Abstract
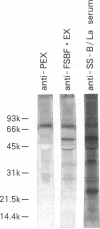
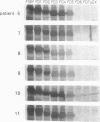
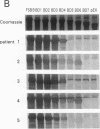
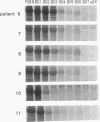
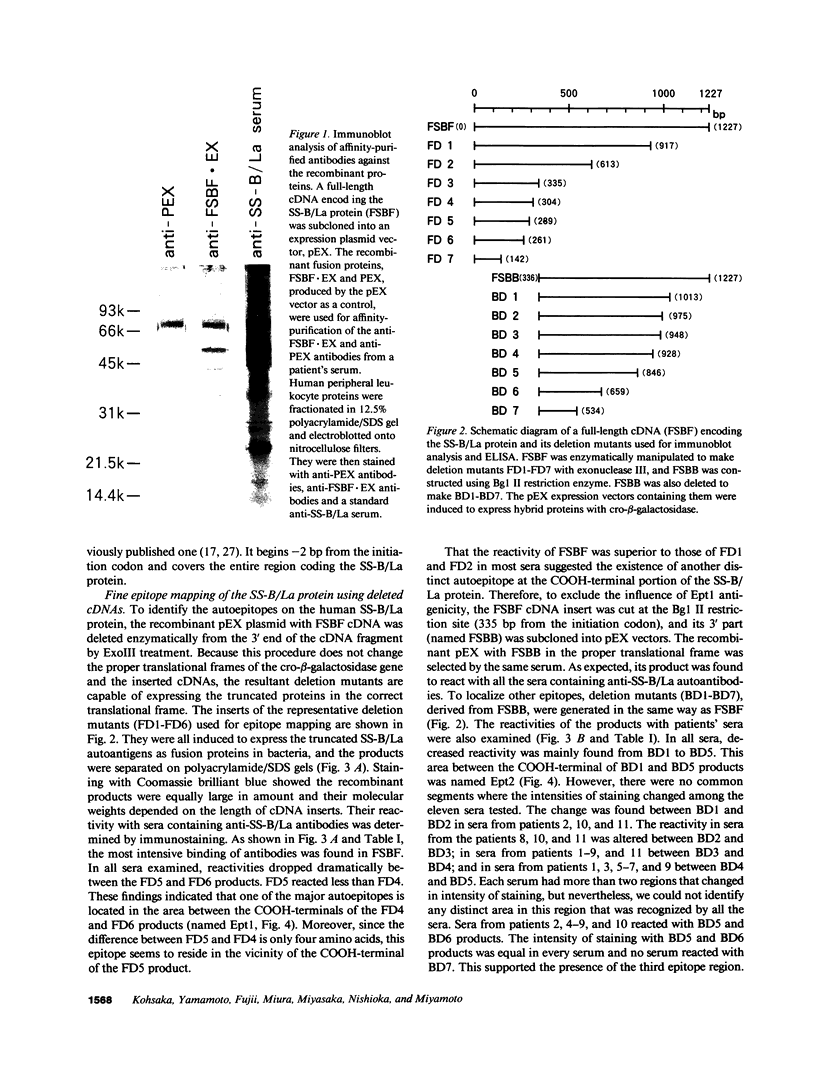
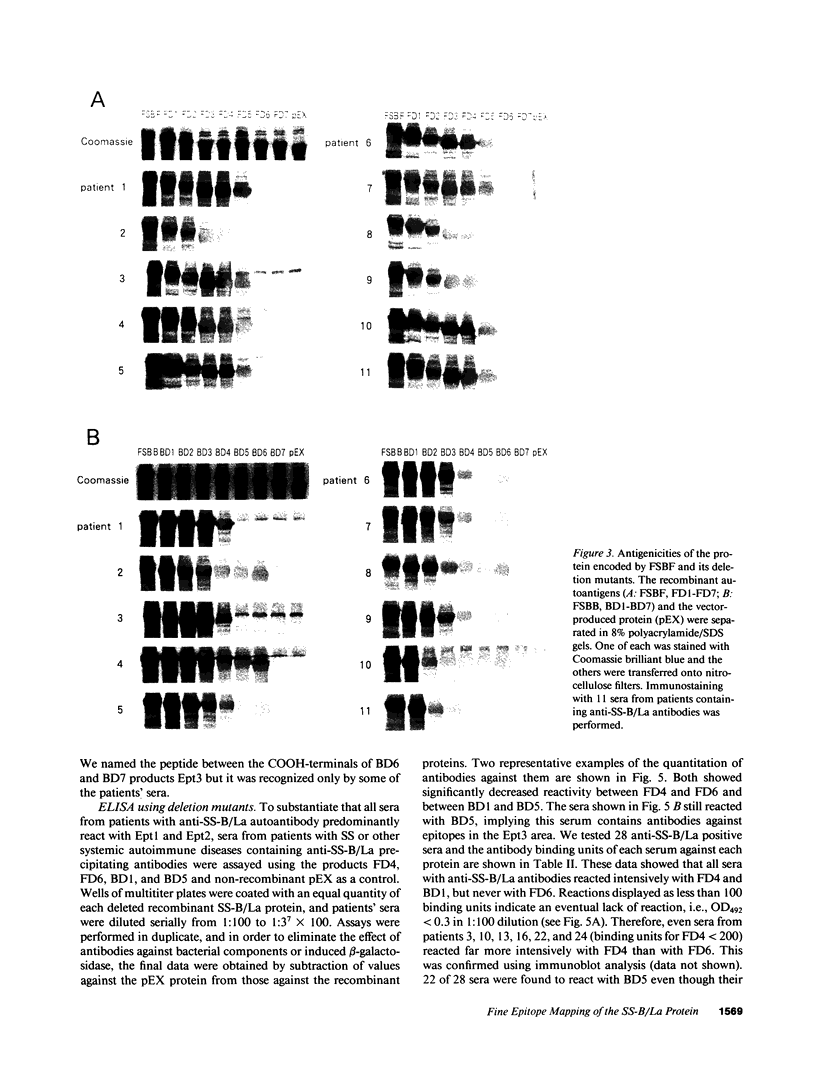
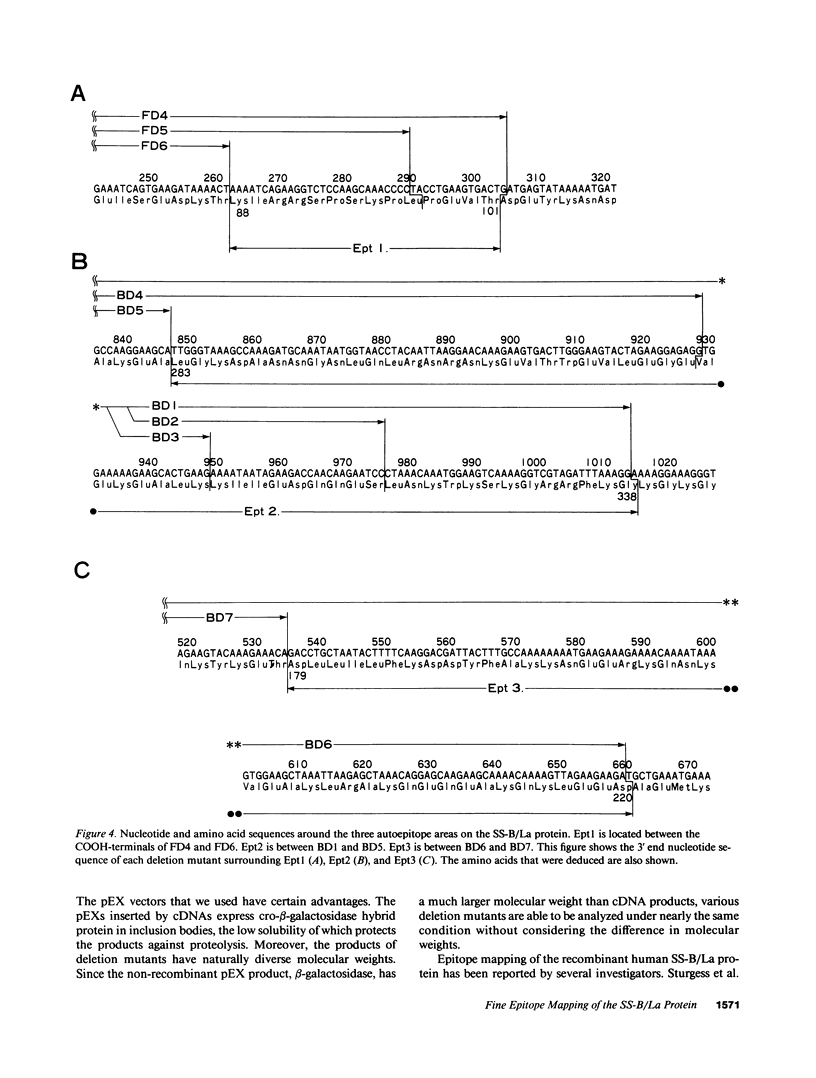
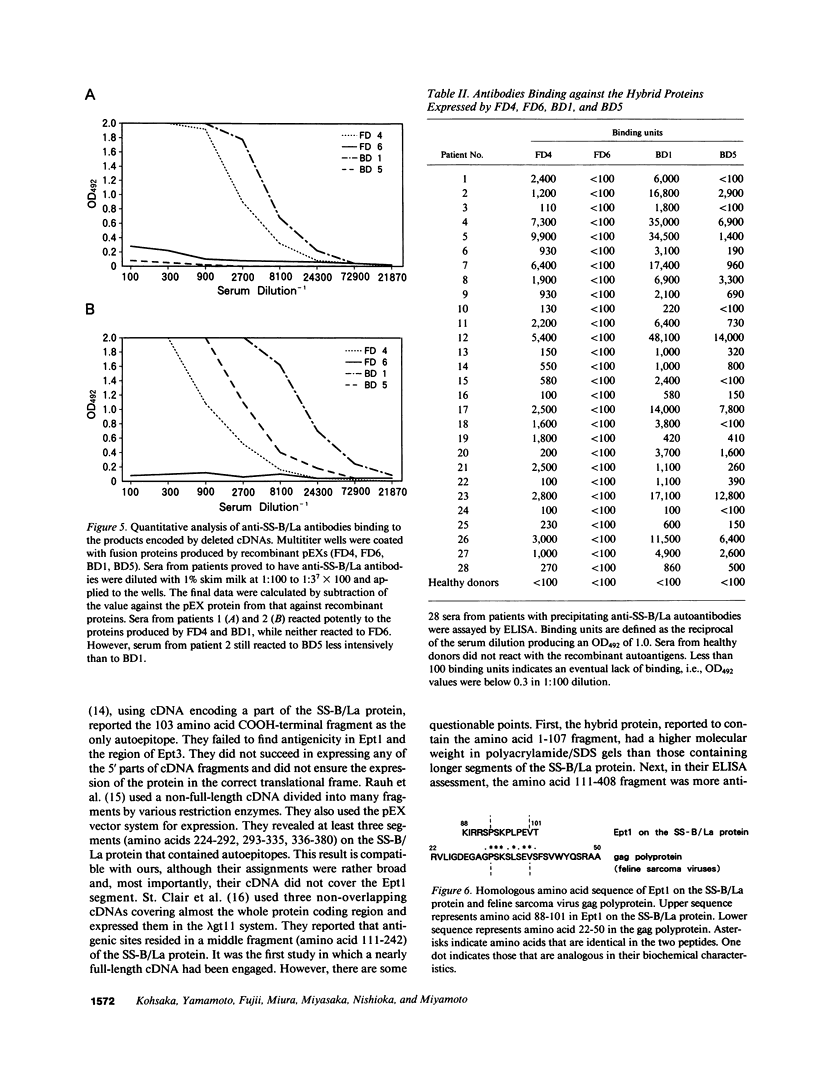
To analyze the autoepitopes on the SS-B/La protein, a cDNA covering the entire region coding the protein was isolated from a human cDNA library. The cDNA was subcloned into an expression plasmid vector, pEX, to express its protein product as a fusion protein with cro-beta-galactosidase in Escherichia coli. A recombinant pEX plasmid expressing three-fourths of the protein (amino acid 112-408) was also constructed. The antigenicities of these recombinant proteins were confirmed with a patient's serum. Their various deletion mutants were produced with exonuclease III treatment from the 3' ends of the cDNAs without changing the proper translational frame. Immunoblot analysis and enzyme-linked immunosorbent assay were used to evaluate the reactivities of the recombinant proteins with patients' sera to determine the autoepitopes. A narrow segment (amino acid 88-101) and the region where several epitopes were located (amino acid 283-338) on the SS-B/La protein were universally recognized by all the sera with anti-SS-B/La antibodies examined. An additional epitope region (amino acid 179-220) was recognized by some