Abstract
Natural dietary agents have drawn a great deal of attention toward cancer prevention because of their wide safety margin. However, single agent intervention has failed to bring the expected outcome in clinical trials; therefore, combinations of chemopreventive agents are gaining increasingly popularity. In the present study, we investigated a combinatorial approach using two natural dietary polyphenols, luteolin and EGCG, and found that their combination at low doses (at which single agents induce minimal apoptosis) synergistically increased apoptosis (3–5-fold more than the additive level of apoptosis) in both head and neck and lung cancer cell lines. This combination also significantly inhibited growth of xenografted tumors in nude mice. The in vivo findings also were supported by significant inhibition of Ki-67 expression and increase in TUNEL-positive cells in xenografted tissues. Mechanistic studies revealed that the combination induced mitochondria-dependent apoptosis in some cell lines and mitochondria-independent apoptosis in others. Moreover, we found more efficient stabilization and ATM-dependent Ser15 phosphorylation of p53 due to DNA damage by the combination, and ablation of p53 using shRNA strongly inhibited apoptosis as evidenced by decreased poly(ADP-ribose) polymerase and caspase-3 cleavage. In addition, we observed mitochondrial translocation of p53 after treatment with luteolin or the combination of EGCG and luteolin. Taken together, our results for the first time suggest that the combination of luteolin and EGCG has synergistic/additive growth inhibitory effects and provides an important rationale for future chemoprevention trials of head and neck and lung cancers.
Keywords: Apoptosis, Cancer Therapy, Gene Regulation, Mitochondrial Apoptosis, p53
Introduction
Drug-associated toxicities pose continuous challenges to the success of cancer therapy. Drug-associated toxicities arise mainly due to the use of higher drug doses to achieve effective responses. The application of combination approaches to chemotherapy has led to enhanced clinical responses, reduced toxicities, and lower probabilities of developing drug resistance. Chemoprevention is a cost-effective alternative to chemotherapy and may offer greater potential in the long run than the use of chemotherapeutic agents (1). Chemoprevention strategies have achieved success in colorectal cancer using nonsteroidal anti-inflammatory drugs (2, 3) and breast cancer using tamoxifen (reviewed in Refs. 4, 5). Like chemotherapy, combinatorial chemoprevention strategies also have shown early success (6–10), although such strategies have not been pursued as aggressively as in chemotherapy.
An ideal chemopreventive agent should be nontoxic, orally active, effective at low doses, economical, and easily available. Recently, natural dietary agents present in fruits, vegetables, and spices have drawn much attention from researchers and the general public for their potential in chemoprevention and therapy, and many of them are currently under early phase clinical trials (reviewed in Ref. 11). The advantage of dietary agents over currently used chemotherapy drugs is their high margin of safety. The dietary agent curcumin was found to be safe up to 8 g/day in subjects at high risk for premalignant lesions (12). The phase I clinical trial conducted by Pisters et al. (13) suggested that 1.0 g/m2 t.i.d. of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG)3 was safe for patients with solid tumors. A recent study conducted at the Mayo Clinic using polyphenon E (PPE) suggested that EGCG is safe even at 2000 mg twice a day in patients with lymphocytic leukemia (14). Another phase II randomized, placebo-controlled trial of green tea extract (GTE) in patients with high risk oral premalignant lesions supported the above safety profile of EGCG (15). Although GTE or green tea polyphenols have shown promising results in preclinical studies and a high degree of safety in clinical trials, because of low oral bioavailability, plasma tea catechin concentrations determined in humans after oral administration of GTE or green tea catechins were 5–50 times lower than the concentrations shown to exert biological activities in vitro (16–19). The combination of green tea with other agents having synergistic growth inhibitory properties might reduce the concentration of green tea required to exert biological activities, which could be more readily achieved in vivo or in patients. However, the challenge is to identify an effective combination. A study conducted by Hou et al. (20) demonstrated that addition of superoxide dismutase strongly increased the growth inhibitory activity of EGCG by increasing its half-life, suggesting that combination with an antioxidant might increase the activity of EGCG. In the present study, for the first time, we have shown that combination of EGCG with luteolin, another dietary polyphenol, at lower doses tremendously increased the apoptotic potential of both compounds as compared with either single agent.
Luteolin, 3′,4′,5,7-tetrahydroxyflavone, is a natural antioxidant that usually occurs in its glycosylated form in several green vegetables such as artichoke, celery, broccoli, cauliflower, green pepper, cabbage, and spinach (21). It exhibits a wide range of pharmacological properties ranging from anti-inflammation to anticancer effects (22). Our studies show that the combination of luteolin and EGCG more effectively induced apoptosis of both lung cancer and squamous cell carcinoma of the head and neck (SCCHN) cancer cell lines and inhibited tumor growth in nude mouse xenograft models. We also showed that the combination activated both mitochondria-dependent and -independent pathways of apoptosis to varying degrees in the cell lines tested. Moreover, lung cancer cell lines expressing wild-type p53 showed higher sensitivity to the combination than those with mutant or no p53. Finally, we showed that, as a consequence of DNA double strand break (DSB), the combination more efficiently induced stabilization, phosphorylation, and mitochondrial translocation of p53. Moreover, knockdown of p53 using shRNA strongly inhibited apoptosis, suggesting activation of p53-dependent apoptotic pathways by the combination of luteolin and EGCG.
EXPERIMENTAL PROCEDURES
Cell Lines
The Tu212 cell line is established from a hypopharyngeal tumor and was kindly provided by Dr. Gary L. Clayman (University of Texas MD Anderson Cancer Center, Houston, TX). Tu686 and 686LN are paired cell lines from a primary tongue cancer and its lymph node metastasis, respectively. These were gifts from Dr. Peter G. Sacks (New York University College of Dentistry, New York, NY). The 886LN cell line, also provided by Dr. Peter G. Sacks, was derived from lymph node metastasis of squamous cell carcinoma of the larynx. The human lung cancer cell lines used in this study were obtained from the laboratory of Dr. Sun (Emory University) and described previously (23). Normal diploid human fibroblast BJ was obtained from the laboratory of George Stark (Cleveland Clinic Foundation) and maintained in DMEM containing 10% FBS. The immortalized bronchial epithelial cell line BEAS-2B was obtained from the laboratory of Dr. Xingming Deng (Winship Cancer Institute of Emory University) and maintained in DMEM containing 10% FBS. The SCCHN cell lines were maintained in DMEM/F12 (1:1) medium supplemented with 10% heat-inactivated fetal bovine serum in a 37 °C, 5% CO2 humidified incubator. RPMI 1640 media supplemented with 5% FBS was used for lung cancer cell lines.
