins and Mutant Variants
The genes encoding 2-Cys Prx of Anabaena and Synechocystis were amplified from genomic DNA by PCR using gene-specific oligonucleotides, which included NdeI and XhoI sites for cloning. The sequences of these oligonucleotides were as follows: Ana2CP, forward (5′-GAATTAAGCATATGTCCATCACC-3′) and Ana2CP reverse (5′-GTTCTCGAGACGCGATCGCCAAC-3′) for the Anabaena 2-Cys Prx, and 2-Cys forward (5′-GCTACATATGACAGAGGTATTAAGGGTAG-3′) and 2-Cys reverse (5′-GCTACTCGAGCTAAGGTTCCGCCACTGTCTC-3′) for the Synechocystis 2-Cys Prx. The NdeI and XhoI sites are underlined. The PCR products were digested with both enzymes and cloned into the expression vector pET28 to produce the pET-Anab2CP and pET-Syn2CP plasmids, which were introduced into Escherichia coli BL21 (DE3)-pLysS (Promega). Expression was induced by 1 mm isopropyl-l-d-thiogalactoside (IPTG), and the recombinant proteins were purified by nickel-nitrilotriacetic acid affinity chromatography (Qiagen).
Site-directed mutagenesis was performed by PCR using pET-Anab2CP as the template DNA. In each case, mutations were produced with oligonucleotides that included a single change (underlined), which caused the C56S substitution (5′-GACTTTACCTTTGTTTCCCCCACGGAGATC-3′; 5′-GATCTCCGTGGGGGAAACAAAGGTAAAGTC-3′) and the C178S substitution (5′-CCCAGATGAAGTTTCCCCTGCTGGTTGGC-3′; 5′-GCCAACCAGCAGGGGAAACTTCATCTGGG-3′), respectively. The PCR products were digested with DpnI for 1 h at 37 °C to eliminate the methylated template DNA. Non-digested plasmids were thereafter used to transform E. coli BL21(DE3) pLysS. The correct introduction of mutations was verified by DNA sequencing. Expression of the mutated versions of 2-Cys Prx in E. coli and purification of the recombinant enzymes were carried out as described for the wild type enzymes.
One- and Two-dimensional Gel Electrophoresis and Western Blot Analysis
Cytosolic extracts of cyanobacterial strains were prepared as described (25). One-dimensional SDS-PAGE was performed using 15% polyacrylamide gels under non-reducing or reducing conditions. For reducing conditions, the loading buffer included 1 mm DTT and 14 mm β-mercaptoethanol. All samples contained 10 μg of protein and were boiled for 5 min prior to electrophoresis. For isoelectric focusing, protein extracts were precipitated with a final concentration of 5% (v/v) trichloroacetic acid, and immobilized pH gradient (pH range 4–7) strips of 11 cm (IPG strips, Bio-Rad) were applied according to the manufacturer's recommendations. IPG strips were thereafter subjected to SDS-PAGE.
For Western blot analysis, proteins resolved on two-dimensional or one-dimensional SDS-PAGE (15% acrylamide) gels were transferred to nitrocellulose membranes and probed with antibodies raised against the 2-Cys Prx from rice (7) or the 2-Cys Prx from Synechocystis, which was produced by immunization of rabbits with the purified recombinant protein. Antibodies against overoxidized 2-Cys Prx were purchased from LabFrontier (Seoul, Korea).
Mass Spectrometry Analysis
Purified recombinant proteins treated or not with hydrogen peroxide were subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry on an Autoflex apparatus (Bruker Daltonics, Bremen, Germany). External calibration was performed using the peptide calibration standard (Bruker Daltonics), and the trypsin autodigestion products of m/z values 842.5094 and 2211.1046 were used for internal calibration.
Determination of Growth and Viability of Cyanobacterial Cultures in the Presence of Hydrogen Peroxide
To determine growth rates in the presence of hydrogen peroxide, Anabaena and Synechocystis cultures in exponential growth phase (3–5 μg of chlorophyll ml−1) were adjusted to a density of 2.5 μg of chlorophyll ml−1. Hydrogen peroxide, at final concentrations ranging from 0 to 10 mm, was added to 30-ml aliquots of cultures, which were then incubated for 24 h under standard growth conditions (50 μmol photons m−2 s−1).
Analysis of Hydrogen Peroxide Decomposition in Vivo
For analysis of hydrogen peroxide decomposition, 20-ml cultures were inoculated at a concentration of 5 μg of chlorophyll ml−1 and kept under standard growth conditions. Thereafter, hydrogen peroxide was added to final concentrations of 0.5 mm, and aliquots were withdrawn at various time points for measurements of peroxide concentration. The amount of hydrogen peroxide remaining in the cultures was determined by oxidation of Fe2+ in the presence of xylenol orange using the ferrous oxidation of xylenol orange assay (26).