patients' sera. Computer analysis revealed that the most distinct autoepitope, amino acid 88-101, had a striking homology to a retroviral gag polyprotein. These findings indicate that exogenous or endogenous retroviruses may play a role in initiation of the anti-SS-B/La autoimmunity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
- Chan E. K., Francoeur A. M., Tan E. M. Epitopes, structural domains, and asymmetry of amino acid residues in SS-B/La nuclear protein. J Immunol. 1986 May 15;136(10):3744–3749. [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Tan E. M. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. doi: 10.1093/nar/17.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. E., Hinrichs S. H., Vogel J., Jay G. Exocrinopathy resembling Sjögren's syndrome in HTLV-1 tax transgenic mice. Nature. 1989 Sep 7;341(6237):72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Even J., Sherr C. J., Galibert F. Nucleotide sequences of feline sarcoma virus long terminal repeats and 5' leaders show extensive homology to those of other mammalian retroviruses. J Virol. 1983 Jan;45(1):466–472. doi: 10.1128/jvi.45.1.466-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L., Slack J. H., Amundson C., Izui S., Theofilopoulos A. N., Dixon F. J. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983 Mar 1;157(3):874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kohsaka H., Yamamoto K., Fujii H., Miyasaka N., Miura H., Tanaka Y., Nishioka K., Miyamoto T. Molecular cloning of cDNAs expressing SS-B/La protein. J Autoimmun. 