Reagents
EGCG and luteolin (Sigma-Aldrich, St. Louis, MO) were dissolved in autoclaved water and DMSO, respectively, as stock solutions for in vitro studies. The reagents were further diluted in cell culture media immediately before use. The final concentration of DMSO was <0.1%.
Annexin V-phycoerythrin Staining for Apoptosis
Cells were treated with EGCG, luteolin, or their combination as indicated in the figure legends, trypsinized, and washed in cold 1× PBS. The cells were then resuspended in 1× annexin binding buffer and stained with annexin V-phycoerythrin and 7-AAD (all from BD Pharmingen, San Diego, CA) for 15 min at room temperature. The stained samples were measured using a fluorescence-activated cell sorting caliber bench-top flow cytometer (BD Biosciences). FlowJo software (Tree Star, Ashland, OR) was used for apoptosis analysis.
Western Blot Analysis
Whole cell lysates were extracted from drug-treated cells using lysis buffer. 20–30 μg of protein was separated on 8–12% SDS-PAGE, transferred onto a polyvinylidene difluoride membrane (Millipore Corp., Billerica, MA) and immunoblotted with specific antibodies. Mouse anti-β-actin antibody (Trevigen, Gaithersburg, MD) was used as a sample loading control. Immunostained protein bands were detected with an enhanced chemiluminescence kit (Thermo Scientific, Rockfield, IL).
Transfection of Packaging Cells for Viral Production and Infection of Cells with Virus
Packaging cells 293T were plated in 10-cm plates at a cell density of 5 × 106 a day prior to transfection in DMEM containing 10% heat-inactivated fetal bovine serum without antibiotics. shp53 and shGFP constructs in lentivirus vector were generous gifts from Dr. Didier Trono (Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland). Transfection of packaging cells and infection of mammalian cells were carried out using standard protocols (24). In brief, 293T cells were transfected with ∼6 μg of plasmids (1.6 μg pCMV-dR8.74, 1 μg pMD2G, and ∼3 μg of lentiviral vector) using lipid transfection (Lipofectamine/Plus reagent, Invitrogen) according to the manufacturer's protocol. The virus-containing medium was used to infect target cells.
Immunofluorescence Staining
H460 cells grown on coverslips were treated with 30 μm EGCG, 20 μm luteolin, or a combination of both for 24 h. Cells were exposed to 25 nm MitoTracker Red CMXRos (Invitrogen) at 37 °C for 30 min before being fixed with warm PHEMO buffer (68 mm PIPES, 25 mm HEPES, pH 6.9, 15 mm EGTA, 3 mm MgCl2, 10% (v/v) DMSO containing 3.7% formaldehyde and 0.05% glutaraldehyde). Cells were then permeabilized with 0.5% Triton X-100 for 10 min and incubated with primary antibody (anti-p53, Santa Cruz Biotechnology, Santa Cruz, CA) and fluoroconjugated secondary antibodies. Images were taken with a Zeiss LSM510 META confocal microscope at 400× magnifications.
Nude Mouse Xenograft Model
The animal experiments were approved by the Animal Care and Use Committee of Emory University. Thirty two nude mice (athymic nu/nu, Taconic, NY), aged 4–6 weeks (∼20 g weight), were randomly divided into four groups. Each mouse was orally gavaged for 7 days with vehicle control (n = 8), EGCG (125 mg/kg, n = 8), luteolin (10 mg/kg, n = 8), or the combination of EGCG (125 mg/kg) and luteolin (10 mg/kg) (n = 8) using a blunt-tipped 20-gauge 1.1/2 needle (Popper and Sons, New Hyde Park, NY) before inoculation of 2 × 106 Tu212 cells by subcutaneous injection into the right flank. The animals were continuously administered the agents 5 days a week. The tumor size was measured 3 times a week. The tumor volume was calculated using the formula: V = π/6 × larger diameter × (smaller diameter)2, as reported previously (25). Growth curves were plotted using average tumor size within each experimental group at the set time points. For the A549 study, the luteolin dose was 20 mg/kg for both the single and combination groups.
Immunohistochemistry and TUNEL Assay
Immunohistochemistry analysis for Ki-67 staining on formalin-fixed, paraffin-embedded nude mouse xenograft tissue was performed using the R.T.U. Vectastain kit following the standard manufacturer's protocol (Vector Laboratories, Burlingame, CA). Tissue sections were incubated with mouse anti-human Ki-67 (prediluted; Biomeda, Foster, CA) overnight at 4 °C. The slides were stained with 3,3′-diaminobenzidine (Sigma-Aldrich) and counterstained with hematoxylin (Vector Laboratories). TUNEL assay was performed by immunofluorescence using the same specimens as above following the manufacturer's procedure (Promega, Madison, WI). The slides were counterstained with DAPI (Vector Laboratories).
Statistical Analysis
The method of repeated measures analysis of variance with between-subject factors was used to evaluate the significance of tumor cell growth inhibition among each group.
