Abstract
Amyloid-β (Aβ) peptide is thought to have a significant role in the progressive memory loss observed in patients with Alzheimer disease and inhibits synaptic plasticity in animal models of learning. We previously demonstrated that brain-derived neurotrophic factor (BDNF) is critical for synaptic AMPA receptor delivery in an in vitro model of eyeblink classical conditioning. Here, we report that acquisition of conditioned responses was significantly attenuated by bath application of oligomeric (200 nm), but not fibrillar, Aβ peptide. Western blotting revealed that BDNF protein expression during conditioning is significantly reduced by treatment with oligomeric Aβ, as were phosphorylation levels of cAMP-response element-binding protein (CREB), Ca2+/calmodulin-dependent protein kinase II (CaMKII), Ca2+/calmodulin-dependent protein kinase IV (CaMKIV), and ERK. However, levels of PKA and PKCζ/λ were unaffected, as was PDK-1. Protein localization studies using confocal imaging indicate that oligomeric Aβ, but not fibrillar or scrambled forms, suppresses colocalization of GluR1 and GluR4 AMPA receptor subunits with synaptophysin, indicating that trafficking of these subunits to synapses during the conditioning procedure is blocked. In contrast, coapplication of BDNF with oligomeric Aβ significantly reversed these findings. Interestingly, a tolloid-like metalloproteinase in turtle, tTLLs (turtle tolloid-like protein), which normally processes the precursor proBDNF into mature BDNF, was found to degrade oligomeric Aβ into small fragments. These data suggest that an Aβ-induced reduction in BDNF, perhaps due to interference in the proteolytic conversion of proBDNF to BDNF, results in inhibition of synaptic AMPA receptor delivery and suppression of the acquisition of conditioning.
Keywords: Alzheimers Disease; Amyloid; Glutamate Receptors Ionotropic (AMPA, NMDA); Metalloprotease; Neurotrophic Factor; Classical Conditioning
Introduction
Alzheimer disease (AD)2 is a neurodegenerative disorder resulting in a progressive decline in cognitive function and is characterized pathologically by the presence of senile plaques and intracellular neurofibrillary tangles. Amyloid-β peptides (Aβ), the peptide deposited as amyloid plaques, have long been implicated as an important component underlying neural dysfunction associated with AD. Recent evidence indicates that soluble forms of Aβ, such as small and large oligomers that are accumulated before plaque formation, contribute significantly to the pathogenesis of AD by producing excitatory synaptic dysfunction, although the mechanisms are poorly understood (1). Postsynaptic AMPA receptor number and trafficking may be a prime target in AD. A recent study using double knock-in mice carrying mutated human amyloid precursor protein (APP) and presenilin-1 genes exhibited a reduction in AMPA receptor number and AMPA receptor-mediated synaptic currents in CA1 hippocampal neurons that strongly corresponded with increased age, Aβ levels, and plaque deposition (2). Moreover, the double knock-in mice showed age-related deficits in long term potentiation, long term depression, and memory performance. Working memory and spatial learning assessed with the Morris water maze appeared normal, but performance of a more complex spatial learning task was significantly impaired in the older mice with greater plaque deposition (2). Cultured neurons from APP mutant transgenic mice (Tg2576) showed significantly reduced surface expression of GluR1-containing AMPA receptors that was paralleled by exogenous application of Aβ to wild-type neurons (3) and might be mediated at least in part through a reduction in Ca2+/calmodulin-dependent protein kinase (CaMK) II activity (4). Hsieh et al. (5) demonstrated that Aβ overexpression resulted in AMPA receptor endocytosis, a decrease in synaptic AMPA receptors, depression of synaptic transmission, and dendritic spine loss. It is unclear how Aβ induces the loss of AMPA receptors at synapses, but mechanisms similar to those that produce long term depression are implicated.
Growing evidence suggests that brain-derived neurotrophic factor (BDNF) plays a critical role in regulating expression and synaptic delivery of AMPA receptor subunits (6–10). Application of BDNF resulted in up-regulation of AMPA receptor subunit expression through TrkB receptors and promoted synaptic delivery of GluR1 in cultured organotypic hippocampal slices (6). Moreover, application of BDNF to brain slices from BDNF knock-out mice resulted in conversion of silent synapses into AMPA receptor-containing synapses (11). Most studies agree that BDNF is dramatically reduced in brain from AD patients and normal neural tissue exposed to Aβ peptides (12–16). The major BDNF transcripts are specifically down-regulated in brain of AD patients or after treatment with oligomeric Aβ (13, 15). Significantly, the reduction in BDNF protein may occur in the preclinical stages of AD, making it a potential marker for AD onset (14). In contrast, BDNF expression is implicated as a marker for high cognitive function in the normally aging brain (17). Therefore, a reduction in BDNF during early stages of AD may lead to a loss in AMPA receptor trafficking mechanisms and synaptic dysfunction.