RESULTS
Cyanobacterial 2-Cys Prxs Possess the Sequence Motifs Characteristic for Sensitive Eukaryotic Enzymes
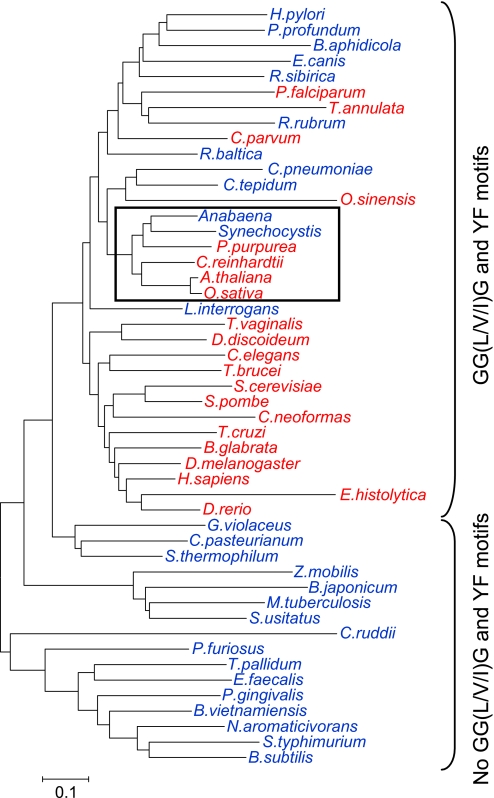
It has been proposed that the susceptibility to overoxidation of 2-Cys Prx, specific for eukaryotic organisms, depends on the presence of the GGLG and YF motifs (12). A phylogenetic analysis of 2-Cys Prx from diverse species distinguished 2-Cys Prxs lacking the GGLG and YF motifs, all of which are from prokaryotic organisms (Fig. 1 and supplemental Table 1). However, 2-Cys Prxs containing these motifs, thus representing sensitive enzymes, were found in all eukaryotic organisms analyzed but also in a number of prokaryotes (Fig. 1 and supplemental Table 1). It should be noted that although the first two residues of the GGLG motif were highly conserved, the third and fourth residues showed some variability. As the enzymes of the cyanobacterial strains here analyzed (see below) contained valine or isoleucine as the third residue, this motif is herein denoted GG(L/V/I)G. In addition, 2-Cys Prx from both cyanobacterial strains showed the conserved YF motif (supplemental Table 1). Moreover, the phylogenetic analysis revealed that plant, algal, and cyanobacterial 2-Cys Prxs are closely related and belong to the group of enzymes predicted to be sensitive (Fig. 1, box).
FIGURE 1.
Phylogenetic analysis of 2-Cys Prxs from eukaryotic and prokaryotic organisms. Amino acid sequences were obtained from the National Center for Biotechnology Information (NCBI) protein database, and accession numbers of these sequences are presented in supplemental Table 1. The tree was obtained by the MEGA4 package, and the neighbor-joining method was applied with 1000 bootstrap replications. The scale bar indicates the frequency of substitutions per site. Blue, prokaryotic organisms; red, eukaryotic organisms. The box indicates the clade formed by cyanobacterial, algal, and plant enzymes. Anabaena represents sp. PCC 7120, and Synechocystis represents sp. PCC 6803 are represented. H. pylori, Helicobacter pylori; P. profundum, Photobacterium profundum; B. aphidicola, Buchnera aphidicola; E. canis, Ehrlichia canis; R. sibirica, Rickettsia sibirica; P. falciparum, Plasmodium falciparum; T. annulata, Theileria annulata; R. rubrum, Rhodospirillum rubrum; C. parvum, Cryptosporidium parvum; R. baltica, Rhodopirellula baltica; C. pneumoniae, Chlamydophila pneumoniae; C. tepidum, Chlorobium tepidum; O. sinensis, Odontella sinensis; P. purpurea, Porphyra purpurea; C. reinhardtii, Chlamydomonas reinhardtii; A. thaliana, Arabidopsis thaliana; O. sativa, Oryza sativa; L. interrogans, Leptospira interrogans; T. vaginalis, Trichomonas vaginalis; D. discoideum, Dictyostelium discoideum; C. elegans, Caenorhabditis elegans; T. brucei, Trypanosoma brucei; S. cerevisiae, Saccharomyces cerevisiae; S. pombe, Schizosaccharomyces pombe; C. neoformas, Cryptococcus neoformas; T. cruzi, Trypanosoma cruzi; B. glabrata, Biomphalaria glabrata; D. melanogaster, Drosophila melanogaster; H. sapiens, Homo sapiens; E. histolytica, Entamoeba histolytica; D. rerio, Danio rerio; C. pasteurianum, Clostridium pasteurianum; S. thermophilum, Symbiobacterium thermophilum; Z. mobilis, Zymomonas mobilis; B. japonicum, Bradyrhizobium japonicum; M. tuberculosis, Mycobacterium tuberculosis; S. usitatus, Solibacter usitatus; C. ruddii, Candidatus Carsonella ruddii; P. furiosus, Pyrococcus furiosus; T. pallidum, Treponema pallidum; E. faecalis, Enterococcus faecalis; P. gingivalis, Porphyromonas gingivalis; B. vietnamiensis, Burkholderia vietnamiensis; N. aromaticivorans, Novosphingobium aromaticivorans; S. typhimurium, Salmonella typhimurium; B. subtilis, Bacillus subtilis.