RESULTS
Synergistic Apoptosis Induced by Combination of Luteolin and EGCG
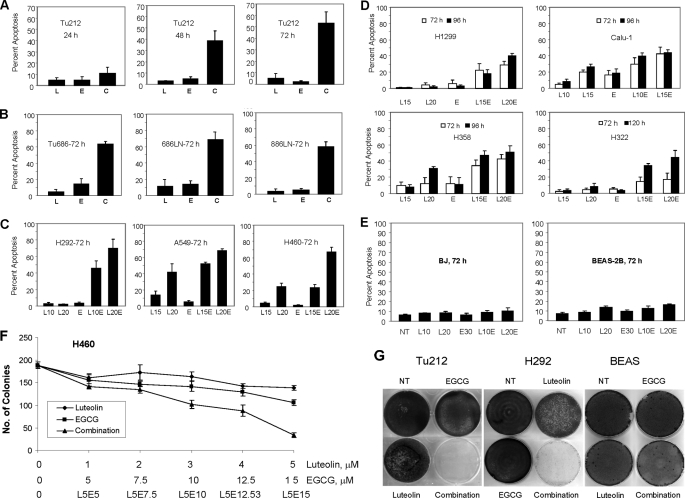
To study whether luteolin and EGCG have synergistic apoptotic effects, we first examined the sensitivity of Tu212, a SCCHN cell line, to different doses of luteolin and EGCG and found dose-dependent apoptosis induced by the compounds (supplemental Fig. S1, A and B). We next examined the apoptotic activities of different doses of luteolin in combination with a fixed dose of EGCG, 30 μm. As shown in supplemental Fig. S1C, 30 μm EGCG, which itself induced very minimal apoptosis, tremendously increased the apoptotic activity of luteolin. Particularly, the combination of low doses of luteolin (5, 10, and 15 μm, at which luteolin induced minimal apoptosis) and EGCG exhibited highly synergistic apoptotic effects. The combination of luteolin and EGCG exerted 3–5-fold more apoptosis than the additive level of apoptosis induced by luteolin and EGCG when used separately. Combination of 15 μm or higher doses of luteolin with 30 μm EGCG induced almost 100% apoptosis. We also examined the kinetics of apoptosis in Tu212 cells and found that the combination also time-dependently increased apoptosis (Fig. 1A).
FIGURE 1.
Synergistic apoptosis induced by the combination of EGCG and luteolin. A, Tu212 cells were treated with 10 μm luteolin (L), 30 μm EGCG (E), or a combination of 10 μm luteolin and 30 μm EGCG (C) for 24, 48, and 72 h. Apoptosis was measured by annexin V-phycoerythrin staining. B, SCCHN cell lines were treated with 10 μm luteolin, 30 μm EGCG, or a combination of 10 μm luteolin and 30 μm EGCG for 72 h, and apoptosis was measured as above. C, lung cancer cell lines expressing wild-type p53 were treated with luteolin, 30 μm EGCG, or a combination of luteolin and EGCG, and apoptosis was measured. The number after L indicates the dose of luteolin in μm. D, lung cancer cell lines expressing mutant or no p53 were treated with luteolin, 30 μm EGCG, or a combination of luteolin and EGCG, and apoptosis was measured. E, noncancerous BEAS-2B and BJ cells were treated with the compounds for 72 h, and apoptosis was measured. F, H460 cells were seeded at a concentration of 250 cells/well in six-well plates and treated with the indicated concentration of luteolin, EGCG, or a combination of 5 μm luteolin plus varying concentrations of EGCG until colonies were visible. Finally, colonies were counted after methylene blue staining. G, Tu212, H292, and BEAS-2B cells were seeded at a density of 2.5 × 105 cells/well in six-well plates, treated twice (72 h each) with 10 μm luteolin, 30 μm EGCG, or their combination, and cultured in drug-free media for an additional 2 weeks and stained with methylene blue. Apoptosis occurring in untreated cells was subtracted from apoptosis occurring after compound treatment, and net apoptosis occurring after treatment with the compounds was presented. All experiments were repeated at least three times. Error bars represent S.D. from at least three independent experiments. NT, no treatment.
On the basis of these observations, we next tested the sensitivity to this combination of a panel of SCCHN, lung cancer and two noncancerous cell lines with varying histological and biochemical status (supplemental Table S1). As shown in Fig. 1, A–E, combination of the two compounds showed synergistic or additive activities against all of the cell lines tested, except the two noncancerous cell lines, as compared with either single agent. However, all SCCHN cell lines (Fig. 1, A and B) showed greater sensitivity than the lung cancer cell lines tested, except H292. Lung cancer cell lines with wild-type p53 (Fig. 1C) showed greater sensitivity than those with mutant or no p53 (Fig. 1D). Noncancerous cell lines were almost resistant to the compounds (Fig. 1E). This is consistent with many other reports, which suggest that dietary agents do not harm normal cells (26, 27). A dose-dependent colony forming assay also suggested that the combination of two compounds inhibited colony formation more effectively than either single agent (Fig. 1F).
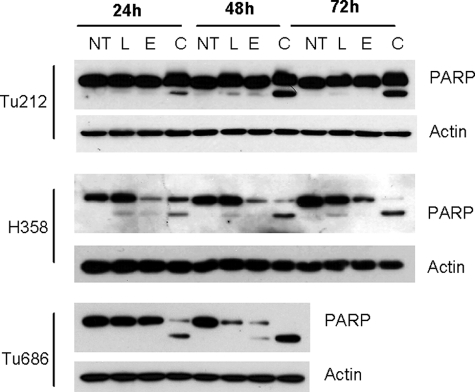
To study the long term effect of the combination, we undertook a cell growth assay, in which cells were treated with 10 μm luteolin, 30 μm EGCG, or a combination of the two. As shown in Fig. 1G, cancer cells (Tu212 and H292) treated with luteolin or EGCG alone reached confluency, like the untreated cells. However, in plates treated with the combination of luteolin and EGCG, no survival colonies were formed, suggesting that the combination might be suitable for complete eradication of tumors. In contrast, only a slight growth inhibition was observed in noncancerous BEAS-2B cells, suggesting that normal cells are not affected by the combination of EGCG and luteolin. To further confirm the synergistic apoptotic effect, we examined PARP cleavage in three cell lines after treatment with luteolin, EGCG, or their combination. Efficient PARP cleavage was observed only after combined treatment, further suggesting that the combination of luteolin and EGCG showed synergistic apoptotic effects (Fig. 2).
FIGURE 2.
Cleavage of PARP after treatment with the combination of EGCG and luteolin. Tu212, H358, and Tu686 cells were treated with luteolin (L), EGCG (E), or their combination (C) for different time periods. Total cell lysates were immunoblotted with antibody that detects both full-length and cleaved PARP (Cell Signaling Technologies, Danvers, MA). Representative data from three independent experiments are shown. NT, no treatment.
