Abstract
A meta-cleavage pathway for the aerobic degradation of aromatic hydrocarbons is catalyzed by extradiol dioxygenases via a two-step mechanism: catechol substrate binding and dioxygen incorporation. The binding of substrate triggers the release of water, thereby opening a coordination site for molecular oxygen. The crystal structures of AkbC, a type I extradiol dioxygenase, and the enzyme substrate (3-methylcatechol) complex revealed the substrate binding process of extradiol dioxygenase. AkbC is composed of an N-domain and an active C-domain, which contains iron coordinated by a 2-His-1-carboxylate facial triad motif. The C-domain includes a β-hairpin structure and a C-terminal tail. In substrate-bound AkbC, 3-methylcatechol interacts with the iron via a single hydroxyl group, which represents an intermediate stage in the substrate binding process. Structure-based mutagenesis revealed that the C-terminal tail and β-hairpin form part of the substrate binding pocket that is responsible for substrate specificity by blocking substrate entry. Once a substrate enters the active site, these structural elements also play a role in the correct positioning of the substrate. Based on the results presented here, a putative substrate binding mechanism is proposed.
Keywords: Crystal Structure, Enzyme Catalysis, Enzyme Mechanisms, Enzyme Structure, Protein Metal Ion Interaction, Catechol, Extraldiol Dioxygenase, Substrate Binding
Introduction
A general comparison of the major pathways for the aerobic degradation of aromatic hydrocarbons in bacteria has revealed that the initial conversion steps are carried out by different enzymes, but that the compounds are transformed into a limited number of central intermediates, including (substituted) catechols (1). These dihydroxylated intermediates are channeled into one of two possible pathways: an ortho-cleavage pathway or a meta-cleavage pathway (2). The former pathway is catalyzed by intradiol dioxygenases, which utilize a non-heme ferric iron to cleave the aromatic ring ortho to the hydroxyl substituents, while the latter pathway is catalyzed by extradiol dioxygenases, which utilize a non-heme ferrous iron or other divalent metal ion to cleave the aromatic ring meta to the hydroxyl substituents. Additionally, it is generally understood that extradiol dioxygenase is more versatile than its intradiol counterpart (3).
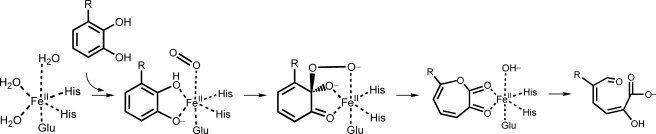
Two decade-long research on the reaction mechanism of extradiol dioxygenases has revealed that the catalytic process occurs via a two-step mechanism: binding of catechol substrates and incorporation of the dioxygen atoms of molecular oxygen into the substrate (3–7). As depicted in Fig. 1, the iron atom in the active site is coordinated by the so-called 2-His-1-carboxylate facial triad motif (two histidines and one glutamate (or aspartate)), which occupies only one face of the coordination sphere. The opposite face is occupied by a displaceable water molecule and the hydroxyl oxygens of the catechol substrate. The binding of the substrate triggers the release of the water, thereby opening a coordination site for molecular oxygen, which is then apparently activated by an electron transferred from the substrate through the iron. Several conserved amino acid residues at the active site, including tyrosine and histidine, are known to play important roles in oxygen activation by deprotonating the substrate. Both substrate deprotonation and oxygen activation allow recombination to form an alkylperoxo intermediate, which then undergoes a Criegee rearrangement to yield a seven-membered lactone. The extradiol cleavage is completed as the lactone is hydrolyzed by the second oxygen atom of O2.
FIGURE 1.
Proposed mechanism for extradiol aromatic ring-cleaving dioxygenases (adapted and modified from Lipscomb, Ref. 5).
Rhodococcus sp. strain DK17 is able to grow on various alkylbenzenes (o-xylene, toluene, ethylbenzene, isopropylbenzene, and n-propyl to n-hexylbenzenes) (8). We previously reported that a single extradiol dioxygenase (methylcatechol 2,3-dioxygenase encoded by akbC) is involved in alkylbenzene metabolism by DK17 (9, 10). Extradiol dioxygenases can be grouped into at least three evolutionarily independent families based on structure (3). Type I extradiol dioxygenases, belonging to the vicinal oxygen chelate superfamily, include two-domain and one-domain enzymes. Type II extradiol dioxygenases include enzymes consisting of one or two different subunits. Type III extradiol dioxygenases belong to the cupin superfamily. Amino acid sequence comparisons of AkbC and other related enzymes indicate that AkbC belongs to the type I extradiol dioxygenase group, which is representatively exemplified by 2,3-dihydroxybiphenyl 1,2-dioxygenase (DHBD)5 from Burkholderia cepacia LB400. In fact, AkbC is closely related (e.g. 70% identity and 80% similarity) to DHBDs from Sphingomonas strains. Interestingly, however, despite high sequence conservation, including important residues for activity, AkbC is able to cleave 2,3-dihydroxybiphenyl (DHB) only at a significantly lower rate (e.g. 15% of that for 3-methylcatechol (3-MC)). Even more interesting is that 3-MC acts as a potent suicide inhibitor of the DHBD enzyme from B. cepacia LB400 (11). These observations strongly suggest that the DK17 AkbC and the DHBPs have critical differences in their substrate recognition properties. This hypothesis led us to investigate the structural basis of substrate binding and the underlying mechanism of AkbC catalysis. During the past two decades, much research has centered on elucidating the ring cleavage mechanism of extradiol dioxygenases, and many of its details have been well documented (5, 12). In contrast, there has been little in-depth work examining the substrate binding process. Here, based on the crystal structure and functional studies of AkbC, we propose a substrate binding process for type I extradiol dioxygenases.