The 2-Cys Prxs from Anabaena and Synechocystis Undergo Overoxidation in Vitro
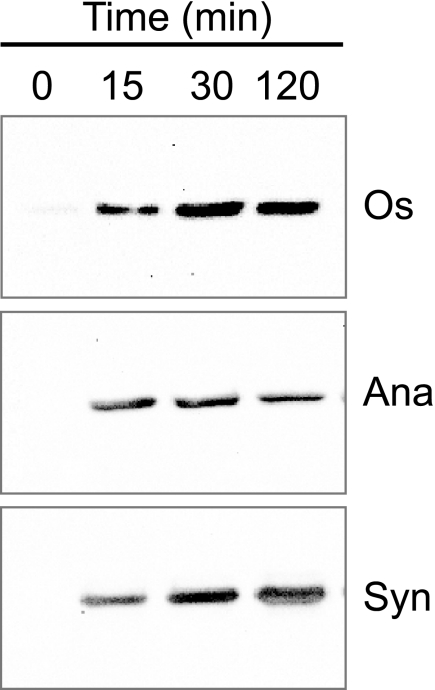
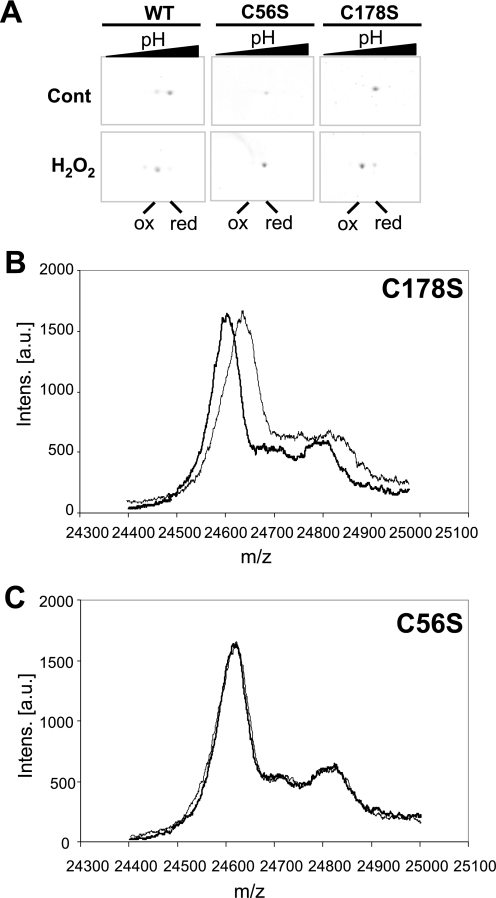
The above observations raised the possibility that cyanobacterial 2-Cys Prxs, despite being prokaryotic enzymes, might undergo overoxidation. To analyze this possibility, the 2-Cys Prxs from the cyanobacteria Anabaena sp. PCC 7120 and Synechocystis sp. PCC 6803 were expressed in E. coli with a His tag at the N terminus and purified by nickel-nitrilotriacetic acid chromatography (not shown). As an initial approach, we tested the cross-reactivity of an antibody raised against overoxidized 2-Cys Prx toward the purified cyanobacterial enzymes treated with DTT and hydrogen peroxide. Because the plant chloroplast 2-Cys Prx has been previously reported to undergo overoxidation, as detected by these antibodies (22), the purified rice 2-Cys Prx was included as a positive control. Indeed, all three enzymes showed clear signals after peroxide treatment, whereas no signals were observed before treatment (Fig. 2). Aiming at a more detailed analysis of the overoxidation of a prokaryotic 2-Cys Prx, we generated site-directed mutant versions of the enzyme from Anabaena replacing either the peroxidatic Cys-56 or the resolving Cys-178 residues by Ser (C56S and C178S mutants, respectively). Purified wild type and mutant enzymes were incubated with hydrogen peroxide in the presence of DTT, and subsequently the formation of cysteine sulfinic acid was examined by two-dimensional gel electrophoresis and mass spectrometry. The wild type 2-Cys Prx was shifted toward the acidic side of the gel, which is indicative of sulfinic acid formation (Fig. 3A). The C178S mutant lacking the resolving cysteine exhibited a similar shift; however, no shift was observed for the C56S mutant lacking the peroxidatic cysteine (Fig. 3A). Furthermore, mass spectrometry analysis of peroxide-treated C178S protein showed a shift of the mass to charge ratio (m/z) of 32.3 Daltons (Fig. 3B), consistent with the additional mass of two oxygen atoms, not observed in the C56S mutant (Fig. 3C). Thus, it may be concluded that the peroxidatic cysteine residue of the Anabaena 2-Cys Prx undergoes overoxidation to sulfinic acid under oxidizing conditions and, therefore, behaves as a sensitive, eukaryotic-type enzyme.
FIGURE 2.
Overoxidation of purified plant and cyanobacterial 2-Cys Prx in vitro. Purified recombinant 2-Cys Prx (25 μg of protein) from rice (Os), Anabaena (Ana), and Synechocystis (Syn) were incubated with 10 mm DTT and 5 mm hydrogen peroxide. At the indicated times, the reaction was stopped by protein precipitation with 5% trichloroacetic acid and washed twice with ice-cold acetone. Samples (0.3 μg of protein of the rice enzyme and 5 μg of Anabaena and Synechocystis enzymes) were then subjected to SDS-PAGE under reducing conditions. Western blot analysis was performed using antibodies against overoxidized 2-Cys Prx.
FIGURE 3.
Overoxidation of purified Anabaena 2-Cys Prx in vitro. A, purified recombinant wild type and mutant versions of Anabaena 2-Cys Prx (4 μm final concentration) was incubated for 5 min in the absence or presence of 0.5 mm hydrogen peroxide. Thereafter, DTT was added to each sample at a final concentration of 10 mm, and samples were incubated for another 10 min and precipitated with 5% trichloroacetic acid. Samples (1 μg of protein) were subjected to two-dimensional gel electrophoresis and proteins visualized with Coomassie Brilliant Blue. Cont, control; red, reduced; ox, overoxidized. B and C, MALDI-TOF analysis of mass-to-charge ratios (m/z) of mutant versions, as indicated, of 2-Cys Prx from Anabaena. Continuous lines, control; dashed lines, H2O2-treated. Intens. [a.u.], intensity (arbitrary units).