BDNF is a key signal transduction element in postsynaptic events that mediate in vitro eyeblink classical conditioning using a brainstem preparation from turtles. Conditioned responses (CRs) are accompanied by enhanced levels of BDNF protein, and application of BDNF alone induces synaptic delivery of GluR1- and GluR4-containing AMPA receptors through TrkB- and extracellular signal-regulated kinase (ERK)-mediated mechanisms (8, 9). Inhibition of BDNF function by application of BDNF antibodies or siRNAs that prevent conversion of mature BDNF from its precursor proBDNF suppresses conditioning and synaptic incorporation of AMPA receptors (10). The aim of this study was to determine whether oligomeric or fibrillar forms of Aβ suppress BDNF expression, AMPA receptor synaptic delivery, and conditioning using this in vitro model system. Application of oligomeric Aβ, but not the less toxic fibrillar form, significantly reduced BDNF protein levels, activation of several protein kinase signaling molecules, synaptic insertion of AMPA receptors, and acquisition of conditioning. Delivery of AMPA receptors could be rescued by coapplication of oligomeric Aβ with BDNF. Interestingly, in vitro cleavage assays showed that a secreted tolloid-like metalloproteinase, tTLLs (turtle tolloid-like protein), which is up-regulated in conditioning and processes proBDNF into mature BDNF (10), cleaves oligomeric Aβ at lower concentrations than proBDNF. Preparations incubated with tTLLs alone expressed BDNF, whereas levels of BDNF significantly declined in the presence of Aβ, suggesting that oligomeric Aβ competes with proBDNF as a target for cleavage by proteases. Together, these data suggest a novel mechanism whereby oligomeric Aβ inhibits AMPA receptor trafficking and conditioning by interfering with the extracellular proteolytic processing of proBDNF into mature BDNF.
EXPERIMENTAL PROCEDURES
Conditioning Procedures
Freshwater pond turtles (Pseudemys scripta elegans) obtained from commercial suppliers were anesthetized by hypothermia and decapitated. Protocols involving the use of animals complied with the guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee. The brain stem was transected at the levels of the trochlear and glossopharyngeal nerves, and the cerebellum was removed as described previously (18). Therefore, the preparation consisted of only the pons with the cerebellar circuitry removed. The brain stem was continuously bathed in physiological saline (2–4 ml/min) containing (in mm): 100 NaCl, 6 KCl, 40 NaHCO3, 2.6 CaCl2, 1.6 MgCl2, and 20 glucose, which was oxygenated with 95% O2, 5% CO2 and maintained at room temperature (22–24 °C) at pH 7.6 (18). Suction electrodes were used for stimulation and recording of cranial nerves. The US was a 2-fold threshold single-shock stimulus applied to the trigeminal nerve; the CS was a subthreshold 100-Hz, 1-s train stimulus applied to the ipsilateral posterior root of the eighth nerve that was below the threshold amplitude required to produce activity in the abducens nerve (19). The latter nerve will be referred to as the auditory nerve because it carries predominantly auditory fibers. Neural activity was recorded from the ipsilateral abducens nerve that normally projects to the extraocular muscles controlling movements of the eye, nictitating membrane, and eyelid. The CS-US interval was 20 ms, which is defined as the time between the offset of the CS and the onset of the US. This brief delay was found to be optimal for conditioning; however, conditioning is not supported using longer CS-US intervals (20). The intertrial interval between the paired stimuli was 30 s. A pairing session consisted of 50 CS-US presentations followed by a 30-min rest period in which there was no stimulation (19). Conditioned responses were defined as abducens nerve activity that occurred during the CS and exceeded an amplitude of double the baseline recording level. Conditioned preparations were those that received paired CS-US stimulation, whereas pseudoconditioned control preparations received the same number of CS and US exposures that were explicitly unpaired using a CS-US interval randomly selected between 300 ms and 25 s. All data were analyzed using a one-way analysis of variance followed by Fisher's and Bonferroni's tests for post hoc comparisons. Means ± S.E. are plotted except where indicated.
Preparation of Oligomeric and Fibrillar Aβ
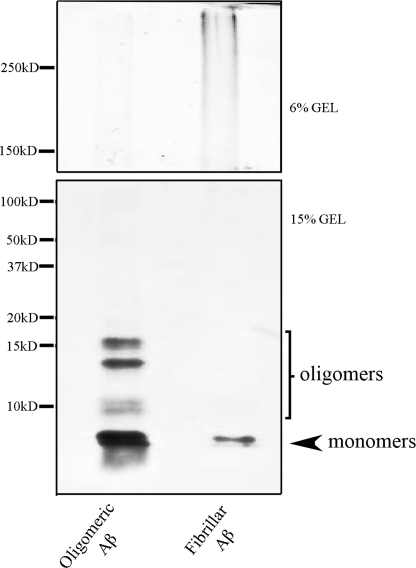
Preparation of oligomeric and fibrillar Aβ was from Dahlgren et al. (21). The human Aβ1–42 peptide and scrambled Aβ1–42 peptide (having the same amino acid composition as Aβ1–42 but in random sequence) used here was produced by chemical synthesis (rPeptide, Bogart, GA). Peptide was diluted in 1,1,1,3,3,3-hexafluoro-2-propanol (Sigma) to 1 mm. The 1,1,1,3,3,3-hexafluoro-2-propanol was allowed to evaporate in a fume hood, and the resulting peptide film that was formed was stored at −70 °C. Aβ oligomers were prepared by diluting 5 mm Aβ1–42 or scrambled Aβ1–42 in DMSO to 100 μm in ice-cold cell culture medium (Ham's F-12; BioSource), vortexed for 10 min, and incubated at 4 °C for 24 h. Aβ1–42 fibrils were prepared by diluting 5 mm Aβ1–42 in DMSO to 100 μm in 10 mm HCl, vortexed for 10 min, and incubated at 37 °C for 24 h. Western blots showing the resultant Aβ1–42 oligomers and fibrils detected by the monoclonal antibody 6E10 (recognizing residues 1–16 of β amyloid; Covance, Emeryville, CA) are shown in Fig. 1. The solutions of soluble oligomeric Aβ used in this study contained a mixture of Aβ monomers and soluble low n-oligomers, whereas fibrillar Aβ contained monomers and high molecular weight fibrils (Fig. 1). We used a concentration of 200 nm Aβ, which is lower than that typically used in neurotoxicity studies and inhibits long term potentiation in hippocampal slices (22). Oligomeric, fibrillar, or scrambled Aβ was bath-applied for 1.5 h prior to beginning the conditioning and continued throughout the training procedures.