EXPERIMENTAL PROCEDURES
Expression and Purification of the AkbC Protein
The akbC gene was amplified from DK17 genomic DNA by polymerase chain reaction (PCR) with forward and reverse primers carrying NcoI and EcoRI restriction sites (5-CATGCCATGGCAAAAGTGACCG-3 and 5-CCGGAATTCTTATGCGGGGATGTCG-3), respectively. The thermocycler program used for PCR was as follows: 95 °C for 2 min, 30 cycles (95 °C for 1 min, 60 °C for 1.5 min, 72 °C for 1 min), and 72 °C for 10 min. The PCR product was cloned into a pGST-parallel vector (13), a GST fusion protein expression vector containing a recombinant TEV protease (rTEV) cleavage site. Recombinant plasmid was transformed into Escherichia coli strain BL21 (DE3). Transformants were grown in LB medium containing 50 μg/ml ampicillin at 37 °C until an A600 between 0.6 and 0.8 was reached. After induction with 0.2 mm isopropyl d-1-thiogalactopyranoside (IPTG) and 2% ethanol, cultures were grown for a further 20 h at 18 °C. The expressed protein was purified by affinity chromatography using a glutathione-Sepharose 4B (GE Healthcare), and subsequently digested with rTEV at 21 °C. After complete digestion, the GST tag was removed using a glutathione-Sepharose 4B column. The purified protein was then dialyzed against 20 mm Tris-HCl, pH 7.5 and concentrated to 15 mg/ml using a vivaspin 20 (30,000 MWCO) for crystallization. The SeMet-substituted protein was expressed using the E. coli methionine auxotroph strain B834 in M9 medium supplemented with 50 mg/ml SeMet a 25 °C. The purification procedure for the SeMet-substituted protein was identical to that of the native protein.
Crystallization and Data Collection
Crystallization of the purified protein was initially performed using commercially available, sparse-matrix screens (Hampton Research and Emerald Biostructures) and the sitting-drop vapor diffusion method at 21 °C. Crystals were observed after an overnight incubation in a drop containing 28% (v/v) PEG 400 and 0.2 m calcium chloride. After an optimization procedure, the best crystals were obtained under conditions of 30% (v/v) PEG 400 in 0.1 m HEPES pH 7.5 containing 0.2 m calcium chloride. SeMet-labeled AkbC was crystallized under the same conditions by the microseeding method using crushed native crystals as the crystal seeds. The crystals were grown to ∼0.2 × 0.15 × 0.15 mm of maximum size within several days. Before mounting, the crystals were soaked in a cryoprotectant solution consisting of the crystallization solution and 10% glycerol. To obtain a substrate-bound complex, the crystallization solution containing 3-MC was added to the drop containing the native crystals and the cryoprotectant solution at a final concentration of 20 mg/ml.
After a fluorescence scan, single anomalous x-ray dispersion data for a SeMet crystal were collected at a wavelength corresponding to the Se absorption peak (0.9796 Å) using an ADSC Quantum 210 CCD detector on the beam line 4A at the Pohang Accelerator Laboratory (Pohang, Korea). The data for native AkbC containing 3-MC were collected at Argonne Advanced Photon Source (Chicago, IL) at a wavelength of 1.0000 Å. The data were indexed, integrated, and scaled using the HKL2000 package (14). The SeMet crystal belongs to the space group P4 with unit cell parameters of a = b = 102.4 Å and c = 142.4 Å. There are four AkbC molecules in the asymmetric unit with a packing density of 2.74 Å3/Da, corresponding to an estimated solvent content of 55%. The native crystal containing 3-MC also belongs to a P4 space group and shows similar unit cell size to the SeMet crystal. The statistics of the crystals are summarized in Table 1.
TABLE 1.
Data collection and refinement statistics
r.m.s.d., root mean square deviation.
| Data set | SeMet | Native bound 3MC |
|---|---|---|
| Experimental data | ||
| X-ray source | PAL 4A | APS |
| Wavelength (Å) | 0.9796 | 1.0000 |
| Space group | P4 | P4 |
| Unit cell parameters (Å) | 102.4 × 102.4 × 142.4 | 102.6 × 102.6 × 142.1 |
| Resolution limit (Å) | 50–2.2 (2.28–2.20)a | 40–1.9 (1.97–1.90)a |
| Total reflections | 509,802 | 415,917 |
| Unique reflections | 74,911 (7,352) | 114,402 (11,347) |
| Redundancy | 6.8 (6.1) | 3.7 (3.6) |
| Completeness (%) | 99.8 (98.2) | 99.2 (98.9) |
| Rsymb | 0.096 (0.329) | 0.056 (0.333) |
| Average I/σ (I) | 25.4 (5.6) | 23.7 (3.6) |
| Refinement details | ||
| Space group | P4 | P4 |
| Resolutions (Å) | 30-2.2 | 30-1.9 |
| Reflections (working) | 69,108 | 108,666 |
| Reflections (test) | 3,650 | 5,735 |
| Rworkc | 0.155 | 0.151 |
| Rfreec | 0.178 | 0.176 |
| Number of water molecules | 251 | 302 |
| r.m.s.d. from ideal geometry | ||
| Bond length (Å) | 0.009 | 0.007 |
| Bond angle (°) | 1.202 | 1.070 |
| Average B factors (Å) | ||
| Molecule A (main/side chain) | 18.9 (18.6/19.1) | 17.4 (17.8/17.6) |
| Molecule B (main/side chain) | 17.8 (17.4/18.3) | 18.1 (18.8/18.4) |
| Molecule C (main/side chain) | 19.9 (19.6/20.3) | 18.7 (18.5/19.0) |
| Molecule D (main/side chain) | 19.8 (20.1/20.0) | 20.1 (20.0/20.3) |
| Waters | 18.7 | 21.5 |
a The numbers in parentheses describe the relevant value for the last resolution shell.
b Rsym = Σ|Ii − 〈I〉|/ΣI, where Ii is the intensity of the ith observation and 〈I〉 is the mean intensity of the reflections.
c Rwork = Σ‖Fobs| − |Fcalc‖/Σ|Fobs|, crystallographic R factor, and Rfree = Σ‖Fobs| − Fcalc‖/Σ|Fobs| when all reflections belong to a test set of randomly selected data.