The 2-Cys Prxs from Anabaena and Synechocystis Display Different Sensitivity to Overoxidation in Vivo
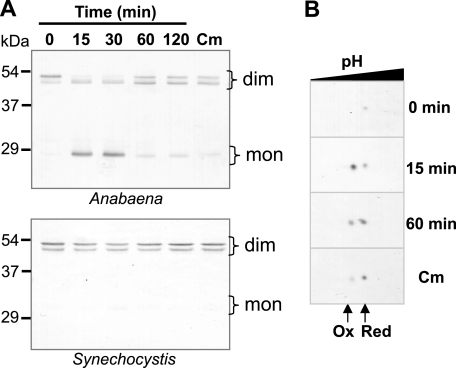
To assess the physiological relevance of 2-Cys Prx overoxidation in cyanobacteria, the redox status of the enzyme was determined in vivo under standard growth conditions and upon exposure of cultures to high light intensity or hydrogen peroxide treatments. Because NTRC and Srx exert a great influence on chloroplast 2-Cys Prx overoxidation, this analysis was performed in two representative cyanobacterial strains, Anabaena sp. PCC 7120, which contains NTRC and Srx, and Synechocystis sp. PCC 6803, which lacks both enzymes (supplemental Table 2). The two cyanobacterial strains were first grown under standard conditions at a photon flux density of 50 μE m−2 s−1 (control light) and then transferred to a light intensity of 800 μE m−2 s−1 (high light). Under control conditions, 2-Cys Prx was detected exclusively in the dimeric, oxidized form both in Anabaena and in Synechocystis (Fig. 4A). The detection of dimeric 2-Cys Prx as a double band has been attributed to the simultaneous presence of dimers linked by two disulfides (lower band) or one disulfide (upper band) (27). Fifteen min following the onset of high light, most of the Anabaena 2-Cys Prx was found as a monomer, which is indicative of extensive overoxidation of the enzyme (Fig. 4A). Despite continuous illumination with high light, after 60–120 min, the monomeric form disappeared gradually, hence showing the reversibility of the process. Reversion was not inhibited by the addition of chloramphenicol, showing that it was independent of protein synthesis. Surprisingly, the same high light treatment did not produce detectable overoxidation of 2-Cys Prx in Synechocystis (Fig. 4A). The antibody used for detection of overoxidation of the purified enzymes required at least 1 μg of overoxidized cyanobacterial 2-Cys Prx (Fig. 2) and was therefore not sensitive enough to detect these proteins in vivo, taking into account their relative abundance (supplemental Fig. 2). Therefore, to confirm the high light-induced overoxidation of the Anabaena 2-Cys Prx and its reversion, extracts were analyzed by two-dimensional gel electrophoresis (Fig. 4B). These results show that despite the fact that both Anabaena and Synechocystis have 2-Cys Prx with the eukaryotic-type motifs, GG(L/V/I)G and YF, and undergo overoxidation in vitro, the enzyme from Anabaena is more sensitive in vivo.
FIGURE 4.
Effect of high light on Anabaena and Synechocystis 2-Cys Prx overoxidation in vivo. A, cells grown to mid-exponential phase at 50 μE m−2 s−1 were diluted to a concentration of 2.5 μg of chlorophyll ml−1 and exposed to a light intensity of 800 μE m−2 s−1. After 30 min of high light exposure, chloramphenicol (200 μg/ml final concentration) was added to one aliquot and incubated for a further 30 min (Cm). Cytosolic extracts of samples from Anabaena (5 μg of protein) and Synechocystis (25 μg of protein) harvested at the times indicated were subjected to SDS-PAGE under non-reducing conditions and Western blot analysis. The overoxidized form of the 2-Cys Prxs is distinguished as a monomer. Molecular mass markers, in kDa, are indicated on the left. mon, monomer; dim, dimer. B, extracts (15 μg of protein) from Anabaena cultures at the indicated times of high light treatment were subjected to two-dimensional gel electrophoresis and Western blot. Ox, overoxidized; Red, reduced.
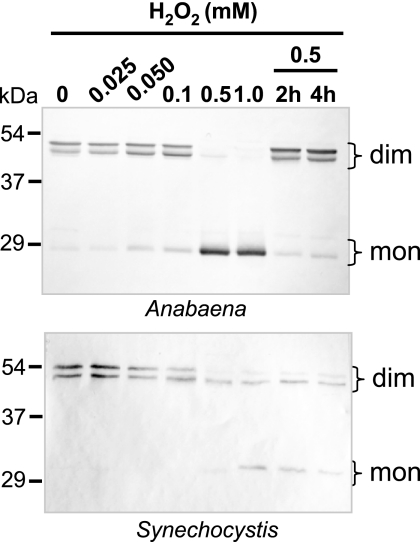
To analyze further the differences in sensitivity between these cyanobacterial 2-Cys Prxs, cultures were treated with increasing concentrations of exogenously added hydrogen peroxide under control light conditions. This treatment provoked overoxidation of the Synechocystis 2-Cys Prx, although it was apparently less sensitive than the Anabaena enzyme (Fig. 5). Moreover, most of the dimeric form was recovered in Anabaena after 2 h of further incubation without peroxide, whereas the Synechocystis 2-Cys Prx, which became only partially overoxidized, retained essentially the same proportion of dimeric and monomeric forms even after 4 h of further incubation (Fig. 5), thus suggesting that overoxidation is not a reversible process in Synechocystis.
FIGURE 5.
Effect of hydrogen peroxide on Anabaena and Synechocystis 2-Cys Prx overoxidation in vivo. Cultures grown at 50 μE m−2 s−1 to a concentration of 5 μg of chlorophyll ml−1 were incubated for 15 min with hydrogen peroxide at the indicated concentrations. Cultures treated with 0.5 mm hydrogen peroxide were also harvested 2 and 4 h after the addition of hydrogen peroxide. Western blot analysis was performed using antibodies raised against rice and Synechocystis 2-Cys Prx for Anabaena and Synechocystis samples, respectively. Molecular mass markers, in kDa, are indicated on the left. mon, monomer; dim, dimer.