FIGURE 1.
Monomeric and oligomeric Aβ preparation. Western blots displaying the formation of Aβ1–42 monomers, oligomers, and fibrils in the preparations of Aβ used to test conditioning are shown. Oligomeric Aβ1–42 and fibrillar Aβ1–42 were prepared as described.
Western Blot Analysis
Turtle brain stems were frozen in liquid nitrogen immediately after the physiological experiments and stored at −70 °C. Tissue was homogenized in lysis buffer (20 mm Tris, pH 8.0; 1 mm EDTA, 1% Nonidet P-40, 0.15 m NaCl, 10 mm Na4P2O7, and 5% glycine) with a protease inhibitor (Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitor mixture (Sigma), rotated at 4 °C for 2 h, and centrifuged at 14,000 ×g for 20 min at 4 °C, and the supernatants were aliquoted and stored at −70 °C. Protein sample concentrates were solubilized in 2× SDS/β-mercaptoethanol and boiled for 5 min before separation by 10 or 15% SDS-PAGE. After electrophoresis, membranes were blocked with 5% nonfat dry milk in Tris-buffered saline/0.1% Tween 20 for 1 h at room temperature. We used the phosphorylation site-directed antibodies against protein kinase A (PKA) Thr-197 (p-PKA; Cell Signaling Technology, Danvers, MA), CaMKII Thr-286 (p-CaMKII; Cell Signaling Technology), cAMP-response element-binding protein (CREB) Ser-133 (p-CREB; Cell Signaling Technology), CaMKIV Thr-196 (p-CaMKIV; Santa Cruz Biotechnology, Santa Cruz, CA), ERK Thr-183/Tyr-185 (p-ERK; Promega, Madison, WI), or protein kinase C ζ/λ (PKCζ/λ) Thr-410/403 (p-PKCζ/λ; Cell Signaling Technology). We also used PKA (t-PKA; Upstate Biotech Millipore, Lake Placid, NY), CaMKII (t-CaMKII; Chemicon), CREB (t-CREB; Cell Signaling Technology), CaMKIV (t-CaMKIV; Abcam, Cambridge, MA), ERK (t-ERK; Chemicon), PKCζ (t-PKCζ; Zymed Laboratories Inc., San Francisco, CA), and BDNF (Santa Cruz Biotechnology). Membranes were incubated with primary antibodies overnight at 4 °C, washed, and incubated with horseradish peroxidase-conjugated (1:10,000) or fluorescently tagged secondary antibodies (1:5,000) for 1 h at room temperature. Loading controls were performed using primary antibodies to actin (1:1,000; Chemicon). Proteins were detected by the ECL Plus chemiluminescence system (Amersham Biosciences) or the Odyssey infrared imaging system (Li-Cor Biotechnology, Lincoln, NE). Immunoreactive signals were captured on Hyperfilm ECL film and quantified by computer-assisted densitometry. Optical densities of the bands were determined relative to background levels. Quantifications of total and phosphoprotein were determined relative to actin. Ratios of phospho- to total protein were obtained for each experiment and averaged. Data are displayed as a percentage of normalized values from pseudoconditioned controls.
Glutamate Receptor Localization, Confocal Imaging, and Data Analysis
Immediately after the physiological experiments, brain stems were immersion-fixed in cold 0.5% paraformaldehyde. Tissue sections were cut at 30 μm and incubated in primary antibody overnight at 4 °C with gentle shaking. The primary antibodies used were a polyclonal antibody raised in goat that recognizes the GluR4 subunit of AMPA receptors (1:100, catalogue number 7614, Santa Cruz Biotechnology), a polyclonal antibody raised in rabbit that recognizes GluR1 (1:200, catalogue number 1504, Chemicon), and a monoclonal antibody raised in mouse that recognizes synaptophysin (1:1,000, catalogue number 5768, Sigma). The specificity of the antibodies was confirmed by Western blot. After the primary antibodies, sections were rinsed and incubated with secondary antibodies for 2 h using a concentration of 1:100 for GluR4 and GluR1 or 1:50 for synaptophysin. The secondary antibodies were a Cy3-conjugated goat anti-rabbit IgG for GluR1, a Cy3-conjugated rabbit anti-goat IgG for GluR4, and a Cy2-conjugated goat anti-mouse IgG for synaptophysin (Jackson ImmunoResearch Laboratories, West Grove, PA) that were used to visualize the primary antibodies. After incubation in the secondary antibodies, sections were rinsed, mounted on slides, and covered with a coverslip. Images of labeled neurons in the principal or accessory abducens motor nuclei were obtained using an Olympus Fluoview 1000 laser scanning confocal microscope. Tissue samples were scanned using a 60× 1.4 NA oil immersion objective with dual excitation using a 488 nm argon laser and a 543 nm HeNe laser. Quantification of punctate staining of at least 2-fold greater intensity above background was performed using stereological procedures (23) with MetaMorph software (Universal Imaging Corp., Downingtown, PA). Images of two consecutive optical sections were taken using confocal microscopy. Protein puncta were counted in one optical section (sample section) if they were not present in the optical section immediately below the sample section (lookup section) and if they were within the inclusion boundaries of the unbiased counting frame. Colocalized staining indicating the presence of glutamate receptor subunits at synaptic sites was determined when red and green puncta were immediately adjacent to one another or if they were overlapping.