Structure Determination
The structure of SeMet-substituted AkbC was determined by the single anomalous dispersion (SAD) method at a resolution of 2.2 Å. Twenty-nine Se atoms were identified in the asymmetric unit using SOLVE (15), and the initial phase had a figure of merit (FOM) of 0.252. Density modification and subsequent automated model building were performed with RESOLVE (16), increasing FOM to 0.642 with 57% of the residues built. The initial RESOLVE model was used as a guide to build the remainder of the protein manually into density-modified electron density maps with the program COOT (17). Refinement with isotropic displacement parameters was performed initially with Refmac5 in the CCP4 suite (18). Because AkbC crystals were revealed as twined crystals by analyzing the intensity statistics of the diffraction data, further refinement of the twined model was continued with a new version of the CCP4 Refmac5 program (Version 5.5.0088), which can handle the twining problem. Twining fraction of the AkbC crystal was 0.492. Atomic displacement parameters were refined in Refmac5 by the TLS (translation, libration, screw) method, with each of the four monomers in the asymmetric unit treated as a single TLS group. The Rwork and Rfree values of the refined structure were 0.155 and 0.178, respectively. The native AkbC structure was solved by molecular replacement using the refined model of the SeMet-AkbC with the program MOLREP (19). The crystallographic data statistics are summarized in Table 1. The atomic coordinates and structure factor amplitudes of the SeMet proteins and the native protein-bound 3-MC were deposited in the Protein Data Bank under the accession codes 2WL3 and 2WL9, respectively.
Site-directed Substitution and Deletion Mutagenesis
All mutagenesis was performed using PCR-based techniques. The same thermocycler program as described above was used, and PCR primers used in the experiments are listed in supplemental Table S1. The coding sequence of akbC was amplified by PCR using primers akbC-F and akbC-R with DK17 genomic DNA as a template. Site-directed substitution mutagenesis was performed at Tyr-175, Phe-177, His-244, Tyr-253, and Asp-281 via overlap extension PCR as described by Sakamoto et al. (20). The two halves of akbC were amplified in two separate reactions: 1) using the akbC-F primer and either Y175-R, F177-R, H244-R, Y253-R, or D281-R primers to amplify the 5′ portion of the akbC gene and 2) using the akbC-R primer and either Y175-F, F177-F, H244-F, Y253-F, or D281-F primers to amplify the 3′ portion of the gene. One microliter of each reaction was combined for a second reaction using the akbC-F-akbC-R primer pair. The overlap-extension PCR technique was also employed to generate two deletion mutants of the β-hairpin structure (β11–β12). The first round of PCR was performed using the akbC-F primer and either Δβ-1-R or Δβ-2-R primers and the akbC-R primer and either Δβ-1-F or Δβ-2-F primers, followed by the second round of PCR using the akbC-F-akbC-R primer pair. Eight C-terminal truncation mutants were constructed by PCR with the akbC-F primer and either G291*-R, L294*-R, D295*-R, I296*-R, P297*-R, L298*-R, K299*-R, or G300*-R primers. The final PCR products were cloned using the pCRT7/CT-TOPO TA expression kit (Invitrogen) according to the manufacturer's instructions. Each recombinant plasmid was transformed into E. coli strain TOP10 (Invitrogen) competent cells for propagation. The insertional orientation of the cloned fragment was confirmed by PCR, and the properly oriented clones were then sequenced for the confirmation of each mutation. Sequence-confirmed clones were transformed into E. coli BL21(DE3) for expression. E. coli strains were grown on LB medium at 37 °C.
Enzyme Assay
Preculture of E. coli BL21(DE3) containing the akbC gene was prepared by inoculating three or four colonies into 4 ml of LB medium supplemented with ampicillin (100 μg/ml) and incubated overnight at 37 °C. All of the overnight culture was transferred to 200 ml LB supplemented with ampicillin and further incubated at 37 °C. To overexpress akbC in the E. coli host system, 1.0 mm (final concentration) IPTG was added when bacterial cells reached the exponential phase (A600 = 0.5–0.8). The incubation was prolonged at 37 °C for 2.5 h, and the induced cells were harvested and washed in one-half volume of 1× PBS (phosphate-buffered saline; 140 mm NaCl, 2.7 mm KCl, 10 mm NaHPO4, and 1.8 mm KH2PO4 (pH 7.4)). Cells were chemically homogenized in Bugbuster Master Mix (Novagen) according to the manufacturer's instructions. Unbroken cells and cell debris were removed by centrifugation at 14,000 × g for 30 min. The resulting supernatant was used as the crude enzyme solution. The extradiol dioxygenase activity was assayed spectrophotometrically by measuring, at the corresponding wavelength, the increase in absorbance of each meta-cleavage product formed from the following substrates: 3-MC, λmax = 388 nm and ϵ = 13,800 cm−1 m−1 (21); 2,3-DHB, λmax = 434 nm and ϵ = 22,000 cm−1 m−1 (22). The reaction mixture contained 100 mm phosphate buffer (pH 7.4) and an appropriate substrate at a final concentration of 0.4 mm. One unit of enzyme activity is defined as the amount of enzyme required to form 1 μmol product per minute. Protein content was determined by the method of Bradford with bovine serum albumin as the standard. Substrates were obtained from Sigma-Aldrich Korea.
RESULTS
Overall Structure and Oligomerization of AkbC
The crystal structure of selenomethionine (SeMet)-substituted AkbC from Rhodococcus sp. strain DK17 was determined at a resolution of 2.2 Å (Table 1). A three-dimensional native structure of the enzyme-substrate (3-MC) complex was also determined at a resolution of 1.9 Å. In both structures, four AkbC molecules (A, B, C, and D) form the asymmetric unit. By the 4-fold rotational symmetry in the P4 space group, 16 AkbC molecules were arranged as two octameric complexes in a unit cell. Molecules A and B formed one octamer (supplemental Fig. S1), while molecules C and D completed the other octamer. Two molecules in each pair were related to each other by 2-fold symmetry in which the axis was perpendicular to the 4-fold symmetrical axis. Thus, an octameric complex is a stack of two ring structures composed of four subunits. The subunits in a ring structure are connected by hydrogen bonds and ionic interactions while a binding of two ring structures are maintained by hydrophobic interactions and ionic bonds. Because the conceptually calculated molecular weight of this complex matches that of the native AkbC protein in the solution, as estimated by gel filtration, one can reasonably speculate that AkbC exists in vivo as an octameric complex as assembled in the crystal structure. In addition, it is worth noting that all eight active sites face the surface of the octameric complex, although a large cavity (∼45-Å width at the center) exists inside the octameric globular complex with two large openings (∼23-Å diameter) at each end of the complex.