Combination of Luteolin and EGCG Activates Both Mitochondrial-dependent and -independent Apoptosis
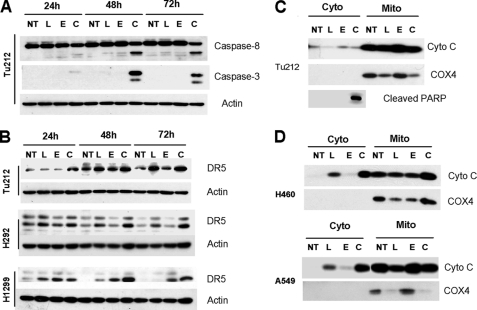
To understand the mechanism of apoptosis induced by the combination of luteolin and EGCG, we treated Tu212 cells with luteolin, EGCG, or their combination. Total cell lysates were immunoblotted for caspase-8 and -3. The combination of EGCG and luteolin more efficiently induced cleavage of caspase-8 and -3 than either single agent (Fig. 3A). Because caspase-8-mediated apoptosis is mainly driven by induction of DR5, we next examined the expression of DR5 and found increased levels of DR5 in multiple cell lines after treatment with luteolin or the combination of luteolin and EGCG (Fig. 3B). These results suggest that the combination of luteolin and EGCG induced death receptor-mediated apoptosis. We next examined release of cytochrome c in the cytoplasm. As shown in Fig. 3C, there was no release of cytochrome c in Tu212 cells, although there was efficient apoptosis induction after combined treatment as evidenced by PARP cleavage. However, cytochrome c release was observed in A549 and H460 cell lines after treatment with luteolin, which was further increased after combined treatment (Fig. 3D). Because death receptor-mediated apoptosis may involve mitochondria via cleavage of BID to tBID, we next examined the expression of BID in H460 and A549 cells using an antibody that can detect both BID and tBID and did not detect tBID in these cells (supplemental Fig. S2). Results shown in Fig. 3 thus suggest the involvement of both mitochondria-dependent and -independent pathways and further support synergistic apoptosis induced by the combination.
FIGURE 3.
Combination of EGCG and luteolin induced both mitochondria-dependent and -independent apoptosis. A, Tu212 cells were treated with 10 μm luteolin (L), 30 μm EGCG (E), or their combination (C) for various time periods, and total cell lysates were immunoblotted with anti-caspase-8 (detects both full-length and cleaved form, Cell Signaling Technologies, Danvers, MA) and anti-caspase-3 (detects only cleaved forms at 19 and 17 kDa, Cell Signaling Technologies). B, cells were treated with luteolin, EGCG, or their combination, and total cell lysates were immunoblotted with anti-DR5 (ProSci, Poway, CA). C–D, Tu212 (C), H460 (D, upper panel), and A549 (D, lower panel) cells were treated with luteolin, EGCG, or their combination. Cytoplasmic and mitochondrial fractions were separated and immunoblotted with cytochrome c (Cyto C) antibody. COX4 (a mitochondrial protein) was used to show efficiency of cell fractionation. For A–D, all experiments were repeated at least three times, and representative data are presented. NT, no treatment.
Phosphorylation, Stabilization, and Mitochondrial Translocation of p53
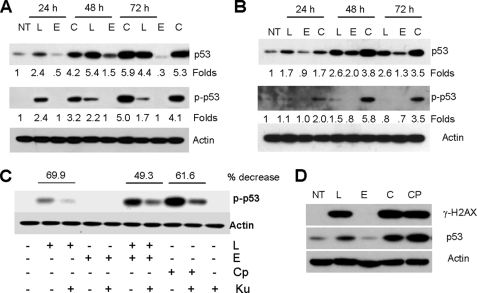
The p53 protein is regulated by complex post-translational modifications, such as phosphorylation and acetylation that contribute to its stabilization and activation (28–30). As lung cancer cell lines expressing wild-type p53 showed greater sensitivity to the combination of EGCG and luteolin, we examined the expression of p53 and its phosphorylation at Ser15 after treatment with luteolin, EGCG, or their combination. Results presented in Fig. 4, A and B, suggest that the combination of EGCG and luteolin more efficiently stabilized p53 and induced its phosphorylation at Ser15 than either single agent. Because Ser15 is a classical site for the ATM (ataxia telangiectasia mutated) kinase, we next examined the involvement of ATM in the phosphorylation of p53 by using the specific ATM inhibitor Ku55933. Pretreatment with Ku55933 strongly inhibited p53 phosphorylation at Ser15 induced by luteolin, the combination of EGCG and luteolin or the DNA-damaging agent camptothecin (Fig. 4C). Finally, we examined the expression of γ-H2AX as a marker of DSB. Immediately after DSB, H2AX is phosphorylated to form γ-H2AX, which is widely used as a marker for DSB (31). Correlating with the expression of p53, luteolin, the combination of EGCG and luteolin and camptothecin strongly induced the expression of γ-H2AX (Fig. 4D). Consistent with our previous results in colon cancer cell line, EGCG did not induce any DSB (32). Thus, the results presented in Fig. 4 suggest that treatment of cells with luteolin or the combination of EGCG and luteolin induced DSB, which phosphorylates p53 at Ser15 in an ATM-dependent manner.
FIGURE 4.
Phosphorylation and stabilization of p53 by the combination of luteolin and EGCG. H460 (A) and A549 (B) cells were treated with luteolin (L), EGCG (E), or their combination (C), and total cell lysates were immunoblotted with anti-p53 (Santa Cruz Biotechnology) and anti-phospho p53 (Ser15, Cell Signaling Technologies). Representative data from three independent experiments are shown. Numbers below each lane represent fold change. C, H460 cells were pretreated with 10 μm Ku55933 for 1 h, followed by treatment with 20 μm luteolin, 30 μm EGCG, their combination, or 2 μg/ml camptothecin (Cp) for 48 h. Cells were treated with capmtothecin for 24 h. Total cell lysates were immunoblotted with antiphospho p53 Ser15. Representative data from three independent experiments are shown. D, H460 cells were treated with luteolin, EGCG, their combination, or camptothecin for 24 h. Total cell lysates were immunoblotted with phospho-H2AX (γ-H2AX) and p53. Experiments were repeated three times. NT, no treatment.