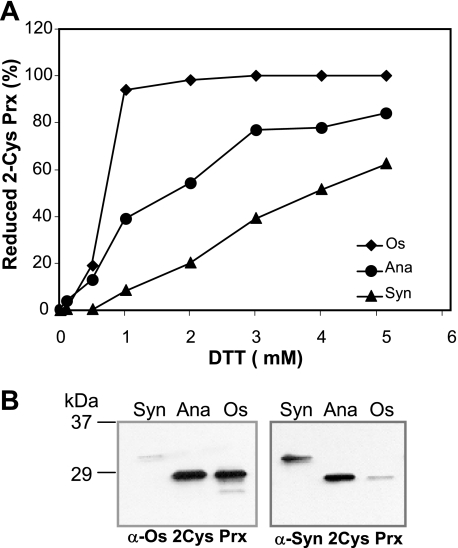
Because the 2-Cys Prxs from both Anabaena and Synechocystis contain the structural GG(L/V/I)G and YF motifs and undergo overoxidation in vitro, the different sensitivity to overoxidation might be due to additional properties of these enzymes. We reasoned that the disulfide between peroxidatic and resolving cysteines should protect the former from reacting with peroxides. To test this possibility, the ability of an artificial disulfide reductant, DTT, to reduce each of these enzymes was analyzed. The rice 2-Cys Prx was highly sensitive to DTT reduction (Fig. 6A). Of the cyanobacterial enzymes, Anabaena 2-Cys Prx was more prone to reduction by DTT than the Synechocystis enzyme. These results suggest that the proportion reduced to disulfide-bound 2-Cys Prx may be a significant determinant of the susceptibility to overoxidation and may underscore the different sensitivity of the enzymes from Anabaena and Synechocystis.
FIGURE 6.
Comparison of plant and cyanobacterial 2-Cys Prx. A, titration in vitro of the purified plant and cyanobacterial 2-Cys Prx with DTT. Samples (0.8 μg of purified protein) of 2-Cys Prx from rice, Anabaena, and Synechocystis, as indicated, were treated with increasing concentrations of DTT for 10 min. Proteins were then subjected to SDS-PAGE, and bands stained with Coomassie Brilliant Blue were quantified with the ImageJ software. B, immunological characterization of plant and cyanobacterial 2-Cys Prx. For Western blot analysis, 50 ng of the purified 2-Cys Prx from Synechocystis (Syn), Anabaena (Ana), or rice (Os) were subjected to SDS-PAGE and probed with antibodies raised against the rice (α-Os 2Cys Prx) and Synechocystis 2-Cys Prx (α-Syn 2Cys Prx), as indicated. Molecular mass markers, in kDa, are indicated on the left.
A sequence comparison of 2-Cys Prx from different sources revealed a high degree of identity between 2-Cys Prxs from plant, algae, and cyanobacteria (supplemental Fig. 1). The percentage of identity between the 2-Cys Prx from Anabaena and Synechocystis (75%) is very similar to the identity between the homologues from Anabaena and rice (73%). However, the identity between the Synechocystis and rice 2-Cys Prx is lower (63%). Most of the differences between Synechocystis, Anabaena, and plant 2-Cys Prxs are located in the vicinity of the resolving cysteine (supplemental Fig. 1). In agreement with these observations, antibodies raised against the rice 2-Cys Prx cross-reacted efficiently with the Anabaena 2-Cys Prx but not with the Synechocystis enzyme (Fig. 6B). Conversely, antibodies raised against the Synechocystis 2-Cys Prx efficiently detected the enzyme from Anabaena but not from rice (Fig. 6B). Remarkably, the 2-Cys Prx from Synechocystis showed a different electrophoretical mobility (Fig. 6B) despite the fact that the molecular mass of the enzyme (24.8 kDa) is similar to the Anabaena and rice enzymes (24.7 and 24.2 kDa, respectively). In addition, quantitative Western blot analysis showed that Synechocystis contains at least 20 times less 2-Cys Prx than does Anabaena (supplemental Fig. 2). The Anabaena enzyme was estimated to be present in a proportion of 10 ng/μg of cytosolic protein, compatible with the abundance of the chloroplast 2-Cys Prx, which constitutes about 0.6% of the total chloroplast protein (23). These results emphasize the similarities between the Anabaena and chloroplast 2-Cys Prxs and the differences between these enzymes and the Synechocystis homologue.
Anabaena and Synechocystis Have Different Strategies to Cope with Hydrogen Peroxide
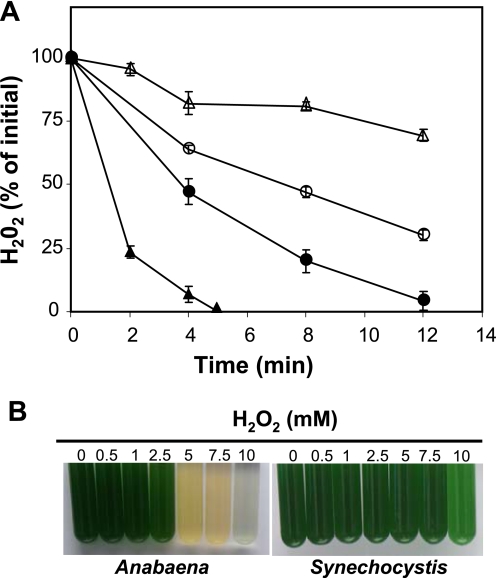
Next, we examined whether the different content and sensitivity of 2-Cys Prx in the cyanobacterial strains had any effect on the response to hydrogen peroxide. Exponentially growing Synechocystis cultures showed a higher rate of hydrogen peroxide decomposition than Anabaena (Fig. 7A). Furthermore, the peroxide removal in Synechocystis was strongly inhibited by NH2OH (Fig. 7A), which blocks the activity of the cyanobacterial catalase-peroxidase KatG (28). In contrast, peroxide removal by Anabaena, which lacks KatG (29) (supplemental Table 2), was much less affected by NH2OH (Fig. 7A). Finally, we explored the consequences of these different cyanobacterial systems for peroxide detoxification with respect to survival to oxidative stress. In agreement with the higher rate of peroxide detoxification, Synechocystis cultures survived higher concentrations of exogenously added hydrogen peroxide than Anabaena (Fig. 7B).