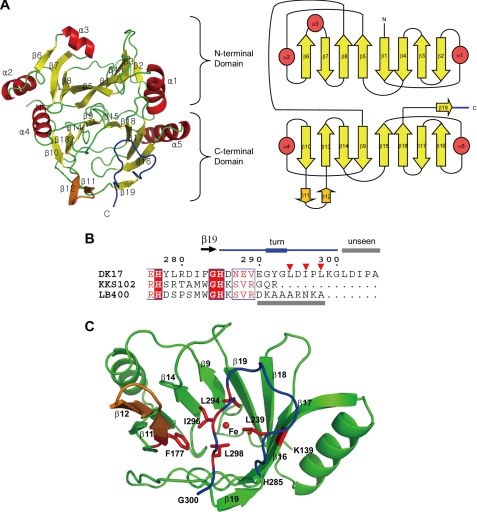
In the native AkbC structure, residues from M1 to G300 were determined in molecule A (Fig. 2). No electron density for the last five residues of the C terminus (LDIPA) was visible, suggesting that this region is flexible. A monomeric AkbC molecule is composed of two topologically similar domains, an N-domain (residues Met-1—Gly-138) and a C-domain (Lys-139—Gly-300), including the C-terminal tail (His-285—Gly-300). Each domain contains two similar sequence motifs (β-α-β-β-β) joined together by a loop to make an eight-stranded mixed β-sheet with α-helices at each end. The two motifs are positioned as an inverted mirror image to each other. In the C-domain, two long loops that connect strands β18 and β19 and strands β16 and β17, respectively, are positioned between the two end strands (β10 and β16) of the C-domain β-sheet, and thereby cover the bottom side of the C-domain half-barrel to complete a bowl structure.
FIGURE 2.
Monomeric structure of AkbC. A, ribbon (left) and topology (right) diagrams of the AkbC monomer. B, sequence alignment of the C-terminal tail. Residues Arg-276—Ala-305 form AkbC is aligned with those of DHBDs from Pseudomonas sp. strain KKS102 (Arg-276—Arg-292) and B. cepacia LB400 (Arg-276—Ala-298). C, C-domain structure of AkbC. Hydrophobic residues in the β-hairpin structure (β11–β12, orange) and the C-terminal tail (blue) are shown as red sticks and marked as inverted triangles. Unseen regions in the crystal structures are indicated with gray bars.
A β-hairpin structure (β11–β12) is inserted between strands β10 and β13 in the C-domain, while in the N-domain, a helix (α3) is incorporated between the corresponding strands β6 and β7, covering the open side of the N-domain half-barrel. The C-terminal tail region (in blue) is antiparallel to strand β16 and makes a turn to come back to strand β11 (Fig. 2C). The C-terminal tail and inserted β-hairpin motif of β11–β12 cover the open side of the C-domain bowl structure whose inside forms the substrate binding site. The hydrophobic side chain of Leu-298 is in close contact with Phe-177 from the β-hairpin structure and with Leu-239 from the β18 strand. Together with Leu-294 and Ile-296, these C-terminal hydrophobic residues contribute to the hydrophobic environment of the substrate binding site.
Iron (Fe2+) Coordination and Active Site Properties
The crystal structure clearly shows that AkbC possesses the 2-His-1-carboxylate facial triad motif for iron binding at the active site (His-149, His-212, and Glu-263), which resides in the C-domain (Fig. 3). However, an additional amino acid residue (Tyr-253) is apparently involved in binding the iron, as indicated by the short distance (2.79–2.95 Å) from the hydroxyl oxygen atom of Tyr-253 to the iron. This tyrosine is also conserved in DHBDs from Pseudomonas sp. strain KKS102 and B. xenovorans strain LB400 at equivalent positions, but they are not close enough to coordinate the iron (3.7–3.9 Å and 3.8–3.9 Å for the KKS102 and the LB400 DHBDs, respectively). Thus, to determine whether Tyr-253 of AkbC is dispensable for iron binding in AkbC, we introduced a point mutation by substituting a phenylalanine for Tyr-253 and found that the mutant enzyme Y253F possesses ∼15% of the activity of the wild-type enzyme. This result shows that the iron can still function without binding to Tyr-253, suggesting that Tyr-253 could retract from the iron. In general, in type I extradiol dioxygenases, this conserved tyrosine is known to be involved in the deprotonation of the hydroxyl group of a substrate (23, 24). It has also been reported that a water molecule can functionally replace the tyrosine (25). One can thus speculate that Tyr-253 contributes to the deprotonation of the substrate hydroxyl group.
FIGURE 3.
Iron coordination in AkbC. The iron is bound by His-149, His-212, Tyr-253, Glu-263, and a water molecule in molecule A. In the second shell, His-244 and Asp-281 and Asp-247 are hydrogen bonded through Tyr-253 and a water molecule, respectively. The iron, water molecule, and residues involved in the interactions are shown as brown and red spheres, and sticks, respectively. The distances between interacting atoms are indicated. Electron density maps for the iron found in the active sites of iron-omitted molecule A. The 2Fo−Fc (blue) and Fo−Fc (red) electron density maps were contoured at the 2.0 and 10.0 σ level, respectively.
Catalysis of ring cleavage by extradiol dioxygenases is accomplished primarily through an acid-base catalysis mechanism, in which suitably positioned histidine residues in the second shell of the iron (i.e. His-197 and His-244 in AkbC) play important roles (26). In fact, in AkbC, there is a hydrogen bond network from the carboxyl group of Asp-281 to the hydroxyl group of Tyr-253 via the imidazole ring of His-244. Subsequently, seven mutant enzymes (H197F, H197I, H244L, H244N, H244Q, D281S, and D281V) were generated by site-directed mutagenesis. Since all seven mutant enzymes were found to completely lose their activities, these residues were deemed essential for enzyme activity, possibly as acid/base catalysts, although the possibility cannot be excluded that the loss of activity results simply from iron loss. To address this issue, attempts were made to measure iron contents in the wild-type and selected mutant (H197F, H244N, and D281S) enzymes. Atomic absorption spectroscopy analysis revealed that the mutant enzymes contain approximately half as much iron as the wild-type one.
