Abstract
Leishmania parasites use polymorphonuclear neutrophils as intermediate hosts before their ultimate delivery to macrophages following engulfment of parasite-infected neutrophils. This leads to a silent and unrecognized entry of Leishmania into the macrophage host cell. Neutrophil function depends on its cytoplasmic granules, but their mobilization and role in how Leishmania parasites evade intracellular killing in neutrophils remain undetermined. Here, we have found by ultrastructural approaches that neutrophils ingested Leishmania major promastigotes, and azurophilic granules fused in a preferential way with parasite-containing phagosomes, without promoting parasite killing. Azurophilic granules, identified by the granule marker myeloperoxidase, also fused with Leishmania donovani-engulfed vacuoles in human neutrophils. In addition, the azurophilic membrane marker CD63 was also detected in the vacuole surrounding the parasite, and in the fusion of azurophilic granules with the parasite-engulfed phagosome. Tertiary and specific granules, involved in vacuole acidification and superoxide anion generation, hardly fused with Leishmania-containing phagosomes. L. major interaction with neutrophils did not elicit production of reactive oxygen species or mobilization of tertiary and specific granules. By using immunogold electron microscopy approaches in the engulfment of L. major and L. donovani by human neutrophils, we did not find a significant contribution of endoplasmic reticulum to the formation of Leishmania-containing vacuoles. Live Leishmania parasites were required to be optimally internalized by neutrophils. Our data suggest that Leishmania promastigotes modulate their uptake by neutrophils, and regulate granule fusion processes in a rather selective way to favor parasite survival in human neutrophils.
Keywords: Neutrophil, Parasitology, Phagocytosis, Protozoan, Subcellular Organelles, Cytoplasmic Granules, Human, Leishmania
Introduction
Leishmaniasis, caused by infection with protozoan Leishmania parasites, is initiated by infected sandfly bites and the deposition of the promastigote form of the parasite in the skin of the vertebrate host. Polymorphonuclear neutrophils prevail within the first hours after infection in the skin (1). In vivo imaging evidence reveals an essential role for neutrophils in sandfly-transmitted Leishmania infections (2). Current evidence suggests that Leishmania parasites are initially engulfed by neutrophils where they survive and retain infectivity, thus using neutrophils as transport vehicles before they enter safely and silently their ultimate host cell, the macrophage (3, 4).
Neutrophils constitute the first line of host defense (5). The major function of neutrophils is phagocytosis and subsequent killing of invading microorganisms, via the generation of oxygen metabolites, and the release of lytic enzymes stored in their granules. Rapidly after their formation, phagosomes fuse with intracellular granules to form phagolysosomes where exposure to hydrolytic enzymes, antimicrobial peptides, acid pH, and reactive oxygen species further contributes to the demise and degradation of microorganisms. How Leishmania parasites avoid intracellular killing in neutrophils remains elusive. Most functions of neutrophils depend on three major cytoplasmic granules, namely: azurophilic, specific, and gelatinase-rich tertiary granules, which differ in their contents and mobilization (6–15). Despite neutrophils phagocytose and destroy a variety of microorganisms through the action of an arsenal of lytic granule components, Leishmania parasites are intriguingly protected from their deadly action (3). On these grounds, we investigated the putative involvement and mobilization of the distinct neutrophil granules in Leishmania infection.
EXPERIMENTAL PROCEDURES
Neutrophil Isolation
Neutrophils were obtained from fresh human peripheral blood by dextran sedimentation and Ficoll-Hypaque centrifugation, followed by hypotonic lysis of residual erythrocytes as previously described (16). The final cell preparation contained more than 99% neutrophils, as assessed by May-Grünwald-Giemsa staining.
Coincubation of Neutrophils with Leishmania major and Leishmania donovani Promastigotes
L. major LV39 (MRHO/SU/59/P strain) parasites were maintained in a virulent state through monthly passage in mice, by infecting 2 × 106 stationary phase parasites subcutaneously into the footpad. Stationary phase promastigotes were collected from in vitro cultures in biphasic NNN blood agar medium. Neutrophils were coincubated with L. major promastigotes at a parasite to neutrophil ratio of 5:1 in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mm l-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin at 37 °C in humidified 95% air and 5% CO2. L. donovani DD8 (MHOM/IN/80/DD8 strain) promastigotes were also incubated with neutrophils as above.
Cell Surface Antigen Detection by Immunofluorescence Flow Cytometry
Cell surface antigen expression was analyzed by flow cytometry as described previously (17), using specific IgG1 mouse monoclonal antibodies against human CD11b (Bear-1) (18), CD66b (clone CLB-B13.9), and CD63 (clone CLB-gran/12,435) (Central Laboratory of the Blood Transfusion Service, Amsterdam, The Netherlands). Neutrophils were incubated for 1 h at 4 °C with the corresponding monoclonal antibodies, washed with cold phosphate-buffered saline (PBS), and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin. After washing, cells were fixed with 1.5% formaldehyde and subjected to immunofluorescence flow cytometry in a BD Biosciences fluorescence-activated cell sorting (FACS) Calibur flow cytometer (Franklin Lakes, NJ). P3X63 myeloma culture supernatant, provided by F. Sánchez-Madrid (Hospital de La Princesa, Madrid, Spain), and an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody (BD Biosciences) were used as negative controls, leading to virtually identical background values. Mean fluorescence intensity in linear scale was obtained from at least 7000 cells in each sample, and the fluorescence produced by the myeloma P3X63 supernatant or an isotype-matched FITC-conjugated nonrelevant IgG monoclonal antibody was considered as background.
Analysis of Neutrophil Infection by Leishmania Parasites through Flow Cytometry
Green fluorescent Leishmania panamensis (MHOM/CO/87/UA140 strain) promastigotes, transfected with p6.5-egfp to express green fluorescent protein (GFP) (provided by R. E. Varela and C. Muskus, Programa de Estudio y Control de Enfermedades Tropicales-PECET, Medellín, Colombia) (19), were used to assess the uptake of Leishmania into neutrophils by flow cytometry.