Real-time RT-PCR
Total RNA was isolated from turtle brain stems using TRI Reagent (Molecular Research Center, Inc.). Real-time RT-PCR was performed using 50 ng of total RNA per reaction. RNA was combined with primer/probe sets and TaqMan Gold RT-PCR master mix (Applied Biosystems, Inc., Foster City, CA). Gene-specific primers and probes were created for P. scripta elegans to total tTLL, both splice variants tTLLs and tTLLc, and turtle actin, using the Primer Express software (Applied Biosystems) as described previously (10). Real-time assays were run on an ABI PRISM 7000 analyzer (Applied Biosystems). The RT-PCR profile consisted of one cycle at 48 °C for 30 min and 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The results of the real-time RT-PCR were normalized to turtle actin. Samples were confirmed to be free of DNA contamination by performing reactions without reverse transcriptase. All reactions were performed twice on each preparation.
Oligomeric Aβ Cleavage Assay
Human synthetic oligomeric Aβ protein (rPeptide; 200 nm) and recombinant proBDNF protein from turtles (50 nm; 10) were incubated together with increasing concentrations of recombinant tTLLs (at 0, 25, 35, 50, 100, 150 nm) in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 5 mm CaCl2 for 1 h at 25 °C. Reactions were stopped by adding SDS-PAGE loading buffer and boiling for 5 min. Assay samples were subjected to SDS-PAGE using 15% gradient gels and stained by primary antibodies to amyloid 1–16 residues (6E10) and proBDNF/BDNF (catalogue number 20981; Santa Cruz Biotechnology). All reactions were performed twice.
RESULTS
Conditioning Is Inhibited by Oligomeric but Not Fibrillar Aβ
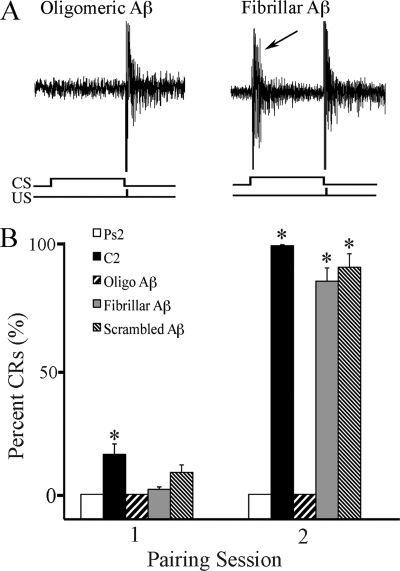
The effects of the different forms of Aβ (oligomeric, fibrillar, and scrambled) on the acquisition of in vitro classical conditioning were examined, and these data are summarized in Fig. 2. Representative abducens nerve recordings are shown from a preparation treated with 200 nm oligomeric Aβ that blocked the expression of CRs but left the unconditioned response unaffected (Fig. 2A, left panel) and a preparation incubated with 200 nm fibrillar Aβ that exhibited conditioning (Fig. 2A, right panel, CR indicated by the arrow). Summaries of the mean percentage of CR acquisition for preparations that received conditioning or pseudoconditioning stimuli and those treated with different forms of Aβ are shown in Fig. 2B. Significant levels of CRs usually begin to be acquired by the second pairing session in normal, untreated preparations (Fig. 2B, C2; n = 10/group) as compared with pseudoconditioning (Ps2; p < 0.0001). Bath application of oligomeric Aβ 1.5 h prior to and during conditioning resulted in no CR acquisition (Fig. 2B; p < 0.0001 versus C2). In contrast, application of fibrillar or scrambled Aβ resulted in a level of CR acquisition similar to untreated preparations (Fig. 2B; p < 0.0001 for both groups versus Ps2). These data provide strong physiological evidence that oligomeric but not fibrillar Aβ suppresses the acquisition of in vitro classical conditioning.
FIGURE 2.