Functional Roles of the C-terminal Tail
Our previous molecular modeling studies suggested that the overall structure of AkbC is similar to those of DHBDs from KKS102 and LB400 except that AkbC has a longer C-terminal tail and shorter α4 helix and β18–β19 loop than the other two enzymes (10). Indeed, the room mean square deviation values of the Cα atoms of AkbC to the KKS102 and LB400 DHBDs were determined to be 1.02 and 1.14 Å, respectively. Superimposition of the active site of AkbC on those of the KKS102 and LB400 DHBDs revealed that 1) residues surrounding the active site of AkbC are primarily located in the conserved regions and 2) the size and shape of the substrate binding cavity of AkbC are similar to those of the other two enzymes (supplemental Fig. S2). Because these results mean that the substrate binding site of AkbC can accommodate a bulky substrate DHB, the question is why AkbC shows a much lower activity against DHB than 3-MC.
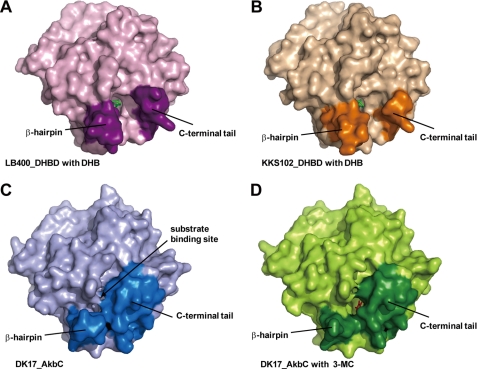
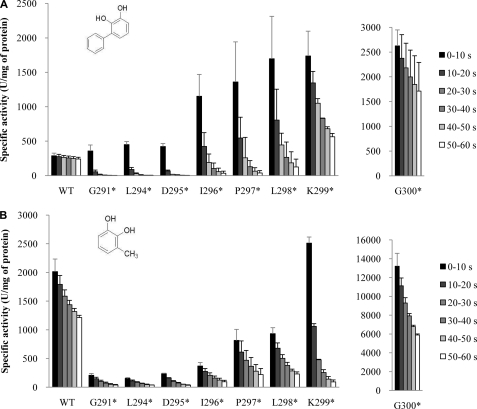
As explained above, the C-terminal tail (His-285—Gly-300) was determined to be positioned over the substrate binding pocket. Considering that the C-terminal regions of DHBDs from KKS102 and LB400 are relatively short compared with that from AkbC and some C-terminal residues were not visible in their previously determined structures, it is possible that this topography results in a smaller entrance to the active site in AkbC compared with the DHBDs (Fig. 4). To address this hypothesis, a series of truncated mutants were generated by introducing stop codons at various positions after residue Gly-291, which is the hinge of the C-terminal tail (Fig. 2B). We first truncated five residues (LDIPA) from the C-terminal end, which is invisible in the crystal structure of AkbC. Interestingly, the G300* mutation caused an approximate 9- and 7-fold increase in the activity of AkbC against DHB and 3-MC respectively, and as a result there is an increase in the preference of AkbC for DHB (Fig. 5). It is even more interesting to see that further sequential deletions (K299*, L298*, P297*, I296*, D295*, L294*, and G291*) lead to a dramatic change in the enzyme activity profile because these mutant AkbC enzymes show maximal activity against DHB. This finding suggests that the C-terminal tail determines the substrate specificity of AkbC by restricting the access of large substrates, such as DHB, to the active site.
FIGURE 4.
Comparison of active site entrances. The surface representation of DHBDs from B. cepacia LB400 (A) and Pseudomonas sp. strain KKS102 (B) and AkbC (C and D). The substrate binding sites are located between the β-hairpin structures and the C-terminal tails, which are indicated by dark colors. DHB and 3-MC are shown as green and red sticks, respectively. The substrate binding site of AkbC in molecule A (C) is closed whereas that in molecule B with 3-MC (D) is opened.
FIGURE 5.
Comparison of enzyme activities of wild-type AkbC and its truncated mutant enzymes. Six bars for wild-type AkbC and each mutant represent specific activity values in six sequential 10 s period during 1 min reaction time against 2,3-DHB (A) and 3-MC (B).
Assays of mutant enzymes showed pronounced nonlinear kinetics. Namely, rapid decays of initially high activity were observed. As shown in Fig. 5, the mutant enzymes K299*, L298*, P297*, and I296* initially exhibited much higher activities against DHB than wild-type enzymes, but their activities suffered sharp drops. When assayed with 3-MC, the same four enzymes showed more pronounced drops and retained less than 5% of their initial values after 1 min. Because preincubation of the assay mixture without substrate for 1 min at 30 °C did not show any negative effect on initial enzyme activities, these observed decays were suspected to be due to substrate inhibition. Moreover, additional deletion mutants of the C terminus (G295*, L294*, and G291*) showed lower activities against 3-MC from the beginning compared with the wild-type enzyme, although their initial activities against DHB were a little bit higher than that of the wild-type control. These results suggest that the C-terminal tail plays additional role(s) in the reaction with 3-MC.
Identification of Structural Intermediates in Substrate Binding
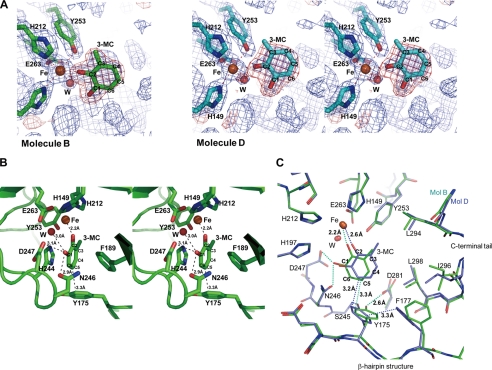
In the native three-dimensional structure of the enzyme-substrate complex, 3-MC was found in the active sites in molecules B and D, respectively (Fig. 6A). In all other currently known structures of type I extradiol dioxygenases, both oxygen atoms from the two hydroxyl groups of the catechol substrates were bound to the iron. Interestingly, however, in our structure of the complex, 3-MC was found to interact with the iron using only the oxygen atom at C2 in 3-MC. The oxygen atom of the hydroxyl group at the C2 position coordinates the iron (2.21 Å), while the other hydroxyl group at the C1 position has a hydrogen bond with Asn-246 (2.86 Å) instead of the iron (Fig. 6B) and was also closely located with the water molecule (3.02 Å), binding the iron and carboxyl group of D247 (3.13 Å). The ring of 3-MC closely contacts the side chain of Tyr-175 in the β-hairpin structure. The distance between the C5 atom of 3-MC and Cϵ2 of Tyr-175 is 3.26 Å. The C5 atom of 3-MC also closely contacts the side chain Asn-246. The methyl group of 3-MC faces the hydrophobic residues, including Leu-294 in the C-terminal loop that covers the active site (Fig. 6C).