To prepare non-viable parasites, Leishmania promastigotes in the logarithmic phase of growth were centrifuged at 2500 × g and resuspended in 1% paraformaldehyde for 10 min. The lack of parasite viability was assessed by a drastic decrease in parasite mobility. Untreated viable and paraformaldehyde-treated non-viable Leishmania promastigotes were incubated with human neutrophils (5:1, parasite to neutrophil ratio) for 3 h at 37 °C. Then, infection of human neutrophils by viable and non-viable Leishmania promastigotes was analyzed by flow cytometry. Paraformaldehyde was washed away extensively with PBS (at least 4 washes). Neutrophils remained viable after incubation with paraformaldehyde-treated parasites, as assessed by flow cytometry analysis (16).
Respiratory Burst Activity
Generation of reactive oxygen species by neutrophils was evaluated following dihydroethidine (red fluorescence after oxidation) (Sigma) incubation and analysis by flow cytometry as described (20).
Respiratory burst was also examined by luminol-enhanced chemiluminescence, to detect both intracellular and extracellular oxidants, as described (21). Neutrophils were incubated with 10 μm luminol (Sigma) and the appropriate stimulus: 100 ng/ml of phorbol 12-myristate 13-acetate (PMA),3 1 mg/ml of opsonized zymosan, or L. major parasites at the 1:5 neutrophil to parasite ratio. Zymosan was opsonized with pooled human serum as previously reported (22).
Confocal Laser Scanning Microscopy
Resting neutrophils (5 × 106) were incubated with L. major promastigotes at a parasite to neutrophil ratio of 5:1 in 5 ml of complete RPMI 1640, 10% FBS culture medium. After different incubation times, aliquots (100 μl) of the cell suspension were settled onto poly-l-lysine-coated slides, fixed in 4% formaldehyde, washed exhaustively with PBS, and incubated for 5 min with 50 μl of 5 μm SYTOX® Green dye (Molecular Probes, Eugene, OR), previously prepared in water. Samples were then washed twice with PBS, covered with a drop of Slowfade gold antifade reagent (Molecular Probes), and fluorescence was visualized with a Zeiss LSM 510 laser scanning confocal microscope (Oberkochen, Germany).
Immunoelectron Microscopy
Resting human neutrophils incubated with L. major or L. donovani for 3 h were fixed and processed for ultrathin cryosectioning as previously described (14, 23). For immunolabeling, ultrathin cryosections were incubated with anti-lactoferrin (Cappel Laboratories, West Chester, PA), anti-myeloperoxidase (DAKO, Glostrup, Denmark), or anti-gelatinase (24) (provided by N. Borregaard, The National University Hospital, Copenhagen, Denmark) rabbit antibodies, followed with 10-nm protein A-conjugated colloidal gold (EM Laboratories, Utrecht University, The Netherlands). For endoplasmic reticulum detection, rabbit anti-calreticulin polyclonal antibody (ABR-Affinity BioReagents, Golden, CO) and rabbit anti-protein disulfide isomerase (PDI) polyclonal antibody (25) (provided by H. L. Ploegh, Whitehead Institute for Biomedical Research, Cambridge, MA) were used. After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and examined with a Philips CM 10 electron microscope (Eindhoven, The Netherlands). Negative controls, prepared by replacing the primary antibody with a nonrelevant antibody, showed no staining. Images are representative of three independent experiments, and about 50 phagosome sections were observed for each experiment.
Determination of the Viability of Intracellular L. major in Neutrophils
To estimate the viability of intracellular Leishmania following the infection period (3 and 6 h), we first separated neutrophils from non-phagocytosed free Leishmania by centrifugation. After washing off parasites, the viability of ingested parasites in the pelleted neutrophils was examined by adding 300 μl of RPMI 1640 medium containing 0.008% SDS to lyse neutrophils, a process that was observed under the miscroscope. After 10–15 min, 600 μl of RPMI containing 20% FBS was added to stop lysis, and then serial 4-fold dilutions were plated in 96-well flat-bottom microtiter plates containing 50 μl of NNN blood agar and 100 μl of Schneider's medium supplemented with 10% FBS. The blood agar was dispensed as a slant in the wells of the microtiter plates, so that a window remained in each well, through which the plates could be later microscopically examined for parasite growth. After culture for 9–14 days, the highest dilution yielding growth of viable parasites was determined using a phase-contrast microscope.
Statistical Analysis
All values are expressed as mean ± S.E. Between group statistical differences were assessed using the Mann-Whitney test or the Student's t test. A p value of less than 0.05 was considered statistically significant.
RESULTS
Human Neutrophils Readily Ingest L. major Parasites
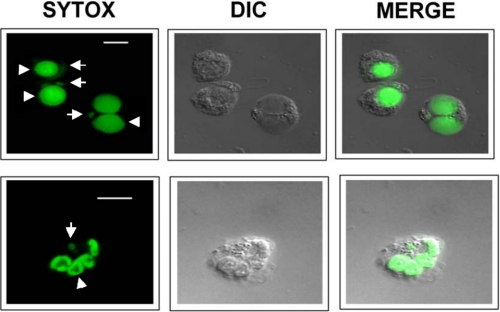
Following incubation of fresh human neutrophils with parasites at different neutrophil to parasite ratios (from 1:1 to 1:10) at different incubation times (1–6 h), we found that optimum parasite engulfment by human neutrophils was reached after incubation of the 1:5 neutrophil to parasite ratio for 3 h. Under these experimental conditions most neutrophils (∼70%) internalized efficiently L. major parasites at a ratio of 1 parasite/neutrophil (Fig. 1), as assessed by confocal microscopy. Kinetoplast and nuclear DNA were visualized in the ingested parasite following DNA staining (Fig. 1 and supplemental Fig. S1). Most of the parasites found inside the human neutrophils (after 3–6 h) remained viable as assessed by limiting dilution assays (about 5–6 × 103 L. major parasites/104 neutrophils).
FIGURE 1.
Uptake of L. major parasites by human neutrophils. Neutrophils and parasite promastigotes were incubated at a parasite to neutrophil ratio of 5:1 in RPMI 1640, 10% FBS culture medium for 3 h, and stained for DNA with SYTOX Green dye (green fluorescence) to visualize both neutrophil (arrowheads) and parasite (arrows) DNA. The corresponding differential interference contrast (DIC) microscopy images are also shown. Interaction and initial phases of parasite engulfment are observed in the two top left neutrophils in the upper panel. A parasite is already inside the right lower neutrophil in the upper panel. A neutrophil has ingested a parasite, and kinetoplast and nuclear DNA of the parasite are readily visualized (lower panel). Images are representative of three independent experiments. Bar, 10 μm.