1989 Aug;2(4):353–357. doi: 10.1016/0896-8411(89)90163-7. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983 Oct;34(3):837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Clonal sketches of the immune response. EMBO J. 1988 Oct;7(10):2945–2951. doi: 10.1002/j.1460-2075.1988.tb03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Jimenez S. A., Riggs E., Ziemnicka-Kotula D. Determination of an epitope of the diffuse systemic sclerosis marker antigen DNA topoisomerase I: sequence similarity with retroviral p30gag protein suggests a possible cause for autoimmunity in systemic sclerosis. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8492–8496. doi: 10.1073/pnas.86.21.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Rauh A. J., Hornig H., Lührmann R. At least three distinct B cell epitopes reside in the C-terminal half of La protein, as determined by a recombinant DNA approach. Eur J Immunol. 1988 Dec;18(12):2049–2057. doi: 10.1002/eji.1830181227. [DOI] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- St Clair E. W., Pisetsky D. S., Reich C. F., Keene J. D. Analysis of autoantibody binding to different regions of the human La antigen expressed in recombinant fusion proteins. J Immunol. 1988 Dec 15;141(12):4173–4180. [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess A. D., Peterson M. G., McNeilage L. J., Whittingham S., Coppel R. L. Characteristics and epitope mapping of a cloned human autoantigen La. J Immunol. 