FIGURE 6.
Substrate 3-MC interaction in the crystal structure of AkbC. A, electron density maps for 3-MC found in the active sites of molecule B (left) and D (right). The 2Fo−Fc (blue) and Fo−Fc (red) electron density maps were contoured at the 1.5 and 3.0 σ level, respectively. B, 3-MC in molecule B is sandwiched between His-244 and Phe-189. It is bound to the iron, a water molecule, Asn-246 and Asp-247, and in close contact with Tyr-175. C, two 3-MC-bound structures are compared. Molecules B (green) and D (cyan) are superimposed. Residues surrounding 3-MCs including those from the β-hairpin structure, and the C-terminal tail are shown as sticks. The iron and water molecule are shown as brown and red spheres, respectively. The distances between interacting atoms are indicated.
In molecule B, the benzene ring of 3-MC is sandwiched between the side chains of His-244 and Phe-189 parallel to the ring plane of 3-MC and the imidazole ring of His-244. In contrast, in molecule D, 3-MC is located further away by a distance of 2.6 Å between the oxygen atom at C2 and the iron. More specifically, the hydroxyl group at C1 was found to retreat from Asn-246, Asp-247, and a water molecule binding the iron (Fig. 6C). It appears that 3-MC in molecule D does not fit well into the substrate binding site. These differences are related to interactions between 3-MC and the hydrophobic amino acid residues surrounding the substrate binding pocket. The side chains of Leu-294 and Leu-298 from the C-terminal tail closely contact 3-MC in molecule D, while those residues are further from 3-MC in molecule B. The configurations of molecules B and D look like two sequential shot images of 3-MC nudged into the active site. Furthermore, these observations can be interpreted as evidence that the residues of the C-terminal tail move in and out to position 3-MC correctly into the substrate binding pocket.
Comparison of molecules B and D also reveals that a shift of 3-MC to the active site is related to the shift of the side chain of Tyr-175 (Fig. 6C). In molecule D, the side chain of Tyr-175 appears to be moved inward into the substrate binding cavity by 3-MC and the side chain of Phe-177. Distances from the tyrosine ring to the ring structures of Phe-177 and 3-MC were determined to be 3.25 Å and 3.23 Å, respectively. The hydroxyl group of Tyr-175 forms a hydrogen bond with the main chain amide group of Ser-245 (2.79 Å) in molecule D. As shown in molecule B, the retreat of the phenyl ring of Phe-177 causes the hydroxyl group of Tyr-175 to shift away from the substrate binding pocket and form a hydrogen bond with the carboxyl group of Asp-281 (2.61 Å). Thus, while the Cϵ2 atom of Tyr-175 closely contacts the C6 atom of 3-MC (3.23 Å) in molecule D (a less well-fitted structure) and associates with the C5 atom of 3-MC (3.26 Å) in molecule B (a more well-fitted structure), it is apparent that such movement of the Tyr-175 side chain positions 3-MC in the substrate binding pocket.
A total of four substitution mutant enzymes were constructed, and their activities were compared with that of wild type to address the above hypothesis (supplemental Table S2). Substitution of tyrosine at position 175 by a small, hydroxyl-containing serine residue (Y175S) completely abolished enzyme activity, confirming the importance of Tyr-175. In contrast, Y175F, which has a bulky hydrophobic side chain, was found to retain more than 90% of the wild-type control activity, while substitution to a smaller hydrophobic valine residue (Y175V) reduced activity to 7% of wild type. These data suggest that AkbC activity depends much more on the benzene ring structure than on the tyrosine hydroxyl group. Phenylalanine at position 177 is also predicted to contribute to substrate positioning because the benzene ring of Phe-177 apparently rotates the side chain of Tyr-175 to direct 3-MC to the correct site. This prediction is supported by the fact that substitution of alanine for a bulky phenylalanine (F177A) resulted in a 25% decrease in enzyme activity.
Functional Roles of the β-Hairpin Structure
As described in the previous section, molecules B and D were found to contain 3-MC molecules in their active sites, while the active sites of molecules A and C were not occupied by substrate. Interestingly, the electron density for residues Leu-179—Ala-183 and Ala-178—Gly-182 at the end of the β-hairpin structure is missing in molecules B and D, respectively. In contrast, a well-defined electron density was observed for the complete β-hairpin structures of molecules A and C. These observations imply that the β-hairpin structures of molecules B and D are more mobile than those of molecules A and C. Such flexibility was also corroborated by higher B-factor values compared with the other regions in AkbC (supplemental Fig. S3), resulting in a wider entrance to the active site in AkbC (Fig. 4D).
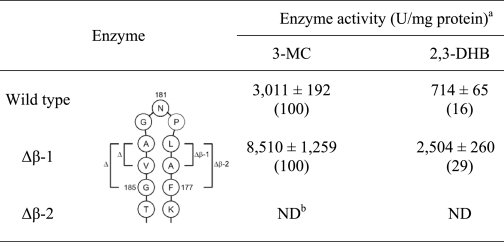
To investigate the functional role of the β-hairpin structure, two deletion mutant enzymes (designated Δβ-1 and Δβ-2, respectively) were constructed by deleting four (ΔA178-L179 and ΔA183-V184) and six (ΔF177-A178-L179 and Δ A183-V184-G185) amino acids from both arms of the β-hairpin, respectively. As summarized in Table 2, Δβ-1 exhibited ∼2.8- and 3.5-fold increased activities against 3-MC and DHB, respectively, indicating that the β-hairpin structure also blocks substrate entry into the active site. It is thus apparent that a smaller β-hairpin structure results in a bigger entrance into the active site and thereby facilitates substrate entry. However, further deletion of Phe-177 and Ala-185 completely abolishes enzyme activity. Deleting too much of the β-hairpin structure could disrupt the folding of the active site. In addition, the elimination of Phe-177 appears to be at least part of the reason for the complete loss of activity.
TABLE 2.