L. major Parasite Ingestion Does Not Mobilize Specific or Tertiary Granules, and Does Not Induce Respiratory Burst
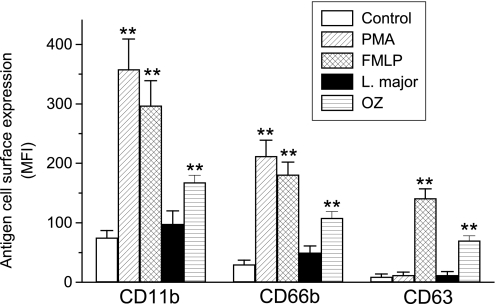
We next examined how engulfment of L. major parasites affected granule mobilization and superoxide anion generation in human neutrophils. CD11b and CD66b are present in the membranes of both specific and tertiary granules (14, 18, 26), whereas CD63 is present in the membranes of azurophilic granules (27) in resting human neutrophils. All these antigens are incorporated into the cell surface facing the extracellular medium following exocytosis of the above neutrophil granules (13, 14, 18). Leishmania interaction with neutrophils did not induce statistically significant CD11b, CD66b, or CD63 cell surface up-regulation (Fig. 2). In contrast, cell surface expression of CD11b and CD66b was increased upon neutrophil activation with PMA, which elicits secretion of specific and tertiary granules, or with N-formyl-methionine-leucine-phenylalanine (fMLP) (Fig. 2), which induces secretion of the three major granules in the presence of cytochalasin B (8, 28). Thus, fMLP treatment also induced up-regulation of cell surface CD63 (Fig. 2). Likewise, incubation of neutrophils with opsonized zymosan to promote phagocytosis led to an increase in cell surface expression of CD11b, CD66b, and CD63 antigens, indicating that the major neutrophil granules fused with plasma membrane at some degree preceding phagosome sealing (Fig. 2). This latter treatment is in agreement with previous reports showing that substantial exocytosis of CD63- and CD66b-bearing granules precedes subsequent phagosomal sealing in opsonized zymosan-treated neutrophils (29). These data suggest that L. major engulfment by human neutrophils follows a pattern of endocytosis that differs from the typical endocytic process.
FIGURE 2.
Lack of granule exocytosis upon internalization of L. major by human neutrophils. The effect of PMA, fMLP, L. major, and opsonized zymosan (OZ) on the cell surface expression of CD11b, CD66b, and CD63 in human neutrophils was analyzed. After neutrophil incubation with L. major as described in the legend to Fig. 1, or with 100 ng/ml of PMA, 10−6 m fMLP (preincubated with 5 μg/ml of cytochalasin B for 5 min), or 1 mg/ml of OZ for 20 min, cell surface expression of the indicated leukocyte antigens was analyzed by flow cytometry, and mean fluorescence intensity (MFI) values were estimated. Data shown are means of 3 independent experiments ± S.E. Asterisks denote significant differences with the control untreated group (**, p < 0.01) by Student's t test.
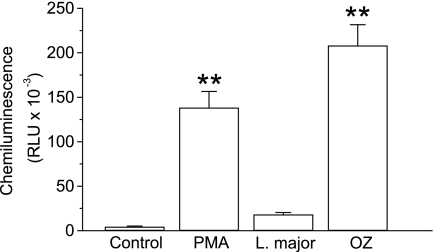
In addition, we found that, unlike soluble (PMA) or particulate (opsonized zymosan) stimuli, L. major parasite engulfment did not induce statistically significant generation of reactive oxygen species (Fig. 3), as assessed under experimental conditions that detected both intracellular and extracellular oxidants. Furthermore, using dihydroethidine to estimate generation of reactive oxygen species by flow cytometry, we found that L. major did not promote statistically significant production of reactive oxygen metabolites (data no shown). Thus, parasites are taken up by neutrophils without triggering granule release and respiratory burst.
FIGURE 3.
Lack of generation of reactive oxygen species upon internalization of L. major parasites by human neutrophils. Neutrophils were untreated (Control) or incubated at room temperature for 45 min with 100 ng/ml of PMA or 1 mg/ml of opsonized zymosan (OZ), or with L. major parasites for 3 h at a parasite to neutrophil ratio of 5:1, and then luminol-enhanced chemiluminescence was measured as relative light units (RLU). Data shown are means of 3 independent experiments ± S.E. Asterisks denote significant differences with the control group (**, p < 0.01) by Student's t test.
Leishmania Parasites Are Located in Vacuoles That Fuse Mainly with Azurophilic Granules
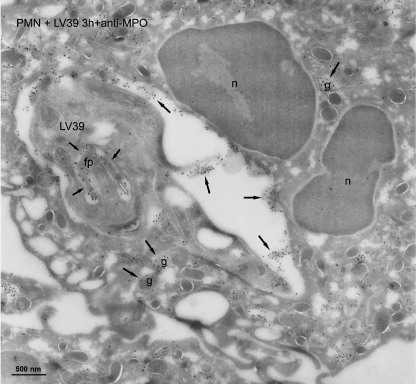
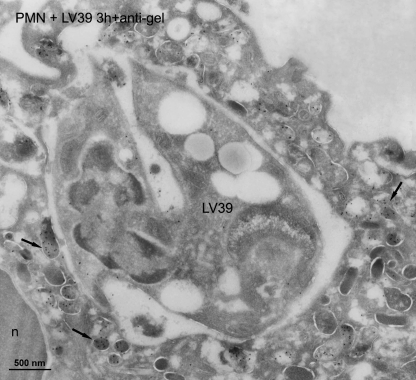
We next examined the intracellular localization of L. major parasites after their incorporation in human neutrophils by immunogold electron microscopy, using gold labels for markers of the distinct granule populations. Electron microscopy images showed that most neutrophil sections contained one parasite in a phagosome (Figs. 4–6, and data not shown). We found that azurophilic granules, assessed by the use of myeloperoxidase granule marker, fused extensively with the L. major-containing vacuole, and gold labeling for myeloperoxidase was shown in the phagosome and in the flagellar pocket of the parasite (Fig. 4). Anti-myeloperoxidase antibodies strongly labeled azurophilic granules, the Leishmania-containing vacuole, as well as azurophilic granules fusing with the vacuole (Fig. 4). However, fusion of specific and tertiary granule with the parasite-containing phagosome was scarce, as assessed by the use of lactoferrin and gelatinase as markers for specific and tertiary granules, respectively (Figs. 5 and 6). Most of the specific and tertiary granules remained intact inside the cell (Figs. 5 and 6). Parasites were morphologically unharmed. This agrees with the above limiting dilution assays, showing that parasites survived inside the neutrophils for at least 3–6 h.