FIGURE 7.
Kinetics of hydrogen peroxide decomposition in cultures of Anabaena and Synechocystis. A, cultures were grown to mid-exponential phase, diluted to a concentration of 3.5 μg of chlorophyll ml−1 and incubated under normal light conditions in the presence or absence of 0.1 mm NH2OH, which inhibits the catalase-peroxidase activity. At time 0, hydrogen peroxide was added to a final concentration of 0.5 mm. The remaining amounts of hydrogen peroxide were monitored using the ferrous oxidation of xylenol orange assay. ▴ and △, Synechocystis in the absence (▴) or presence (△) of NH2OH; ● and ○, Anabaena in the absence (●) or presence (○) of NH2OH. B, effect of hydrogen peroxide on Anabaena and Synechocystis survival. Cyanobacterial cultures in mid-exponential growth phase diluted to a concentration of 2.5 μg of chlorophyll ml−1 were incubated for 24 h under standard light conditions in the presence of up to 10 mm hydrogen peroxide.
DISCUSSION
According to the floodgate hypothesis (12), the different action of hydrogen peroxide in eukaryotic and prokaryotic organisms depends on structural characteristics of 2-Cys Prxs. This means that in contrast to prokaryotic 2-Cys Prxs, eukaryotic enzymes have evolved to become more sensitive to inactivation by overoxidation (30). Plant chloroplasts contain eukaryotic-type 2-Cys Prxs with the GG(L/V/I)G and YF motifs, which undergo overoxidation of the peroxidatic cysteine residue (20–22). As chloroplasts originated from oxygenic photoautotrophic prokaryotes similar to modern cyanobacteria (31), we have analyzed the existence of 2-Cys Prx sensitive to overoxidation in these prokaryotic organisms.
A search for the GG(L/V/I)G and YF motifs characteristic for sensitive 2-Cys Prxs revealed that these are not exclusive to eukaryotic organisms (Fig. 1 and supplemental Table 1). Among the prokaryotes with putative sensitive-type 2-Cys Prx are the cyanobacteria, the enzymes of which are related to those of algae and plants, confirming previous phylogenetic analysis (32). The only exception is the 2-Cys Prx from the cyanobacterium Gloeobacter violaceus, which lacks both the GG(L/V/I)G and the YF motifs (supplemental Table 1) and appears phylogenetically distant to 2-Cys Prxs from algae, plants, and other cyanobacteria (Fig. 1). Notably, G. violaceus is an organism with peculiar properties such as the absence of thylakoid membranes and, in many aspects, differs from other cyanobacteria (33). The wide distribution of 2-Cys Prx predicted to be sensitive among prokaryotes suggests that this structural feature appeared early during evolution.
The cross-reactivity of the peroxide-treated 2-Cys Prx from Anabaena and Synechocystis with an antibody against the overoxidized enzyme (Fig. 2), in addition to the peroxide-induced shift of the pI observed for the wild type and C178S mutant of the Anabaena 2-Cys Prx, but not for the C56S mutant, combined with mass spectrometry (Fig. 3), demonstrated that the cyanobacterial 2-Cys Prx is susceptible to overoxidation of the peroxidatic cysteine residue. Moreover, experiments using Anabaena and Synechocystis cultures showed that overoxidation takes place in vivo in response to high light in Anabaena (Fig. 4) or to hydrogen peroxide treatments both in Anabaena and in Synechocystis (Fig. 5). Therefore, these results show that cyanobacteria constitute a previously unrecognized exception among prokaryotes in having 2-Cys Prx sensitive to overoxidation. In contrast, 2-Cys Prxs from other prokaryotes studied either are not overoxidized or require much higher hydrogen peroxide concentration to become overoxidized in vivo (12, 34–36).
The analysis of 2-Cys Prx overoxidation in vivo revealed remarkable differences between Anabaena and Synechocystis. Despite the fact that the 2-Cys Prxs from both strains possess the structural determinants to allow overoxidation, the enzyme from Synechocystis was much less sensitive (Figs. 4 and 5) and required a higher concentration of DTT for reduction (Fig. 6A). This latter characteristic might explain, at least in part, the lower sensitivity of the enzyme to overoxidation because the disulfide-bonded form of the 2-Cys Prx should be inert to sulfinic acid formation. In this context, it should be noted that the high DTT concentration (10 mm), used for the initial assays for immunological detection of overoxidation (Fig. 2), should have been saturating, and no difference in overoxidation between the rice, Anabaena, and Synechocystis enzymes could be observed. In plant chloroplasts, the most efficient reductant of 2-Cys Prx is NTRC (7, 8). Remarkably, in an Arabidopsis NTRC knock-out mutant, the 2-Cys Prxs are predominantly in the dimeric form and display lower levels of overoxidation (21), which suggests that the enzyme in the dimeric, oxidized form is protected from overoxidation. Another aspect determining the overoxidation status of 2-Cys Prx in vivo is its reversion catalyzed by Srx, a chloroplast-localized enzyme in plants (24). It should be noted that Anabaena contains genes encoding both NTRC and Srx, whereas Synechocystis does not (supplemental Table 2). The reversibility of the 2-Cys Prx overoxidation observed in Anabaena, but not in Synechocystis, would thus be in agreement with the presence of Srx in Anabaena.