Oligomeric but not fibrillar Aβ attenuates the acquisition of in vitro classical conditioning. A, physiological records of abducens nerve recordings taken from experiments in which brainstems were treated with oligomeric or fibrillar Aβ. Both traces show an abducens nerve unconditioned response recorded in the second pairing session after Aβ application. A CR is indicated (arrow) in the record from a preparation treated with fibrillar Aβ. The CS and US are indicated at bottom. B, acquisition curves of the percentage of CRs during the acquisition phase of conditioning. Oligomeric (Oligo), but not fibrillar or scrambled Aβ peptides, significantly suppressed acquisition of CRs as compared with normal conditioning. Asterisks indicate significant differences from pseudoconditioning (p values under “Results”).
Oligomeric Aβ Suppresses BDNF Expression during Conditioning
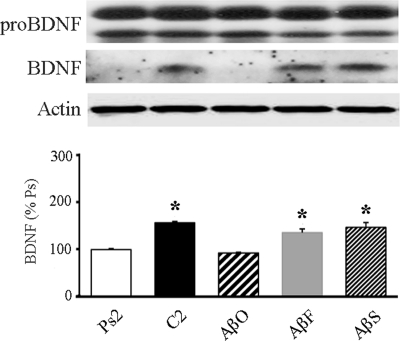
Previous studies showed that expression of BDNF significantly increased during in vitro classical conditioning (10) and plays a key role in signal transduction events leading to synaptic AMPA receptor incorporation and CR acquisition (8, 9). Here, we examined whether oligomeric Aβ inhibits BDNF signaling by reducing its level of expression. Preparations were treated with oligomeric, fibrillar, or scrambled Aβ for 1.5 h prior to and during two pairing sessions of conditioning and subjected to Western blot analysis, which is summarized in Fig. 3 (n = 5/group). The level of BDNF was significantly reduced after oligomeric Aβ treatment as compared with normal conditioning (AβO; p < 0.0001 versus C2). However, neither fibrillar (AβF; p = 0.002 versus Ps2) nor scrambled Aβ (AβS; p < 0.0001 versus Ps2) demonstrated any reduction in BDNF expression during conditioning. These data indicate that oligomeric Aβ treatment interferes with the expression of BDNF during in vitro conditioning.
FIGURE 3.
Oligomeric Aβ attenuates BDNF protein expression during conditioning. Western blots demonstrating that preparations treated by oligomeric Aβ (AβO; 200 nm) for two pairing sessions resulted in suppression of BDNF protein expression are shown, whereas treatment with fibrillar (AβF) or scrambled (AβS) Aβ showed levels of BDNF expression comparable with normal conditioning (C2). Actin loading controls are also shown. % Ps, percentage of pseudoconditioning. Asterisks indicate significant differences from pseudoconditioning (p values under “Results”).
Effect of Oligomeric Aβ on Protein Kinase Phosphorylation during Conditioning
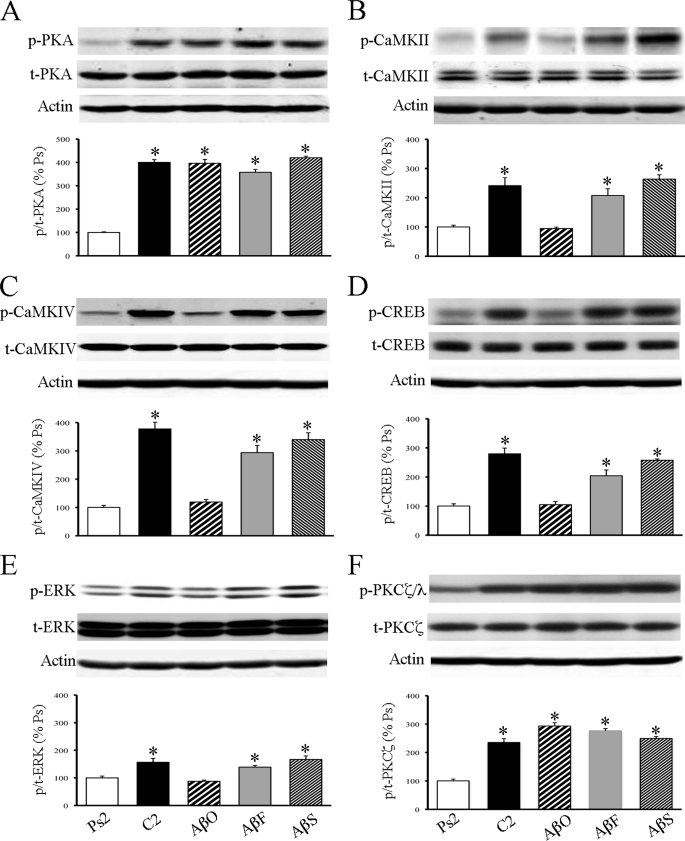
Previously (24, 25), we showed that PKA, CaMKII and CaMKIV, CREB, ERK, and the ζ isoform of PKC were activated during conditioning. To determine whether any of these signaling pathways are affected by oligomeric Aβ treatment during conditioning, Western blot analysis was performed. Incubation of preparations with oligomeric Aβ for 1.5 h prior to and during two sessions of conditioning strongly inhibited the phosphorylation levels of CaMKII, CaMKIV, CREB, and ERK (Fig. 4, B–E; n = 5/group; p < 0.003, AβO versus C2). However, there was no significant effect of oligomeric Aβ on the phosphorylation of PKA (Fig. 4A; p = 0.95, AβO versus C2) or PKCζ/λ (Fig. 4F; p = 0.07, AβO versus C2). In contrast, the phosphorylation levels of all of these signaling proteins were unaffected by treatment with fibrillar or scrambled Aβ and showed high levels of activation after conditioning similar to untreated preparations (Fig. 4, A–F, p < 0.05, AβF and AβS versus Ps2). Taken together, treatment with oligomeric Aβ selectively suppressed the activity-dependent phosphorylation states of several key protein kinases necessary for AMPA receptor trafficking and acquisition of conditioning. Importantly, however, the activity of two other kinases, PKA and PKCζ, at sites phosphorylated by 3-phosphoinositide-dependent protein-kinase-1 (PDK-1) were unaffected by the treatment. We confirmed that the conditioning-related increase in expression of phosphorylated PDK-1 at Ser-241(relative to total PDK-1) was unaffected by treatment with oligomeric, fibrillar, or scrambled Aβ as compared with conditioned preparations (p = 0.42; data not shown).