FIGURE 4.
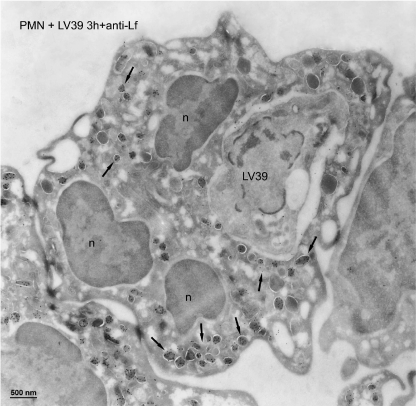
Azurophilic granules fuse with L. major-containing vacuoles in human neutrophils. Cryosections of human polymorphonuclear neutrophils (PMN) incubated with L. major LV39 parasites for 3 h were immunogold labeled with myeloperoxidase (MPO), using specific antibodies. L. major LV39 parasite was shown in the phagosome. After labeling with anti-MPO antibody, a high label (arrows) was present in the phagosome and the flagellar pocket (fp) of the L. major parasite. Macromolecules can diffuse through the opening of the flagellar pocket. A few labeled MPO-positive granules remained in the neutrophil cytoplasm (g, arrows). The nucleus of the neutrophil is indicated, n. Bar, 500 nm.
FIGURE 5.
Subcellular localization of specific granules after L. major internalization in human neutrophils. Cryosections of human polymorphonuclear neutrophils (PMN) incubated with L. major LV39 parasites for 3 h were immunogold labeled with lactoferrin (Lf), using specific antibodies. L. major LV39 parasite was shown in the phagosome. After labeling with anti-lactoferrin antibody, almost no label was detected in the phagosome, whereas abundant labeled lactoferrin-positive granules were observed in the cytoplasm (arrows pointed to clusters of positive granules). The nucleus of the neutrophil is indicated, n. Bar, 500 nm.
FIGURE 6.
Subcellular localization of tertiary granules after L. major internalization in human neutrophils. Cryosections of human polymorphonuclear neutrophils (PMN) incubated with L. major LV39 parasites for 3 h were immunogold labeled with gelatinase (gel), using specific antibodies. L. major LV39 parasite was shown in the phagosome. After labeling with anti-gelatinase antibody, a little label was seen in the phagosomes, whereas abundantly labeled gelatinase-positive granules were observed in the cytoplasm (arrows pointed to clusters of positive granules). The nucleus of the neutrophil is indicated, n. Bar, 500 nm.
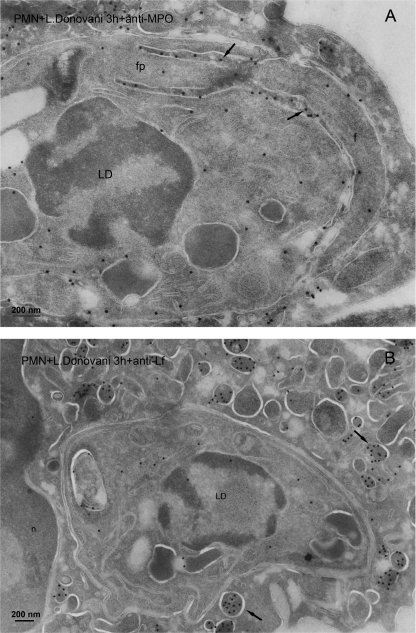
In addition, we also analyzed the location of L. donovani parasites following their uptake by human neutrophils. Electron microscopy images showed that azurophilic granules, assessed by myeloperoxidase granule marker, fused extensively with the L. donovani-containing phagosome, and gold labeling for myeloperoxidase was shown in the phagosome and in the flagellar pocket of the parasite (Fig. 7A). However, most of lactoferrin label was detected in the unfused specific granules, whereas the label in the parasite-containing phagosome was small (Fig. 7B). Thus, we found a predominant fusion of myeloperoxidase-positive azurophilic granules with Leishmania-containing vacuoles, irrespective of using L. major or L. donovani parasites.
FIGURE 7.
Subcellular localization of azurophilic and specific granules after L. donovani internalization in human neutrophils. Cryosections of human polymorphonuclear neutrophils (PMN) incubated with L. donovani parasites (LD) for 3 h were immunogold labeled with myeloperoxidase (MPO) (A) or lactoferrin (Lf) (B). The L. donovani parasite is shown in the phagosome. A, after labeling with anti-MPO antibody, a high label (arrows) was present in the phagosome around the flagellum (f) and in the flagellar pocket (fp) of the L. donovani parasite. B, after labeling with anti-lactoferrin antibody, almost no label was seen in the phagosome, whereas abundantly labeled lactoferrin-positive granules (arrows) were observed in the cytoplasm. The nucleus of the neutrophil is shown, n. Bar, 200 nm.
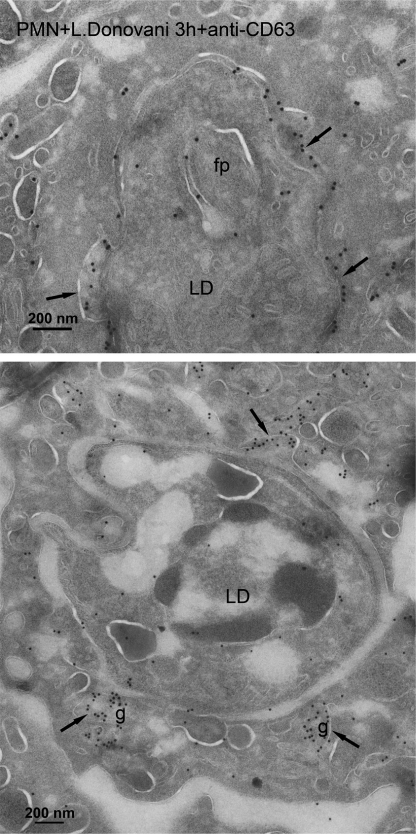
Furthermore, using CD63 as a membrane marker for azurophilic granules, we also detected fusion of CD63-bearing granules with the L. donovani-engulfed phagosome (Fig. 8). CD63 label was found in the vacuole surrounding the parasite and the fusion of azurophilic granules with the parasite-containing vacuole (Fig. 8). These results further support fusion of azurophilic granules with Leishmania-containing vacuoles. Similar results were also achieved with L. major-containing vacuoles and CD63 localization (data not shown). Taken together, our data suggest that engulfment of L. major parasites by neutrophils avoids fusion of parasite-engulfed phagosomes with specific and tertiary granules, which contain the machineries responsible for reactive oxygen species generation and acidification (6–9), two critical noxious processes for parasite survival.