Comparison of extradiol dioxygenase enzyme activities in cell extracts of E. coli containing wild-type akbC, Δβ-1-akbC, and Δβ-2-akbC
a Enzyme activities are the averages from at least three independent experiments.
b ND, not detected.
DISCUSSION
AkbC, the bona fide extradiol dioxygenase in DK17 alkylbenzene metabolism, does not belong to the extradiol dioxygenase subclass for alkylcatechol substrates but instead defines a separate phylogenetic group of extradiol dioxygenases involved in the degradation of bicyclic aromatic compounds, including biphenyl (10). Indeed, our crystallographic data show that the substrate binding site of AkbC is as large as those of related DHBDs. However, AkbC is distinguished from DHBDs by the presence of the C-terminal tail that obstructs the entrance to the active site. Furthermore, high B-factor values and poor electron density suggest that the C-terminal tail is very flexible, which is also reflected by the invisibility of the five residues (LDIPA) at the C-terminal end in our AkbC crystal structure. Deletion of these five residues from the C terminus significantly increased AkbC activity against both DHB and 3-MC by 9- and 7-fold, respectively. Further deletions resulted in additional increases in the preference of AkbC for DHB. A plausible explanation for these results is that shortening of the C-terminal tail leads to a widening of the opening of the substrate binding site, a greater benefit for the larger DHB compared with the smaller 3-MC. Accordingly, it is apparent that the C-terminal tail contributes to the substrate specificity of AkbC by blocking the entrance, discriminating substrates based on size.
A substrate inhibition-like phenomenon was observed for AkbC enzymes. As shown in Fig. 5, during the 1-min enzyme assay, wild-type AkbC activity against DHB and 3-MC during the last 10 s was 84 and 60% of activity during the first 10 s, respectively. Interestingly, this decrease in enzyme activity became greater with the increasing length of truncation. The remaining activities of G300* and K299* against DHB and 3-MC during the final 10-s period were 65 and 45% and 58 and 4% of activities during the first 10 s, respectively. It is generally known that extradiol dioxygenases are subject to two forms of substrate inhibition: reversible substrate inhibition and a mechanism-based inactivation (or suicide inhibition) (27). The molecular basis of the latter mechanism has been relatively well documented although subject to some debate (23, 27, 28). As a representative example, the mechanism-based inactivation of LB400-DHBD involves the dissociation of superoxide from the enzyme-catechol-dioxygen ternary complex with the concomitant oxidation of the active site ferrous iron (11). As explained above, for LB400-DHBD, 3-MC caused a more severe mechanism-based inactivation compared with DHB, likely due to the inability of 3-MC, which is relatively smaller than DHB, to fill the active site and hence protect the iron center from exposure to oxidation (27). Our enzyme assay results, combined with this postulation, suggest a second role for the C-terminal tail; that is, shielding the substrate binding pocket from oxidation.
Another interesting aspect of the enzyme inactivation profile is that the truncated mutants, L298*, L297*, L296*, G295*, L294*, and G291*, showed lower activities against 3-MC than the wild-type enzyme, even from the beginning. In contrast, the same mutant enzymes maintained higher activities against DHB than wild-type AkbC, suggesting that the C-terminal tail has a third role in the ring-cleavage reaction. Our crystallographic results clearly show that the substrate binding pocket is sufficiently large enough to hold 3-MC. Thus, without proper C-terminal tail length, it would be more difficult for 3-MC to be positioned for enzymatic attack.
Like the C-terminal tail, the β-hairpin structure is characterized by high B-factor values and poor electron density. Considering its position and flexibility, the β-hairpin structure was also predicted to restrict substrate entry (Fig. 4, C and D). This prediction was clearly confirmed by the deletion mutagenesis experiments, which showed that deletion of four amino acids from both arms of the β-hairpin caused a significant increase in AkbC enzyme activity (Table 2). As shown in Fig. 6C, comparison of the 3-MC-bound enzyme molecules B and D indicates that Phe-177 plays a role in localizing the substrate at the active site by shifting the side group of Tyr-175. Accordingly, the presence of Tyr-175 and Phe-177 at the β-hairpin suggests that the hairpin structure aids in positioning the substrate for subsequent cleavage.
Extradiol dioxygenases generally initiate the catalysis of ring-cleavage reactions through the bidentate binding of catechol substrates to the ferrous iron at the active site. Substrate binding leads to the displacement of water molecules, and thus activates the iron for O2 binding. Subsequent steps involve acid catalysis of the Criegee rearrangement, followed by lactone hydrolysis (Fig. 1). The present work shows that the catalytic strategy employed by AkbC is essentially the same in terms of ring cleavage, but unique with respect to the substrate binding mode of other known type I extradiol dioxygenases as evidenced by the identification of structural intermediates (Fig. 6). One might question the authenticity of the observed findings, but the intermediate structures can be explained by an analysis of the AkbC crystal structures.
In type I extradiol dioxygenases, prior to substrate binding, the iron is coordinated to a maximum of six ligands, including three amino acid residues of the 2-His-1-carboxylate facial triad motif and two or three water molecules. In the crystal structures of AkbC, one water molecule is almost always bound to the iron on the opposite side of the bound His-212 residue, while the other water molecule is occasionally located in the extended line of Glu-263 bound to the iron, indicating that the latter water molecule is easily released or replaced when substrate binding occurs. Furthermore, combined with the fact that the remaining ligand is occupied by Tyr-253, this topography makes it somewhat unlikely that both hydroxyl groups from 3-MC bind simultaneously to the iron. In such a case, either hydroxyl on 3-MC would first bind to the open coordination site, which is made available by the release of the more displaceable water molecule. This explanation is in a good agreement with the observation of the structural intermediates shown in Fig. 6.
The native crystal was soaked in solution containing 3-MC before beam mounting. In a crystal, four molecules in the asymmetric unit are located at independent positions, suggesting that each molecule is in a different environment. Accordingly, the chances of binding 3-MC to the active sites are predicted to be different. In addition, tightly packed neighboring molecules likely restrict their respective flexibilities of their C-terminal tail and β-hairpin structures, which are essential for substrate binding. Taken together, the identified structural intermediates are not likely artifacts. Rather, they appear to represent phases in the normal sequence of the substrate binding process of AkbC.