The pronounced differences between Anabaena and Synechocystis concerning 2-Cys Prx sensitivity to overoxidation indicates that these cyanobacteria have developed different strategies to cope with oxidative stress; although Synechocystis is equipped with an efficient mechanism to detoxify hydrogen peroxide, the mechanism of Anabaena is less efficient (Fig. 7A). Despite an extensive complement of thiol peroxidases including various types of Prx (37), the antioxidant system of Synechocystis relies mainly on a high KatG catalase activity, as deduced from the almost complete inhibition of hydrogen peroxide decomposition by NH2OH (Fig. 7A) and previous results from Tichy and Vermaas (38) using a KatG deletion mutant. A consequence of such a high rate of hydrogen peroxide detoxification is that peroxide concentrations in vivo would rarely be high enough to cause 2-Cys Prx overoxidation, which is also in line with our observations. In contrast, in Anabaena, the antioxidant mechanism does not rely on catalase (supplemental Table 2), as confirmed by the low inhibitory effect of NH2OH, but probably the 2-Cys Prx, which is about 20 times more abundant than in Synechocystis, plays a more prominent role. These different antioxidant strategies have an important impact on the ability of the cyanobacteria to survive oxidative stress. The efficient system of Synechocystis allows this cyanobacterium to tolerate higher concentrations of hydrogen peroxide than Anabaena. The strategy exemplified by Synechocystis, based on high catalase activity and low level of insensitive 2-Cys Prx, pursues survival by rapid exhaustion of the peroxide, whereas the strategy of Anabaena fails to provide survival. It should be noted that plant chloroplasts have a content of 2-Cys Prx, similar to that of Anabaena (23), and the enzyme is highly sensitive to overoxidation (20–22). Moreover, these organelles are also devoid of catalase but contain NTRC and Srx. Hence, our results are in agreement with the proposal that the endosymbiotic cyanobacterial ancestor, which originated the plant chloroplast, was of the filamentous, heterocyst-forming type, such as the modern Anabaena sp. PCC 7120 and Anabaena variabilis ATCC 29143 (39).
The precise involvement of 2-Cys Prx overoxidation in plant hydrogen peroxide signaling remains to be established. Notably, hydrogen peroxide originating specifically from chloroplasts has been recognized to play a critical role in the response and adaptation of plants to elevated light intensities (40, 41). Moreover, an Srx-deficient mutant of Arabidopsis shows increased expression of the APX2 gene (42), thus suggesting that the overoxidation status of chloroplast 2-Cys Prx is involved in hydrogen peroxide signaling in plants. In mammalian cells, inactivation of Prx I allows hydrogen peroxide accumulation and cell signaling; however, inactivation occurs by phosphorylation, not by overoxidation (43). In this regard, the autophosphorylation of 2-Cys Prx from rapeseed is worth mentioning, although phosphorylation occurs on sulfinic and sulfonic forms of the resolving cysteine residue (44). Therefore, more studies are required to establish the relationship between redox status and phosphorylation and the effect of these modifications on hydrogen peroxide-dependent signaling in plants.
The similarities of the chloroplast and Anabaena antioxidant systems, based on sensitive 2-Cys Prx and the absence of catalase activity, suggest that plants have adopted a low efficiency mechanism for peroxide detoxification. In contrast, this mechanism, which ensures rapid inactivation and reactivation of the peroxidase, would allow the transient increase of hydrogen peroxide levels and thus its use as second messenger, in line with the important signaling activity of this molecule in eukaryotes.
Supplementary Material
Acknowledgment
We thank Rocio Rodriguez for excellent technical assistance.
This work was supported by Grants BIO2007-60644 and BFU2007-60300 from the Ministerio de Educación y Ciencia (Spain) and Grants P06-CVI-01578, BIO-182, and BIO-284 from the Junta de Andalucia (Spain).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1 and 2.
- Prx
- peroxiredoxin
- Srx
- sulfiredoxin
- NTR
- NADPH thioredoxin reductase
- μE
- microeinsteins.