FIGURE 4.
Oligomeric Aβ blocks the conditioning-related phosphorylation of some protein kinases but not others. Western blots show that treatment with the oligomeric Aβ for two pairing sessions inhibited the phosphorylation levels of CaMKII (B), CaMKIV (C), CREB (D), and ERK (E) to pseudoconditioned values, whereas activation of PKA (A) and PKCζ/λ (F) was not significantly affected. Treatment with fibrillar or scrambled Aβ resulted in little change in the elevated state of protein kinase phosphorylation as compared with normal untreated conditioning. % Ps, percentage of pseudoconditioning. Asterisks indicate significant differences from pseudoconditioning (p values under “Results”).
A Tolloid-like Metalloproteinase Critical for Conditioning, tTLLs, Is Not Suppressed by Oligomeric Aβ
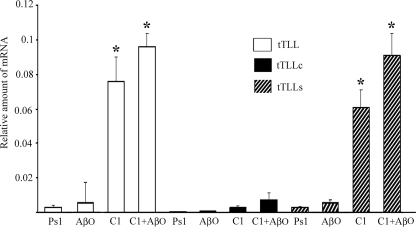
Previously, we showed that a secreted tolloid-like metalloproteinase in turtles, tTLLs, is significantly up-regulated early in conditioning and converts the precursor proBDNF into mature BDNF (10). The conversion of proBDNF to the mature form is a critical step in acquisition of conditioning. Because treatment of preparations with oligomeric Aβ significantly suppressed activity of a number protein kinase signaling pathways described above, we examined the effect of Aβ on the expression of tTLL. Because specific antibodies for the tolloid-like proteins are not available, expression of mRNA levels during conditioning and Aβ treatment was determined by real-time RT-PCR (Fig. 5). After one session of conditioning, the level of total tTLL and tTLLs mRNA was significantly increased above pseudoconditioning levels, whereas a cytosolic form, tTLLc, was not altered (Fig. 5, n = 3/group; tTLLs, p < 0.0001, C1 versus Ps1; tTLLc, p = 0.51, C1 versus Ps1). After treatment with oligomeric Aβ for 1.5 h followed by conditioning, tTLLs was still found to be significantly elevated as compared with pseudoconditioning (p < 0.0001), whereas tTLLc was unchanged by the procedures. Treatment of preparations with oligomeric Aβ alone also failed to alter expression levels of tTLL.
FIGURE 5.
Real-time RT-PCR analysis of the level of the total tolloid-like metalloproteinase (tTLL), cytosolic tTLL (tTLLc), and secreted tTLL (tTLLs) mRNA shows that oligomeric Aβ treatment does not reduce expression of tTLLs. Preparations were conditioned (C1) or pseudoconditioned (Ps1) for one pairing session in normal saline or treated with oligomeric Aβ for the same period of time and not conditioned (AβO) or conditioned in the presence of Aβ (C1+AβO). Error bars are S.D. Asterisks indicate significant differences from pseudoconditioning (p values under “Results”).