FIGURE 8.
Localization of azurophilic granule membrane marker CD63 in L. donovani-containing phagosome. Cryosections of human neutrophils, incubated with L. donovani parasites (LD) for 3 h, were immunogold labeled with anti-CD63 antibody as a membrane marker for azurophilic granules. Lower panel shows fusion of CD63-positive azurophilic granules (g, arrows) with the parasite-containing phagosome. In another area, the CD63-positive granular membrane (arrow) is inserted in the phagosome membrane. The upper panel shows the CD63 label on the phagosome membrane (arrows) containing a L. donovani parasite (LD) with a flagellar pocket (fp). Bar, 200 nm.
Leishmania-containing Vacuoles Do Not Show Endoplasmic Reticulum Markers
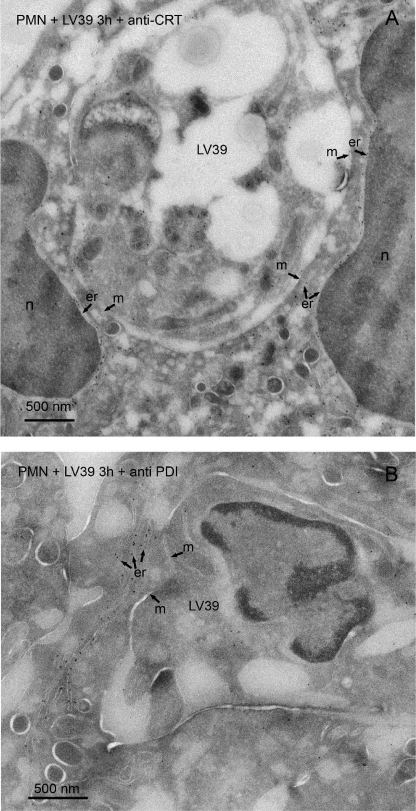
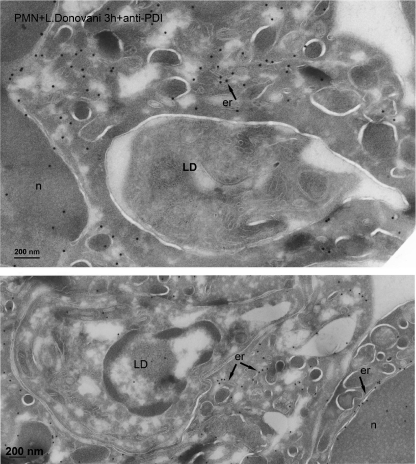
The survival of L. donovani parasites in neutrophils has been recently proposed to be due to their location in non-lytic compartments derived from endoplasmic reticulum (30). This conclusion was reached after characterizing the parasite-containing vacuole at the electron microscopy level using glucose-6-phosphatase cytochemistry (30). To determine whether the endoplasmic reticulum contributed to the phagocytosis of Leishmania by human neutrophils, we performed immunogold labeling for two endoplasmic reticulum resident proteins: calreticulin and PDI, two well established soluble endoplasmic reticulum protein markers (31, 32). After labeling with anti-calreticulin and anti-PDI antibodies, the endoplasmic reticulum cisternae were extensively labeled, whereas the label associated with phagosomes containing L. major parasites was negligible (Fig. 9). Similar results were also achieved with neutrophils containing L. donovani parasites and stained with anti-PDI (Fig. 10) and anti-calreticulin antibodies (supplemental Fig. S2). In some regions, the phagosome membrane was in close proximity to the cisternae of the endoplasmic reticulum, but no fusion was detected (Figs. 9 and 10, and supplemental Fig. S2).
FIGURE 9.
Assessment of the contribution of endoplasmic reticulum to the L. major-containing phagosome. Cryosections of human polymorphonuclear neutrophils (PMN) incubated with L. major (LV39) parasites for 3 h were immunogold labeled with the endoplasmic reticulum-resident proteins calreticulin (CRT) (A) or PDI (B) using specific antibodies. L. major LV39 parasite was shown in the phagosomes. After labeling with anti-CRT (A) and anti-PDI (B) antibodies, the endoplasmic reticulum cisternae (er) were extensively labeled, whereas the label associated with phagosomes was negligible. Some label in the putative endoplasmic reticulum cisternae/vesicles from the parasite was observed. In some areas, the membrane (m) of the phagosome is in close proximity to the cisternae of the endoplasmic reticulum. The nucleus of the neutrophil is shown, n. Bar, 500 nm.
FIGURE 10.
Assessment of the contribution of endoplasmic reticulum to the L. donovani-containing phagosome. Cryosections of human polymorphonuclear neutrophils (PMN) incubated with L. donovani parasites (LD) for 3 h were immunogold labeled with the endoplasmic reticulum-resident PDI, using specific antibodies. The upper panel shows a just internalized L. donovani parasite (LD), where the condensation of actin (electron-dense) below the phagosome membrane is visible. After labeling with anti-PDI antibodies, the endoplasmic reticulum cisternae (er) from the PMN were extensively labeled, whereas the label associated with phagosomes was negligible. The nucleus of the neutrophil is shown, n. Bar, 200 nm.