REFERENCES
- 1.Veal E. A., Day A. M., Morgan B. A. (2007) Mol. Cell. 26, 1–14 [DOI] [PubMed] [Google Scholar]
- 2.Chae H. Z., Chung S. J., Rhee S. G. (1994) J. Biol. Chem. 269, 27670–27678 [PubMed] [Google Scholar]
- 3.Chae H. Z., Kim H. J., Kang S. W., Rhee S. G. (1999) Diabetes Res. Clin. Pract. 45, 101–112 [DOI] [PubMed] [Google Scholar]
- 4.Chae H. Z., Uhm T. B., Rhee S. G. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 7022–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole L. B. (2005) Arch. Biochem. Biophys. 433, 240–254 [DOI] [PubMed] [Google Scholar]
- 6.Serrato A. J., Pérez-Ruiz J. M., Spínola M. C., Cejudo F. J. (2004) J. Biol. Chem. 279, 43821–43827 [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Ruiz J. M., Spínola M. C., Kirchsteiger K., Moreno J., Sahrawy M., Cejudo F. J. (2006) Plant Cell. 18, 2356–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Ruiz J. M., Cejudo F. J. (2009) FEBS Lett. 583, 1399–1402 [DOI] [PubMed] [Google Scholar]
- 9.Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 7017–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biteau B., Labarre J., Toledano M. B. (2003) Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 11.Woo H. A., Chae H. Z., Hwang S. C., Yang K. S., Kang S. W., Kim K., Rhee S. G. (2003) Science 300, 653–656 [DOI] [PubMed] [Google Scholar]
- 12.Wood Z. A., Poole L. B., Karplus P. A. (2003) Science 300, 650–653 [DOI] [PubMed] [Google Scholar]
- 13.Zheng M., Aslund F., Storz G. (1998) Science 279, 1718–1721 [DOI] [PubMed] [Google Scholar]
- 14.Aslund F., Zheng M., Beckwith J., Storz G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 6161–6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foreman J., Demidchik V., Bothwell J. H., Mylona P., Miedema H., Torres M. A., Linstead P., Costa S., Brownlee C., Jones J. D., Davies J. M., Dolan L. (2003) Nature 422, 442–446 [DOI] [PubMed] [Google Scholar]
- 16.Gapper C., Dolan L. (2006) Plant Physiol. 141, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laloi C., Stachowiak M., Pers-Kamczyc E., Warzych E., Murgia I., Apel K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenabeele S., Van Der Kelen K., Dat J., Gadjev I., Boonefaes T., Morsa S., Rottiers P., Slooten L., Van Montagu M., Zabeau M., Inze D., Van Breusegem F. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitzschke A., Forzani C., Hirt H. (2006) Antioxid. Redox Signal. 8, 1757–1764 [DOI] [PubMed] [Google Scholar]
- 20.Broin M., Rey P. (2003) Plant Physiol. 132, 1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchsteiger K., Pulido P., González M., Cejudo F. J. (2009) Mol. Plant. 2, 298–307 [DOI] [PubMed] [Google Scholar]
- 22.Iglesias-Baena I., Barranco-Medina S., Lázaro-Payo A., López-Jaramillo F. J., Sevilla F., Lázaro J. J. (2010) J. Exp. Bot. 61, 1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz K. J., Jacob S., Oelze M. L., Laxa M., Tognetti V., Nunes de Miranda S. M., Baier M., Finkemeier I. (2006) J. Exp. Bot. 57, 1697–1709 [DOI] [PubMed] [Google Scholar]
- 24.Rey P., Bécuwe N., Barrault M. B., Rumeau D., Havaux M., Biteau B., Toledano M. B. (2007) Plant J. 49, 505–514 [DOI] [PubMed] [Google Scholar]
- 25.Lindahl M., Florencio F. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 16107–16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff S. P. (1994) Methods Enzymol. 233, 182–189 [DOI] [PubMed] [Google Scholar]
- 27.Dietz K. J., Horling F., König J., Baier M. (2002) J. Exp. Bot. 53, 1321–1329 [PubMed] [Google Scholar]
- 28.Miller A. G., Hunter K. J., O'Leary S. J., Hart L. J. (2000) Plant Physiol. 123, 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernroitner M., Zamocky M., Furtmüller P. G., Peschek G. A., Obinger C. (2009) J. Exp. Bot. 60, 423–440 [DOI] [PubMed] [Google Scholar]
- 30.Hall A., Karplus P. A., Poole L. B. (2009) FEBS J. 276, 2469–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould S. B., Waller R. F., McFadden G. I. (2008) Annu. Rev. Plant Biol. 59, 491–517 [DOI] [PubMed] [Google Scholar]
- 32.Baier M., Dietz K. J. (1997) Plant J. 12, 179–190 [DOI] [PubMed] [Google Scholar]
- 33.Mimuro M., Tomo T., Tsuchiya T. (2008) Photosynth. Res. 97, 167–176 [DOI] [PubMed] [Google Scholar]
- 34.Weber H., Engelmann S., Becher D., Hecker M. (2004) Mol. Microbiol. 52, 133–140 [DOI] [PubMed] [Google Scholar]
- 35.Parsonage D., Desrosiers D. C., Hazlett K. R., Sun Y., Nelson K. J., Cox D. L., Radolf J. D., Poole L. B. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 6240–6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C. H., Chuang M. H., Wu Y. H., Chuang W. C., Jhuang P. J., Chiou S. H. (2010) J. Biochem. 147, 661–669 [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Pérez M. E., Mata-Cabana A., Sánchez-Riego A. M., Lindahl. M., Florencio F. J. (2009) J. Bacteriol. 191, 7477–7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tichy M., Vermaas W. (1999) J. Bacteriol. 181, 1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deusch O., Landan G., Roettger M., Gruenheit N., Kowallik K. V., Allen J. F., Martin W., Dagan T. (2008) Mol. Biol. Evol. 25, 748–761 [DOI] [PubMed] [Google Scholar]
- 40.Li Z., Wakao S., Fischer B. B., Niyogi K. K. (2009) Annu. Rev. Plant Biol. 60, 239–260 [DOI] [PubMed] [Google Scholar]
- 41.Mullineaux P. M., Karpinski S., Baker N. R. (2006) Plant Physiol. 141, 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galvez-Valdivieso G., Fryer M. J., Lawson T., Slattery K., Truman W., Smirnoff N., Asami T., Davies W. J., Jones A. M., Baker N. R., Mullineaux P. M. (2009) Plant Cell 21, 2143–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo H. A., Yim S. H., Shin D. H., Kang D., Yu D. Y., Rhee S. G. (2010) Cell 140, 517–528 [DOI] [PubMed] [Google Scholar]
- 44.Aran M., Caporaletti D., Senn A. M., Tellez de Iñon M. T., Girotti M. R., Llera A. S., Wolosiuk R. A. (2008) FEBS J. 275, 1450–1463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.