The ferrous iron of AkbC was found to be surrounded by six ligands, one at each apex of the octahedron (Fig. 3). This octahedral geometry is achieved by the coordination of His-149, His-212, Tyr-253, and a water molecule as equatorial ligands, and of Glu-263 as an axial ligand, leaving one site open (Fig. 7A). It has been proposed that substrate binding is a multistep process that includes the displacement of water molecules to yield a bidentate-bound monoanionic catechol (25). Thus, as depicted in Fig. 7B, the identification of a monodentate catechol bound to the iron center is considered experimental evidence for a multistep process. It is apparent that transition from the state shown in Fig. 7B to the state shown in Fig. 7C is accomplished by two flexible motifs, the C-terminal tail and the β-hairpin structure, for bidentate binding. Such a transition results in the displacement of Tyr-253 and a water molecule, which leads to the deprotonation of the hydroxyl group of 3-MC, as well as the provision of a position for an oxygen molecule. This proposed process is in a good agreement with previous reports that the oxygen interaction occurs after the binding of the catechol substrate during the catalytic process of extradiol dioxygenases (3–7). In summary, this work shows that ring cleavage by the DK17 AkbC enzyme is preceded by a series of substrate-positioning steps, such that the flexible motifs direct and correctly position the substrate into the binding pocket/active site, allowing efficient substrate catalysis and product release.
FIGURE 7.
Proposed steps in the substrate binding process of the DK17 AkbC enzyme. A, without substrate, the iron coordinates with four residues and a water molecule. B, 3-MC binds first to one oxygen atom in the empty axial position in the opposite site of Glu-263. C, by correcting the position of 3-MC with both oxygen atoms as equatorial ligands, the water molecule and Tyr-253 are retracted from the iron, leaving the axial binding position empty. D, an oxygen molecule binds the empty position.
Supplementary Material
This work was supported by a grant from the Ministry of Education, Science and Technology of the Republic of Korea through the 21C Frontier Microbial Genomics and Applications Center Program.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Tables S1 and S2.
The atomic coordinates and structure factors (codes 2WL3 and 2WL9) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- DHBD
- 2,3-dihydroxybiphenyl 1,2-dioxygenase
- 3-MC
- 3-methylcatechol
- DHB
- 2,3-dihydroxybiphenyl.
REFERENCES
- 1.Fritsche W., Hofrichter M. (2005) Environmental Biotechnology, Wiley-VCH, Weinheim, Germany [Google Scholar]
- 2.Broderick J. B. (1999) Essays Biochem. 34, 173–189 [DOI] [PubMed] [Google Scholar]
- 3.Vaillancourt F. H., Bolin J. T., Eltis L. D. (2006) Crit. Rev. Biochem. Mol. Biol. 41, 241–267 [DOI] [PubMed] [Google Scholar]
- 4.Bugg T. D., Ramaswamy S. (2008) Curr. Opin. Chem. Biol. 12, 134–140 [DOI] [PubMed] [Google Scholar]
- 5.Lipscomb J. D. (2008) Curr. Opin. Struct. Biol. 18, 644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brivio M., Schlosrich J., Ahmad M., Tolond C., Bugg T. D. (2009) Org. Biomol. Chem. 7, 1368–1373 [DOI] [PubMed] [Google Scholar]
- 7.Ishida T., Tanaka H., Horiike K. (2004) J. Biochem. 135, 721–730 [DOI] [PubMed] [Google Scholar]
- 8.Kim D., Kim Y. S., Kim S. K., Kim S. W., Zylstra G. J., Kim Y. M., Kim E. (2002) Appl. Environ. Microbiol. 68, 3270–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Chae J. C., Zylstra G. J., Kim Y. S., Kim S. K., Nam M. H., Kim Y. M., Kim E. (2004) Appl. Environ. Microbiol. 70, 7086–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D., Chae J. C., Jang J. Y., Zylstra G. J., Kim Y. M., Kang B. S., Kim E. (2005) Biochem. Biophys. Res. Commun. 326, 880–886 [DOI] [PubMed] [Google Scholar]
- 11.Vaillancourt F. H., Han S., Fortin P. D., Bolin J. T., Eltis L. D. (1998) J. Biol. Chem. 273, 34887–34895 [DOI] [PubMed] [Google Scholar]
- 12.Bugg T. D. (2003) Tetrahedron 59, 7075–7101 [Google Scholar]
- 13.Sheffield P., Garrard S., Derewenda Z. (1999) Protein Expr. Purif. 15, 34–39 [DOI] [PubMed] [Google Scholar]
- 14.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 15.Terwilliger T. C., Berendzen J. (1999) Acta. Crystallogr. D 55, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terwilliger T. C. (2000) Acta. Crystallogr. D 56, 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emsley P., Cowtan K. (2004) Acta. Crystallogr. D. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 18.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 19.Navaza J. (1994) Acta. Crytallogr. A. 50, 157–163 [Google Scholar]
- 20.Sakamoto T., Joern J. M., Arisawa A., Arnold F. H. (2001) Appl. Environ. Microbiol. 67, 3882–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayly R. C., Dagley S., Gibson D. T. (1966) Biochem. J. 101, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa K., Simon J. R., Chakrabarty A. M. (1983) J. Bacteriol. 154, 1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N., Uragami Y., Nishizaki T., Takahashi Y., Sazaki G., Sugimoto K., Nonaka T., Masai E., Fukuda M., Senda T. (2002) J. Mol. Biol. 321, 621–636 [DOI] [PubMed] [Google Scholar]
- 24.Vaillancourt F. H., Barbosa C. J., Spiro T. G., Bolin J. T., Blades M. W., Turner R. F., Eltis L. D. (2002) J. Am. Chem. Soc. 124, 2485–2496 [DOI] [PubMed] [Google Scholar]
- 25.Siegbahn P. E., Haeffner F. (2004) J. Am. Chem. Soc. 126, 8919–8932 [DOI] [PubMed] [Google Scholar]
- 26.Bugg T. D. (2004) Bioorg. Chem. 32, 367–375 [DOI] [PubMed] [Google Scholar]
- 27.Vaillancourt F. H., Labbe G., Drouin N. M., Fortin P. D., Eltis L. D. (2002) J. Biol. Chem. 277, 2019–2027 [DOI] [PubMed] [Google Scholar]
- 28.Lin G., Reid G., Bugg T. D. (2001) J. Am. Chem. Soc. 123, 5030–5039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.