Viable Parasites Are Required for Their Efficient Engulfment by Neutrophils
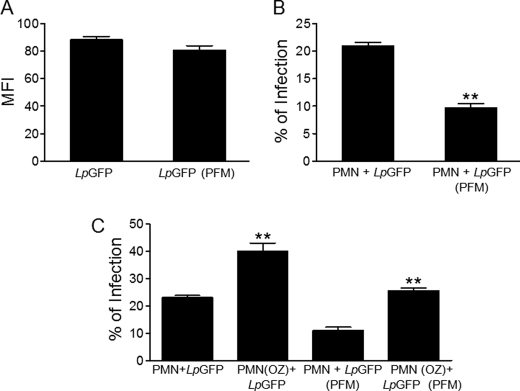
Using infective green fluorescent L. panamensis parasites, transfected with p6.5-egfp to express green fluorescent protein (GFP) (19), we were able to monitor the uptake of Leishmania into neutrophils by flow cytometry. We also treated these green fluorescent L. panamensis parasites with paraformaldehyde under conditions that did not affect the fluorescence signal of the fluorescent parasite (Fig. 11A), but dramatically attenuated cell viability, as assessed by loss of parasite mobility. The uptake of intact Leishmania by neutrophils was higher than that of paraformaldehyde-treated non-viable GFP-expressing Leishmania parasites (Fig. 11B). Similar data were also obtained with Leishmania mexicana transfected with red fluorescent protein (data not shown). These data suggest that the ability of Leishmania to invade neutrophils is largely dependent on live parasites. However, the uptake of Leishmania parasites, either alive or dead, was increased when neutrophils were treated with opsonized zymosan (Fig. 11C).
FIGURE 11.
Live Leishmania parasites are required for their efficient engulfment by naive human neutrophils. A, fluorescent promastigotes of L. panamensis transfected with the p6.5-egfp construct were untreated (LpGFP) or treated with 1% paraformaldehyde for 10 min (LpGFP-PFM), and then the fluorescence signal from the parasites was measured by flow cytometry as mean fluorescence intensity (MFI). B, human polymorphonuclear neutrophils (PMN) were incubated for 3 h with LpGFP or LpGFP-PFM, and the percentage of infection was assessed by flow cytometry. C, human neutrophils were incubated with LpGFP or LpGFP-PFM in the absence or presence of 1 mg/ml of opsonized zymosan (OZ), and then the percentage of infection was assessed by flow cytometry as above. Data shown are means of 3 independent experiments ± S.E. Asterisks denote significant differences with viable parasites in B, and with naive neutrophils in C (**, p < 0.01) by Student's t test.
DISCUSSION
Our results indicate that azurophilic granules mainly fuse with the parasite-containing phagosome during Leishmania infection of human neutrophils, but only a few, if any, specific and tertiary granules fuse with the parasite-bearing phagosome. Fusion of azurophilic granules with Leishmania-engulfed phagosomes was demonstrated by localization of myeloperoxidase and CD63, soluble and membrane markers of azurophilic granules, in the vacuole enclosing the parasite. Leishmania parasites are rapidly killed by a H2O2-dependent process (33). Acid pH activates a number of acid hydrolases and promotes conversion of superoxide anion to H2O2 (34). We have previously found, by subcellular fractionation assays, that the ATP-dependent acidification capacity, required for phagosome acidification, and cytochrome b, required for superoxide anion generation, are mainly located in tertiary granules, with a secondary location in specific granules (6, 7, 9). Fusion of cytochrome b-rich granules with the plasma membrane is required for the production of reactive oxygen species (8, 35). Thus, Leishmania parasites avoid co-localization with granules involved in ATP-dependent acidification and superoxide anion generation. Lack of fusion of proton pump ATPase-containing tertiary granules (7) with phagosomes would prevent phagosome acidification, and consequently the action of azurophilic granule acid hydrolases. Therefore, prevention of tertiary/specific granule fusion with phagosomes containing azurophilic granules may hamper the triggering of major anti-infection mechanisms. Likewise, lack of fusion of tertiary and specific granules, which harbor cytochrome b (6, 8, 9) responsible for the respiratory burst, with parasite-containing phagosome would prevent the triggering of the generation of oxygen metabolites highly toxic for the parasite. In fact, we found that L. major parasites did not trigger the respiratory burst in human neutrophils. In this regard, Leishmania promastigotes and antigens have been reported to inhibit neutrophil respiratory burst (36, 37). Furthermore, Lodge et al. (38, 39) have shown that the surface glycolipid lipophosphoglycan enabled L. donovani to evade the ability of macrophages to produce superoxide anion during its internalization by preventing the assembly of a functional phagosomal NADPH oxidase complex. The lack of mobilization of tertiary and specific granules might underlie the absence of a respiratory burst response, thus allowing survival of the parasites within these cells. In contrast, fusion of solely azurophilic granules with parasite-containing phagosome might release arginase I into the Leishmania-containing vacuole, which, in turn, might support parasite survival. It has been shown that arginase I facilitates Leishmania survival (40), and this enzyme was reported to be present in azurophilic granules (41). Thus, prevention of early fusion of tertiary and specific granules with the phagosome during endocytosis precludes the deleterious action of azurophilic granule constituents against Leishmania parasites. On these grounds, the lack of acidification prevents the action of acid hydrolases, and the absence of superoxide anion generation prevents the action of the highly toxic myeloperoxidase-H2O2-halide antimicrobial system (42).
A previous report showed that non-damaged L. donovani promastigotes were targeted to a lysosome-independent compartment, displaying some features of the endoplasmic reticulum, where parasites were protected from degradation (30). No markers for intracellular neutrophil granules were used in that study, and endoplasmic reticulum was identified by the electron-dense product of the activity of the endoplasmic reticulum enzyme glucose-6-phosphatase. In contrast, the results reported here indicate that L. major and L. donovani parasites are incorporated into vacuoles of human neutrophils that are largely devoid of endoplasmic reticulum markers. Here we identified the endoplasmic reticulum through the use of two soluble endoplasmic reticulum markers, calreticulin and PDI, and found no co-localization of Leishmania-containing vacuoles with endoplasmic reticulum. This discrepancy could be explained in part by the use of different approaches in each study, namely enzymatic staining and immunogold electron microscopy. Cytochemical reactions, such as the cerium capture used for glucose-6-phosphatase determinations, are not as specific as gold immunoelectron microscopy, and may reflect a minor nonspecific contribution of other enzymes, particularly in tissues with low glucose-6-phosphatase activity. Hydrolysis of glucose 6-phosphate can also be catalyzed by other enzymes, including alkaline and acid phosphatases, which are enriched in the plasma membrane and lysosomes, respectively (43–45). In addition, the resolution of the images obtained by enzymatic staining is lower than that achieved by the use of immunogold techniques, and some reaction products might diffuse into nearby organelles. Because endoplasmic reticulum and Leishmania-containing vacuoles can be very close each other (Figs. 9 and 10, and supplemental Fig. S2), a putative diffusion of the enzymatic products into the vacuole could lead to a partial misidentification of the subcellular structures.