Oligomeric Aβ Inhibits Synaptic Delivery of AMPA Receptors
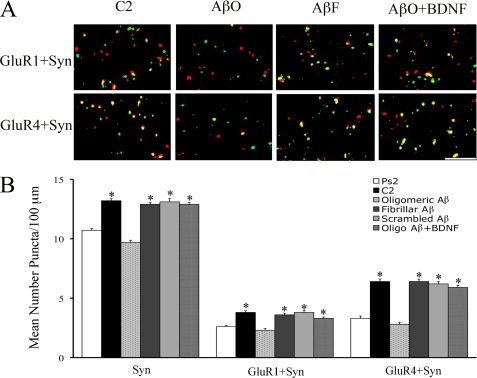
Immunocytochemistry and confocal imaging were performed to assess the synaptic localization of AMPA receptors in abducens motor neurons under conditions in which brain stems were incubated with oligomeric, fibrillar, or scrambled Aβ during the conditioning procedures. Confocal images of punctate staining for the different experimental groups are shown in Fig. 6A, and the results are summarized in Fig. 6B (n = 5/group). Synaptic localization of GluR1- and GluR4-containing AMPA receptors was determined by double-label staining of AMPA receptor subunits (red puncta) with the presynaptic marker synaptophysin (green puncta). Overlapping (yellow puncta) or adjacent puncta indicated colocalization of AMPA receptor subunits at synaptic sites. Conditioning resulted in significantly enhanced punctate staining for synaptophysin (p < 0.0001, C2 versus Ps2) and colocalization of GluR1 (p < 0.0001, C2 versus Ps2) and GluR4 AMPA receptor subunits (p < 0.0001, C2 versus Ps2) with synaptophysin as compared with pseudoconditioning. Bath application of oligomeric Aβ during conditioning not only resulted in complete blockade of the acquisition of CRs but also dramatically suppressed the levels of synaptophysin (p < 0.0001, AβO versus C2) and the synaptic localization of both GluR1 and GluR4 AMPA receptors (p < 0.0001 and p < 0.0001, respectively, versus C2) as compared with the conditioned group (Fig. 6). In contrast, fibrillar and scrambled Aβ had no effect on conditioning-related changes in staining for synaptophysin (p = 0.20, p = 0.69, AβF and AβS, respectively, versus C2) or synaptic delivery of GluR1 and GluR4 AMPA receptors (GluR1, p = 0.56, p = 0.80; GluR4, p = 0.86, p = 0.47; AβF and AβS, respectively, versus C2). Because oligomeric Aβ treatment reduced BDNF expression and BDNF has been previously shown to be essential for synaptic delivery of AMPA receptors during conditioning, we examined whether inhibition of AMPA receptor trafficking by oligomeric Aβ could be rescued by coapplication of human recombinant BDNF. As shown in Fig. 6, oligomeric Aβ plus recombinant human BDNF (100 ng/ml; Santa Cruz Biotechnology) resulted in increased levels of synaptophysin (p < 0.0001) and synaptic incorporation of GluR1- and GluR4-containing AMPA receptors (GluR1, p < 0.0001, GluR4, p < 0.0001, Oligo-Aβ + BDNF versus Ps2) that were similar to conditioned values. Collectively, these findings strongly support the conclusion that disruption of AMPA receptor synaptic delivery by oligomeric Aβ is due, at least in part, to disrupting BDNF signaling during conditioning.
FIGURE 6.
Synaptic localization of GluR1- and GluR4-containing AMPA receptors is inhibited by oligomeric Aβ treatment during conditioning and rescued by BDNF. A, confocal images of GluR1 or GluR4 AMPA receptor subunits (red) and colocalization with synaptophysin (Syn; green) punctate staining. Overlapping (yellow) or adjacent puncta indicate colocalization. C2, normal conditioning. B, quantitative analysis of colocalized staining for GluR1+Syn or GluR4+Syn for the different treatment groups. Treatment with oligomeric Aβ during conditioning resulted in inhibition of CRs and synaptic delivery of GluR1 and GluR4 subunits, whereas application of fibrillar or scrambled Aβ resulted in CR expression and levels of colocalized staining similar to untreated conditioned preparations. The inhibition of colocalized staining observed after oligomeric Aβ treatment was reversed by coapplication of oligomeric Aβ with BDNF. Scale bar = 2 μm. Asterisks indicate significant differences from pseudoconditioning (Ps2) (p values under “Results”).
In Vitro Cleavage Assays of Oligomeric Aβ and proBDNF by tTLLs
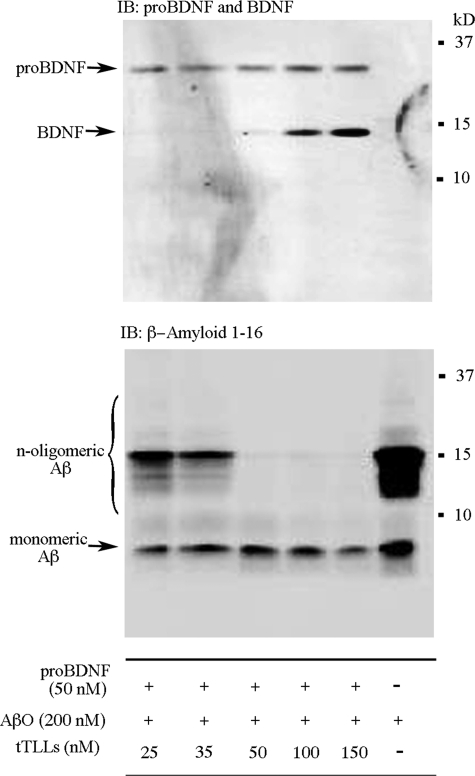
Because some matrix metalloproteinases are known to degrade Aβ (26), we determined whether the tolloid-like metalloproteinase tTLLs could also cleave oligomeric Aβ using in vitro assays. Human synthetic oligomeric Aβ (200 nm) either alone or with recombinant proBDNF from turtle and increasing concentrations of recombinant tTLLs were incubated for 1 h at 25 °C. As shown in Fig. 7 (lower panel), there was a concentration-dependent degradation of oligomeric Aβ into small fragments by tTLLs with effective concentrations beginning at 25 nm (10 nm showed very little cleavage), and complete degradation was observed at 50 nm. Similar results were observed with human plasmin (not shown). Examination of the time course of Aβ (200 nm) degradation by tTLLs (50 nm) in in vitro assays showed that cleavage of oligomers is detectable after 45 min and is complete by 1 h of incubation (data not shown). Interestingly, determination of the levels of proBDNF and mature BDNF in these same assays showed that higher concentrations of tTLLs starting at 100 nm were required for conversion of turtle proBDNF into BDNF (Fig. 7, upper panel). These results suggest that tTLLs preferentially degrades n-meric forms of Aβ, the most toxic forms, at far lower concentrations than proBDNF.