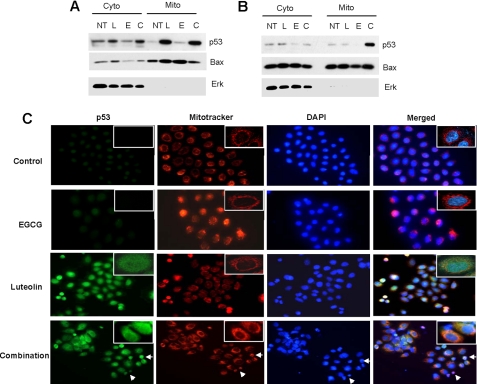
A growing body of evidence suggests that in addition to its transcriptional activation, p53 directly localizes in the mitochondria and induces transcription-independent apoptosis (reviewed in Refs. 33, 34). To test this possibility, we fractionated the cytoplasmic and mitochondrial fractions, separated the proteins with SDS-PAGE, and blotted for p53 and Bax. p53 was detected in the mitochondrial fractions after treatment with luteolin and the combination of luteolin and EGCG (Fig. 5, A and B). Bax was localized in both the cytoplasm and mitochondria. We next confirmed mitochondrial translocation of p53 by immunofluorescence staining, and the results corroborated those of the cell fractionation assay (Fig. 5C). In the control and EGCG-treated cells, p53 was localized in the nucleus. However, colocalization of p53 and mitochondria were observed in cells after treatment with luteolin and the combination of EGCG and luteolin. The immunofluorescence staining also showed nuclear condensation (a hallmark of apoptosis) after treatment with the combination of EGCG and luteolin but not with single agent treatments. These results suggest that the combination of luteolin and EGCG induced mitochondrial translocation of p53 and further support that the combination of the two compounds synergistically induced apoptosis.
FIGURE 5.
Mitochondrial translocation of p53. H460 (A) and A549 (B) cells were treated with luteolin (L), EGCG (E), or their combination (C) for 48 h. Cytoplasmic (Cyto) and mitochondrial (Mito) fractions were immunoblotted with p53, Bax, and Erk2 antibodies (Santa Cruz Biotechnology). Erk2 (a cytoplasmic protein) was used to show efficiency of separation. C. H460 cells were treated with luteolin, EGCG, or their combination for 24 h, and immunofluorescence staining was done as described under “Experimental Procedures.” Arrows in the combination lane indicate nuclear condensation due to apoptosis. For all panels, representative data from at least three independent experiments are shown. NT, no treatment.
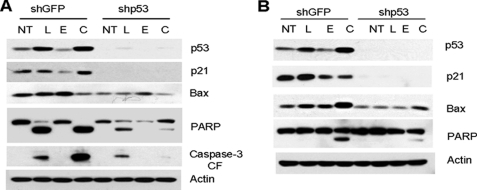
p53 Is Required for Apoptosis Induced by Combined Treatment with Luteolin and EGCG
To further confirm the role of p53 in apoptosis induced by the combination of luteolin and EGCG, we knocked down the expression of p53 using a lentivirus-based shRNA construct, and pools of cells with ablated p53 were established by GFP selection. Cells transduced with shGFP were used as control. The cells were treated with luteolin, EGCG, or their combination, and total cell lysates were used to study the expression of apoptotic markers PARP and caspase-3, and the p53 transcriptional targets p21 and Bax. As shown in Fig. 6, A and B, treatment with the combination of luteolin and EGCG efficiently induced cleavage of caspase-3 and PARP in control cells, which were strongly inhibited after p53 knockdown, suggesting that p53 is required for apoptosis. Transduction of p53-specific shRNA completely knocked down the basal and induced levels of p53 transcriptional targets p21 and Bax in these cell lines. However, there was some residual cleavage of PARP and caspase-3 after p53 knockdown, most probably due to activation of p53-independent apoptotic pathways, as cell lines with mutant or no p53 were also sensitive to the combination of EGCG and luteolin to some extent.
FIGURE 6.
Role of p53 in apoptosis induced by the combination of luteolin and EGCG. p53 was knocked down in H460 (A) and A549 (B) cells as described under “Experimental Procedures” and treated with luteolin (L), EGCG (E), or their combination (C). Total cell lysates were immunoblotted with p53, p21, Bax, PARP, and caspase-3 antibodies. Each experiment was repeated at least three times. NT, no treatment; CF, cleaved form.
Inhibition of Growth of Mouse Xenograft Tumor
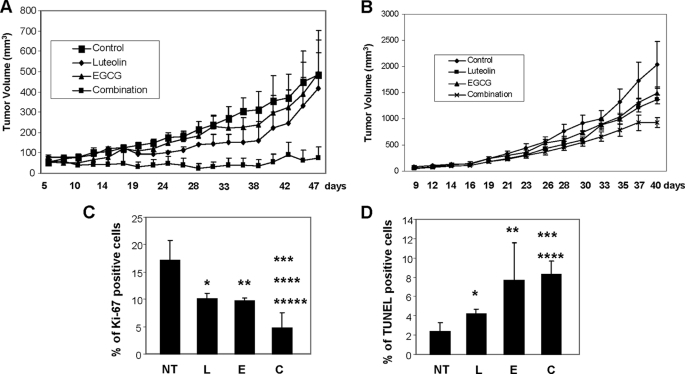
The antitumor efficacy of combined treatment with EGCG and luteolin was investigated in xenografted mice bearing Tu212 and A549 cells. In the Tu212 study, each group consisted of eight mice and after inoculation of cells, six, eight, five, and three mice developed tumors in the vehicle-treated, luteolin, EGCG, and combination groups, respectively (Fig. 7A). One more tumor appeared in the combination group 5 weeks after inoculation of cells. These results suggest that the combination of luteolin and EGCG inhibited tumor development in an intrinsically preventive environment. Although EGCG and luteolin as single agents had minimal effect on tumor growth, their combination inhibited tumor growth to a statistically significant level (p < 0.05). In the A549 model, all of the mice developed tumors. Treatment with single agents moderately inhibited tumor growth (luteolin, 32.4% and EGCG, 26.2% as compared with vehicle control), but this was not statistically significant (Fig. 7B). In contrast, the combination inhibited tumor growth by 54.2% as compared with control; 32.2% as compared with EGCG alone, and 37.3% as compared with luteolin alone. The results were statistically significant only for control versus combination treatment (p = 0.026). Although the synergy is questionable in A549 tumors, the results clearly showed additive effects. The growth behavior of a cell line in vivo typically differs from that in vitro because of the involvement of host factors. Moreover, bioavailability is always a concern with in vivo studies. As Tu212 cells are much more sensitive to treatment than A549, in vivo synergy is clearer in Tu212 tumors.