An alternative model implicating the endoplasmic reticulum as a major component of nascent and maturation of phagosomes has been proposed in phagocytosis (46). However, a subsequent study using different ultrastructural approaches, and a number of distinct endoplasmic reticulum markers, could not detect any significant contribution of the endoplasmic reticulum to forming or maturing phagosomes (47). In this latter study, plasma membrane was found to be the main constituent of nascent and newly formed phagosomes, which were progressively remodeled by fusion with endosomal and eventually lysosomal compartments as phagosomes matured (47). These latter findings are in agreement with our present results, and support that the Leishmania-containing vacuoles in human neutrophils mostly fuse with lysosome-like azurophilic granules, but are largely free of endoplasmic reticulum markers.
Thus, the results shown in this study indicate that Leishmania parasites can survive inside phagosomes in the human neutrophil through a process that directs a preferential mobilization of azurophilic granules to the parasite-containing vacuoles. Contrary to our present results, Streptococcus pyogenes bacteria, expressing surface-associated M and/or M-like proteins, inhibit the fusion of azurophilic granules with phagosomes after internalization by human neutrophils (48). In this way, S. pyogenes survives and replicates intracellularly after being phagocytosed by human neutrophils (48). Thus, it might be envisaged that distinct microorganisms can modulate granule mobilization and fusion with phagosomes in different ways.
Our data indicate that viable Leishmania parasites are predominantly taken up by naive human neutrophils, suggesting that parasites may actively penetrate the neutrophil. This might underlie the lack of neutrophil response to parasite uptake. Treatment with opsonized zymosan increased engulfment of both alive and dead parasites at similar rates. This suggests that induction of phagocytosis in neutrophils facilitates parasite uptake, but parasite incorporation by opsonized zymosan-stimulated neutrophils could proceed through a distinct mechanism from that involving uptake of viable Leishmania by naive neutrophils.
Taken together, our data suggest that engulfment of L. major parasites by neutrophils avoids fusion of parasite-containing vacuole with granules containing the machineries responsible for reactive oxygen species generation and acidification, two critical noxious processes for parasite survival. The results reported here show that L. major promastigotes drive a rather selective fusion of azurophilic granules into parasite-containing phagosomes in human neutrophils, thus eluding microbicidal effector mechanisms by modulating differential granule mobilization.
Supplementary Material
Acknowledgments
We are grateful to Nico Ong for the preparation of micrographs. We thank the University Hospital Blood Bank of Salamanca for blood supply.
This work was supported by Spanish Ministry of Science and Innovation (Grant SAF2008-02251, and Grant RD06/0020/1037 from Red Temática de Investigación Cooperativa en Cáncer, Instituto de Salud Carlos III, co-funded by the Fondo Europeo de Desarrollo Regional of the European Union), and Junta de Castilla y León (CSI01A08, GR15-Experimental Therapeutics and Translational Oncology Program, and Biomedicine Project 2009).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- PMA
- phorbol 12-myristate 13-acetate
- fMLP
- N-formyl-methionine-leucine-phenylalanine
- PDI
- protein disulfide isomerase.
REFERENCES
- 1.Müller K., van Zandbergen G., Hansen B., Laufs H., Jahnke N., Solbach W., Laskay T. (2001) Med. Microbiol. Immunol. 190, 73–76 [DOI] [PubMed] [Google Scholar]
- 2.Peters N. C., Egen J. G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M. P., Germain R. N., Sacks D. (2008) Science 321, 970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laskay T., van Zandbergen G., Solbach W. (2003) Trends Microbiol. 11, 210–214 [DOI] [PubMed] [Google Scholar]
- 4.van Zandbergen G., Klinger M., Mueller A., Dannenberg S., Gebert A., Solbach W., Laskay T. (2004) J. Immunol. 173, 6521–6525 [DOI] [PubMed] [Google Scholar]
- 5.Mollinedo F., Borregaard N., Boxer L. A. (1999) Immunol. Today 20, 535–537 [DOI] [PubMed] [Google Scholar]
- 6.Mollinedo F., Schneider D. L. (1984) J. Biol. Chem. 259, 7143–7150 [PubMed] [Google Scholar]
- 7.Mollinedo F., Manara F. S., Schneider D. L. (1986) J. Biol. Chem. 261, 1077–1082 [PubMed] [Google Scholar]
- 8.Mollinedo F., Pulido R., Lacal P. M., Sanchez-Madrid F. (1991) Scand. J. Immunol. 34, 33–43 [DOI] [PubMed] [Google Scholar]
- 9.Mollinedo F., Gajate C., Schneider D. L. (1991) Mol. Cell. Biochem. 105, 49–60 [DOI] [PubMed] [Google Scholar]
- 10.Mollinedo F., Nakajima M., Llorens A., Barbosa E., Callejo S., Gajate C., Fabra A. (1997) Biochem. J. 327, 917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borregaard N., Cowland J. B. (1997) Blood 89, 3503–3521 [PubMed] [Google Scholar]