1988 May 1;140(9):3212–3218. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Chan E. K., Sullivan K. F., Rubin R. L. Antinuclear antibodies (ANAs): diagnostically specific immune markers and clues toward the understanding of systemic autoimmunity. Clin Immunol Immunopathol. 1988 May;47(2):121–141. doi: 10.1016/0090-1229(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Whittingham S., McNeilage J., Mackay I. R. Primary Sjögren's syndrome after infectious mononucleosis. Ann Intern Med. 1985 Apr;102(4):490–493. doi: 10.7326/0003-4819-102-4-490. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Keene J. D. Interactions of plus and minus strand leader RNAs of the New Jersey serotype of vesicular stomatitis virus with the cellular La protein. Virology. 1984 May;135(1):65–73. doi: 10.1016/0042-6822(84)90117-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Miura H., Moroi Y., Yoshinoya S., Goto M., Nishioka K., Miyamoto T. Isolation and characterization of a complementary DNA expressing human U1 small nuclear ribonucleoprotein C polypeptide. J Immunol. 1988 Jan 1;140(1):311–317. [PubMed] [Google Scholar]
- Yamaoka K., Miyasaka N., Yamamoto K. Possible involvement of Epstein-Barr virus in polyclonal B cell activation in Sjögren's syndrome. Arthritis Rheum. 1988 Aug;31(8):1014–1021. doi: 10.1002/art.1780310812. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]