FIGURE 7.
Inhibition of tumor growth by the combination of luteolin and EGCG in nude mice. Four groups of animals were orally gavaged with vehicle control, luteolin, EGCG, or their combination as described under “Experimental Procedures.” Growth curves were obtained for Tu212 (A) and A549 (B) xenografted tumors. Tu212 tumor growth was significantly inhibited in the combination group as compared with the control (p = 0.031), EGCG (E; p = 0.025), or luteolin (L; p = 0.05) groups. For A549 tumors, the combination of luteolin and EGCG significantly inhibited tumor growth as compared with control group (p = 0.026). Other comparisons were insignificant (p > 0.05). C, quantification of Ki-67 staining. *, statistically significant p values (*, control versus luteolin, p = .0071; **, control versus EGCG, p = .0047; ***, control versus combination (C), p = .0000074; ****, EGCG versus combination, p = .0032; *****, luteolin versus combination, p = .0011). D, quantification of TUNEL staining. An asterisk indicates statistically significant p values (*, control versus luteolin, p = .001; **, control versus EGCG, p = .002; ***, control versus combination, p = .00000019; ****, luteolin versus combination, p = .000069). EGCG versus combination was not statistically significant (p = .69) because one of the mice treated with EGCG had a high number of TUNEL-positive cells. NT, no treatment.
We also stained the Tu212-xenografted tumor tissues for Ki-67 (proliferation marker) and TUNEL (apoptosis marker). Treatment with the agents significantly inhibited Ki-67 expression (Fig. 7C and supplemental Fig. S3A), while increasing the proportion of TUNEL-positive cells (Fig. 7D and supplemental Fig. S3B). We also monitored body weights of the mice throughout the studies. No significant weight loss was observed (supplemental Fig. S4, A and B). H&E staining of the major organs collected at the end of the study also suggested no major organ-related toxicities (supplemental Fig. S5). We carefully examined the organs of all mice from both xenograft studies for signs of toxicity. One mouse from the EGCG group and one from the combination group showed minimal steatosis in the focal area of the liver as shown in supplemental Fig. S5A. One mouse from the luteolin group, one from the combination group, and two from the EGCG group also showed minimal microabscesses in the focal areas of the liver (supplemental Fig. S5B). The histopathologic features of microabscesses and steatosis are considered as acute injury to the liver. However, those observed in the few mice in our study were minimal and generally reversed after discontinuation of treatment. All other mice showed no abnormalities. Taken together, our findings from animal studies suggest that the combination of luteolin and EGCG is more effective in inhibiting tumor growth than either single agent and may have synergistic/additive effects without inducing any notable toxicity in general.
DISCUSSION
Safety is always a primary concern in studies involving human subjects, with the Latin adage primum non nocere (first, do no harm) characterizing medical practice for millenia. Natural dietary agents have been safely consumed over centuries, and preclinical studies suggest that many of them, including green tea polyphenol and luteolin, have strong chemopreventive potential. Recent clinical trials conducted to study the safety and efficacy of natural dietary agents against cancer suggests that their therapeutic index is very high (12–15); however, most have poor bioavailability. Particularly, for green tea polyphenols, the in vivo concentration achieved after oral administration is much lower than that which showed efficacy in vitro (16–19). The recent chemoprevention trial with GTE, which was a four-armed study (placebo control, 500, 750, and 1000 mg/m2 GTE) suggested that the two higher dose GTE arms had higher responses with improved histology, thus a dose-response effect (15). The editorial commentary by Shin (35) on this chemoprevention trial suggests that the results of this study should help guide the design of future clinical research, which should include trials of GTE or green tea polyphenol combined with other natural or synthetic compounds to enhance chemopreventive effects. It is therefore critical to devise strategies either to safely increase their in vivo concentrations or to identify a second agent that has synergistic effect so that a lower in vivo concentration will be effective. In the present study, we have identified a novel combination of two natural dietary agents, EGCG and luteolin, which exhibit highly synergistic apoptotic effects and efficiently inhibited tumor growth in vivo.
Importantly, we also have explored and clarified the mechanism of the observed apoptotic effect. The combination induced mitochondria-dependent apoptosis in some cell lines and mitochondria-independent apoptosis in others, as evidenced by the release or lack of release, respectively, of cytochrome c from the mitochondria to the cytoplasm. In Tu212 cells, the combination efficiently activated caspase-8 and -3 without the release of cytochrome c. The combination, and more importantly luteolin, also induced expression of DR5, the initiator of death receptor-mediated apoptosis. These results suggest the induction of death receptor-mediated, but mitochondria-independent apoptosis. We also have found activation of DR5 in other cell lines. These findings are consistent with previous studies showing that luteolin induced death receptor-mediated apoptosis in some cell lines by inducing DR5 (36, 37). On the other hand, in A549 and H460 lung cancer cell lines, we found efficient release of cytochrome c after luteolin treatment, which was further increased after treatment with the combination of luteolin and EGCG, although EGCG alone had no such effect. These results are consistent with apoptosis induction in these cell lines (Fig. 1), suggesting a mitochondria-mediated apoptosis. Death receptor-mediated apoptosis might link to mitochondria via cleavage of BID to tBID (38, 39); however, our findings suggested no truncation of BID to tBID. Instead, we found translocation of p53 to the mitochondria in these cell lines, which is involved in transcription-independent apoptosis mediated by p53 through mitochondrial depolarization (reviewed in Refs. 33, 34). These results suggest that the combination of luteolin and EGCG induced mitochondria-mediated apoptosis, initiated by the intrinsic pathway.