- 12.Mollinedo F. (2003) Inmunologia 22, 340–358 [Google Scholar]
- 13.Martín-Martín B., Nabokina S. M., Blasi J., Lazo P. A., Mollinedo F. (2000) Blood 96, 2574–2583 [PubMed] [Google Scholar]
- 14.Mollinedo F., Martín-Martín B., Calafat J., Nabokina S. M., Lazo P. A. (2003) J. Immunol. 170, 1034–1042 [DOI] [PubMed] [Google Scholar]
- 15.Mollinedo F., Calafat J., Janssen H., Martín-Martín B., Canchado J., Nabokina S. M., Gajate C. (2006) J. Immunol. 177, 2831–2841 [DOI] [PubMed] [Google Scholar]
- 16.Santos-Beneit A. M., Mollinedo F. (2000) J. Leukocyte Biol. 67, 712–724 [DOI] [PubMed] [Google Scholar]
- 17.Mollinedo F., Burgaleta C., Velasco G., Arroyo A. G., Acevedo A., Barasoain I. (1992) J. Immunol. 149, 323–330 [PubMed] [Google Scholar]
- 18.Lacal P., Pulido R., Sánchez-Madrid F., Mollinedo F. (1988) J. Biol. Chem. 263, 9946–9951 [PubMed] [Google Scholar]
- 19.Varela M, R. E., Muñoz D. L., Robledo S. M., Kolli B. K., Dutta S., Chang K. P., Muskus C. (2009) Exp. Parasitol. 122, 134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajate C., Santos-Beneit A. M., Macho A., Lazaro M., Hernandez-De Rojas A., Modolell M., Muñoz E., Mollinedo F. (2000) Int. J. Cancer 86, 208–218 [DOI] [PubMed] [Google Scholar]
- 21.Liles W. C., Ledbetter J. A., Waltersdorph A. W., Klebanoff S. J. (1995) J. Immunol. 155, 2175–2184 [PubMed] [Google Scholar]
- 22.Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. (1986) J. Immunol. 136, 4220–4225 [PubMed] [Google Scholar]
- 23.Calafat J., Janssen H., Ståhle-Bäckdahl M., Zuurbier A. E., Knol E. F., Egesten A. (1997) Blood 90, 1255–1266 [PubMed] [Google Scholar]
- 24.Kjeldsen L., Bjerrum O. W., Hovgaard D., Johnsen A. H., Sehested M., Borregaard N. (1992) Eur. J. Haematol. 49, 180–191 [DOI] [PubMed] [Google Scholar]
- 25.McGehee A. M., Dougan S. K., Klemm E. J., Shui G., Park B., Kim Y. M., Watson N., Wenk M. R., Ploegh H. L., Hu C. C. (2009) J. Immunol. 183, 3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacal P., Pulido R., Sánchez-Madrid F., Cabañas C., Mollinedo F. (1988) Biochem. Biophys. Res. Commun. 154, 641–647 [DOI] [PubMed] [Google Scholar]
- 27.Kuijpers T. W., Tool A. T., van der Schoot C. E., Ginsel L. A., Onderwater J. J., Roos D., Verhoeven A. J. (1991) Blood 78, 1105–1111 [PubMed] [Google Scholar]
- 28.Mollinedo F., Nieto J. M., Andreu J. M. (1989) Mol. Pharmacol. 36, 547–555 [PubMed] [Google Scholar]
- 29.Tapper H., Grinstein S. (1997) J. Immunol. 159, 409–418 [PubMed] [Google Scholar]
- 30.Gueirard P., Laplante A., Rondeau C., Milon G., Desjardins M. (2008) Cell Microbiol. 10, 100–111 [DOI] [PubMed] [Google Scholar]
- 31.Nauseef W. M., McCormick S. J., Clark R. A. (1995) J. Biol. Chem. 270, 4741–4747 [DOI] [PubMed] [Google Scholar]
- 32.Noiva R., Lennarz W. J. (1992) J. Biol. Chem. 267, 3553–3556 [PubMed] [Google Scholar]
- 33.Pearson R. D., Steigbigel R. T. (1981) J. Immunol. 127, 1438–1443 [PubMed] [Google Scholar]
- 34.Styrt B., Klempner M. S. (1988) J. Med. Microbiol. 25, 101–107 [DOI] [PubMed] [Google Scholar]
- 35.Mollinedo F., Schneider D. L. (1987) FEBS Lett. 217, 158–162 [DOI] [PubMed] [Google Scholar]
- 36.el-On J., Zvillich M., Sarov I. (1990) Parasite Immunol. 12, 285–295 [DOI] [PubMed] [Google Scholar]
- 37.al Tuwaijri A. S., al Mofleh I. A., Mahmoud A. A. (1990) J. Med. Microbiol. 32, 189–193 [DOI] [PubMed] [Google Scholar]
- 38.Lodge R., Diallo T. O., Descoteaux A. (2006) Cell. Microbiol. 8, 1922–1931 [DOI] [PubMed] [Google Scholar]
- 39.Lodge R., Descoteaux A. (2006) Eur. J. Immunol. 36, 2735–2744 [DOI] [PubMed] [Google Scholar]
- 40.Iniesta V., Gómez-Nieto L. C., Corraliza I. (2001) J. Exp. Med. 193, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munder M., Mollinedo F., Calafat J., Canchado J., Gil-Lamaignere C., Fuentes J. M., Luckner C., Doschko G., Soler G., Eichmann K., Müller F. M., Ho A. D., Goerner M., Modolell M. (2005) Blood 105, 2549–2556 [DOI] [PubMed] [Google Scholar]
- 42.Klebanoff S. J. (1982) in Phagocytic Cells (Gallin J. I., Fauci A. S. eds) pp. 111–162, Raven Press, New York [Google Scholar]
- 43.Ulsamer A. G., Wright P. L., Wetzel M. G., Korn E. D. (1971) J. Cell Biol. 51, 193–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols B. A., Setzer P. Y., Bainton D. F. (1984) J. Histochem. Cytochem. 32, 165–171 [DOI] [PubMed] [Google Scholar]
- 45.Matsubara S., Yamada T., Minakami H., Watanabe T., Takizawa T., Sato I. (1999) Placenta 20, 185–188 [DOI] [PubMed] [Google Scholar]
- 46.Gagnon E., Duclos S., Rondeau C., Chevet E., Cameron P. H., Steele-Mortimer O., Paiement J., Bergeron J. J., Desjardins M. (2002) Cell 110, 119–131 [DOI] [PubMed] [Google Scholar]
- 47.Touret N., Paroutis P., Terebiznik M., Harrison R. E., Trombetta S., Pypaert M., Chow A., Jiang A., Shaw J., Yip C., Moore H. P., van der Wel N., Houben D., Peters P. J., de Chastellier C., Mellman I., Grinstein S. (2005) Cell 123, 157–170 [DOI] [PubMed] [Google Scholar]
- 48.Staali L., Bauer S., Mörgelin M., Björck L., Tapper H. (2006) Cell. Microbiol. 8, 690–703 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.