The tumor suppressor protein p53 plays a crucial role in controlling the cell cycle, apoptosis, genomic integrity, and DNA repair in response to various forms of stress. Post-translational modifications such as phosphorylation and acetylation are critical for stabilization and activation of p53 (28–30). Recent studies suggest that the translocation of p53 to mitochondria also is important for its apoptotic effects (reviewed in Refs. 33, 34). Our findings demonstrate several important roles for p53-mediated signaling in this context. First, the combination induced more efficient stabilization and phosphorylation of p53 protein at Ser15. Phosphorylation of p53 at Ser15 occurs under stress conditions in cells undergoing growth arrest and apoptosis (40, 41). We also have demonstrated that DSB is the initiator of ATM-dependent p53 phosphorylation and activation. Second, p53 is required for apoptosis induction by these two compounds in cells expressing wild-type p53. The combination of luteolin and EGCG also increased the expression of proapoptotic Bax, which is dependent on p53. Finally, for the first time, we observed translocation of p53 to the mitochondria after treatment with these compounds. An increasing body of evidence has demonstrated the existence of a transcription-independent pathway of p53-mediated apoptosis in addition to its transcription-dependent pathway (reviewed in Refs. 33, 34). Together, our results suggest that p53 regulates luteolin and EGCG-induced apoptosis by increasing the expression of proapoptotic Bax and by translocating itself to mitochondria.
Employing a panel of SCCHN and lung cancer cell lines with varying histological origins such as squamous cell carcinoma of the head and neck, and nonsmall cell lung cancer, including adenocarcinoma, squamous cell and large cell lung cancer, and two different xenograft models, we have shown that the combination of EGCG and luteolin demonstrates synergistic or enhanced anti-tumor activity both in vitro and in vivo. In particular, these natural compounds were effective at lower concentrations when combined together, which may potentially allow these concentrations to be achieved in vivo and may bypass toxicities associated with high dose single agent treatments. Although we have focused only on p53, studies suggest that both EGCG and luteolin modulate multiple targets to induce their growth inhibitory activities, which is desirable for ideal chemoprentive agents (reviewed in Ref. 11). By describing the mechanism of synergy between these compounds as well as uncovering how they induce p53-dependent apoptosis, we have made the case for the rational combination of luteolin with green tea polyphenol-containing EGCG for further preclinical and clinical development. Studies suggest that natural compounds show greater activity when they are present in a complex mixture than as pure compounds (for a comprehensive review, see Ref. 42). Indeed, a phase I clinical trial using a fixed dose of polyphenon E (a green tea formulation containing 50–60% EGCG) plus varying doses of luteolin (using a formulation called Lutimax in which the main component is luteolin) has been planned at the Winship Cancer Institute of Emory University in an attempt to prevent carcinogenesis in patients with premalignant lesions of the lung epithelium and to determine the maximum tolerated dose and pharmacokinetic profile.
Supplementary Material
Acknowledgments
We thank Drs. Didier Trono for providing the lentivirus-based gene silencing system and Anthea Hammond for critical and editorial review of this article.
This work was supported, in whole or in part, by National Institutes of Health Grants P50 CA128613, U01 CA101244, and R01 CA112643.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S5.
- EGCG
- (−)-epigallocatechin-3-gallate
- GTE
- green tea extract
- PPE
- polyphenon E
- SCCHN
- squamous cell carcinoma of the head and neck
- DSB
- double strand break
- PIPES
- 4-piperazinediethanesulfonic acid
- PARP
- poly(ADP-ribose) polymerase.
REFERENCES
- 1.Hong W. K., Sporn M. B. (1997) Science 278, 1073–1077 [DOI] [PubMed] [Google Scholar]
- 2.Hawk E., Lubet R., Limburg P. (1999) Cancer 86, 2551–2563 [DOI] [PubMed] [Google Scholar]
- 3.Antonakopoulos N., Karamanolis D. G. (2007) Hepatogastroenterology 54, 1694–1700 [PubMed] [Google Scholar]
- 4.Fabian C. J., Kimler B. F. (2005) J. Clin. Oncol. 23, 1644–1655 [DOI] [PubMed] [Google Scholar]
- 5.Peng J., Sengupta S., Jordan V. C. (2009) Anticancer Agents Med. Chem. 9, 481–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torrance C. J., Jackson P. E., Montgomery E., Kinzler K. W., Vogelstein B., Wissner A., Nunes M., Frost P., Discafani C. M. (2000) Nat. Med. 6, 1024–1028 [DOI] [PubMed] [Google Scholar]
- 7.Meyskens F. L., Jr., McLaren C. E., Pelot D., Fujikawa-Brooks S., Carpenter P. M., Hawk E., Kelloff G., Lawson M. J., Kidao J., McCracken J., Albers C. G., Ahnen D. J., Turgeon D. K., Goldschmid S., Lance P., Hagedorn C. H., Gillen D. L., Gerner E. W. (2008) Cancer Prev. Res. 1, 32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin A. R., Khuri F. R., Chen Z. G., Shin D. M. (2009) Cancer Prev. Res. 2, 538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Zhang H., Tighiouart M., Lee J. E., Shin H. J., Khuri F. R., Yang C. S., Chen Z. G., Shin D. M. (2008) Int. J. Cancer 123, 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Chen Z. G., Choe M. S., Lin Y., Sun S. Y., Wieand H. S., Shin H. J., Chen A., Khuri F. R., Shin D. M. (2005) Clin. Cancer Res. 11, 6261–6269 [DOI] [PubMed] [Google Scholar]
- 11.Amin A. R., Kucuk O., Khuri F. R., Shin D. M. (2009) J. Clin. Oncol. 27, 2712–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng A. L., Hsu C. H., Lin J. K., Hsu M. M., Ho Y. F., Shen T. S., Ko J. Y., Lin J. T., Lin B. R., Ming-Shiang W., Yu H. S., Jee S. H., Chen G. S., Chen T. M., Chen C. A., Lai M. K., Pu Y. S., Pan M. H., Wang Y. J., Tsai C. C., Hsieh C. Y. (2001) Anticancer Res. 21, 2895–2900 [PubMed] [Google Scholar]
- 13.Pisters K. M., Newman R. A., Coldman B., Shin D. M., Khuri F. R., Hong W. K., Glisson B. S., Lee J. S. (2001) J. Clin. Oncol. 19, 1830–1838 [DOI] [PubMed] [Google Scholar]
- 14.Shanafelt T. D., Call T. G., Zent C. S., LaPlant B., Bowen D. A., Roos M., Secreto C. R., Ghosh A. K., Kabat B. F., Lee M. J., Yang C. S., Jelinek D. F., Erlichman C., Kay N. E. (2009) J. Clin. Oncol. 27, 3808–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao A. S., Liu D., Martin J., Tang X. M., Lee J. J., El-Naggar A. K., Wistuba I., Culotta K. S., Mao L., Gillenwater A., Sagesaka Y. M., Hong W. K., Papadimitrakopoulou V. (2009) Cancer Prev. Res. 2, 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa K., Okuda S., Miyazawa T. (1997) Biosci. Biotechnol. Biochem. 61, 1981–1985 [DOI] [PubMed] [Google Scholar]
- 17.Yang C. S., Chen L., Lee M. J., Balentine D., Kuo M. C., Schantz S. P. (1998) Cancer Epidemiol. Biomarkers. Prev. 7, 351–354 [PubMed] [Google Scholar]
- 18.Lee M. J., Maliakal P., Chen L., Meng X., Bondoc F. Y., Prabhu S., Lambert G., Mohr S., Yang C. S. (2002) Cancer. Epidemiol. Biomarkers. Prev. 11, 1025–1032 [PubMed] [Google Scholar]
- 19.Chow H. H., Cai Y., Alberts D. S., Hakim I., Dorr R., Shahi F., Crowell J. A., Yang C. S., Hara Y. (2001) Cancer Epidemiol. Biomarkers. Prev. 10, 53–58 [PubMed] [Google Scholar]
- 20.Hou Z., Sang S., You H., Lee M. J., Hong J., Chin K. V., Yang C. S. (2005) Cancer Res. 65, 8049–8056 [DOI] [PubMed] [Google Scholar]
- 21.Miean K. H., Mohamed S. (2001) J. Agric. Food. Chem. 49, 3106–3112 [DOI] [PubMed] [Google Scholar]
- 22.Shimoi K., Saka N., Kaji K., Nozawa R., Kinae N. (2000) Biofactors 12, 181–186 [DOI] [PubMed] [Google Scholar]
- 23.Sun S. Y., Yue P., Dawson M. I., Shroot B., Michel S., Lamph W. W., Heyman R. A., Teng M., Chandraratna R. A., Shudo K., Hong W. K., Lotan R. (1997) Cancer Res. 57, 4931–4939 [PubMed] [Google Scholar]
- 24.Wiznerowicz M., Trono D. (2003) J. Virol. 2, 8957–8961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciardiello F., Bianco R., Damiano V., De Lorenzo S., Pepe S., De Placido S., Fan Z., Mendelsohn J., Bianco A. R., Tortora G. (1999) Clin. Cancer Res. 5, 909–916 [PubMed] [Google Scholar]
- 26.Ahmad N., Feyes D. K., Nieminen A. L., Agarwal R., Mukhtar H. (1997) J. Natl. Cancer Inst. 89, 1881–1886 [DOI] [PubMed] [Google Scholar]
- 27.Sahu R. P., Srivastava S. K. (2009) J. Natl. Cancer Inst. 101, 176–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng L., Lin T., Uranishi H., Gu W., Xu Y. (2005) Mol. Cell. Biol. 25, 5389–5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bode A. M., Dong Z. (2004) Nat. Rev. Cancer 4, 793–805 [DOI] [PubMed] [Google Scholar]
- 30.Carter S., Vousden K. H. (2009) Curr. Opin. Genet. Dev. 19, 18–24 [DOI] [PubMed] [Google Scholar]
- 31.Bonner W. M., Redon C. E., Dickey J. S., Nakamura A. J., Sedelnikova O. A., Solier S., Pommier Y. (2008) Nat. Rev. Cancer 8, 957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur V. S., Ruhul Amin A. R., Paul R. K., Gupta K., Hastak K., Agarwal M. K., Jackson M. W., Wald D. N., Mukhtar H., Agarwal M. L. (2010) Cancer Lett. 296, 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaseva A. V., Moll U. M. (2009) Biochim. Biophys. Acta 1787, 414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchenko N. D., Moll U. M. (2007) Cell Cycle 6, 1718–1723 [DOI] [PubMed] [Google Scholar]
- 35.Shin D. M. (2009) Cancer Prev. Res. 2, 919–921 [DOI] [PubMed] [Google Scholar]
- 36.Horinaka M., Yoshida T., Shiraishi T., Nakata S., Wakada M., Nakanishi R., Nishino H., Matsui H., Sakai T. (2005) Oncogene 24, 7180–7189 [DOI] [PubMed] [Google Scholar]
- 37.Horinaka M., Yoshida T., Shiraishi T., Nakata S., Wakada M., Nakanishi R., Nishino H., Sakai T. (2005) Biochem. Biophys. Res. Commun. 333, 833–838 [DOI] [PubMed] [Google Scholar]
- 38.Roy S. S., Ehrlich A. M., Craigen W. J., Hajnóczky G. (2009) EMBO. Rep. 10, 1341–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y., Li R., Xia W., Neuzil J., Lu Y., Zhang H., Zhao X., Zhang X., Sun C., Wu K. (2010) Cancer Lett. 288, 42–49 [DOI] [PubMed] [Google Scholar]
- 40.Qin J., Chen H. G., Yan Q., Deng M., Liu J., Doerge S., Ma W., Dong Z., Li D. W. (2008) Cancer Res. 68, 4150–4162 [DOI] [PubMed] [Google Scholar]
- 41.Meslin F., Thiery J., Richon C., Jalil A., Chouaib S. (2007) J. Biol. Chem. 282, 32991–32999 [DOI] [PubMed] [Google Scholar]
- 42.Bode A. M., Dong Z. (2009) Cancer Prev. Res. 2, 514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.