FIGURE 7.
The tolloid-like metalloproteinase, tTLLs, cleaves oligomeric Aβ at lower concentrations than proBDNF in in vitro cleavage assays. Synthetic human oligomeric Aβ was incubated either alone or with recombinant turtle proBDNF and increasing concentrations of tTLLs for 1 h at 25 °C. Soluble Aβ, particularly the oligomeric forms, was likely cleaved into small fragments by tTLLs, which was complete at 50 nm. In the same assays, tTLLs began to cleave proBDNF into BDNF at about 100 nm. IB, immunoblot.
Oligomeric Aβ Interferes with Proteolytic Conversion of BDNF in Brainstem Preparations
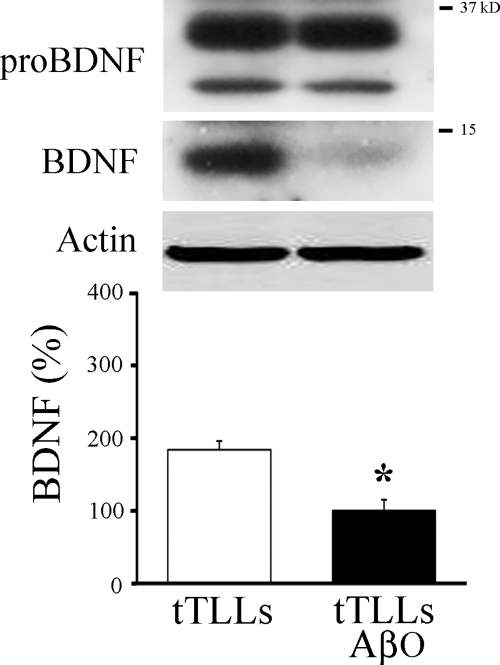
Previously, we showed that incubation of preparations in tTLLs alone resulted in expression of mature BDNF (10). To determine whether there was any apparent competition between oligomeric Aβ and proBDNF for cleavage by tTLLs in brainstem preparations, they were incubated either for 3 h in tTLLs (20 nm) alone or in oligomeric Aβ (200 nm) for 1.5 h followed by tTLLs for an additional 3 h (Fig. 8). Western blot analysis confirmed that incubation of brainstems in tTLLs resulted in expression of BDNF. However, the addition of oligomeric Aβ resulted in a significant reduction in the production of BDNF by tTLLs (Fig. 8; p = 0.04, n = 3/group).
FIGURE 8.
Incubation of in vitro brainstem preparations in tTLLs resulted in expression of mature BDNF protein that was inhibited by oligomeric Aβ. Western blots of proBDNF and mature BDNF show expression of BDNF protein after incubation in tTLLs alone. Incubation in tTLLs and oligomeric Aβ (200 nm) resulted in significant attenuation of BDNF expression. Asterisks indicate significant differences from pseudoconditioning (p values under “Results”).
DISCUSSION
Using an in vitro model of classical conditioning, application of oligomeric Aβ blocked acquisition of conditioning that was associated with inhibition of mature BDNF protein expression, suppressed phosphorylation of key signal transduction elements, and reduced AMPA receptor synaptic delivery. Our previous studies (8–10) revealed a critical role for BDNF signaling in AMPA receptor trafficking during conditioning. Therefore, the Aβ-induced inhibition of synaptic incorporation of these receptors is a likely consequence of the significant reduction in BDNF. Phosphorylation of the critical protein kinases CaMKII, CaMKIV, CREB, and ERK was also reduced by oligomeric Aβ that contributed to inhibition of BDNF expression and AMPA receptor trafficking. Importantly, we also found that oligomeric Aβ appears to interfere with the normal function of a learning-induced extracellular tolloid-like protease, tTLLs, which is to cleave proBDNF to mature BDNF. Therefore, the reduction in mature BDNF induced by Aβ may be a secondary effect of its ability to “bait” proteolytic processing machinery away from its normal targets during learning.
We previously reported that synaptic delivery of GluR1-containing AMPA receptors to activate silent auditory nerve synapses occurs during early stages of conditioning. This step is followed by synthesis and insertion of GluR4 AMPA receptor subunits that support the acquisition of CRs (24, 25, 27). The mature form of BDNF is required for delivery of AMPA receptors and subsequent CR acquisition (8–10). The present study shows that oligomeric Aβ, but not the less toxic fibrillar form, significantly inhibits the synaptic delivery of GluR1- and GluR4-containing AMPA receptors during conditioning. Significantly, preincubation of preparations with BDNF reversed the effects of oligomeric Aβ on AMPA receptor trafficking and conditioning. Consistent with these findings, studies using transgenic mice that overexpress APP showed deficits in learning behavior, long term potentiation, a selective reduction in AMPA receptor-mediated currents, and decreased synaptic AMPA receptor number (2–5). Evidence suggests that the Aβ-induced decline in synaptic AMPA receptors may be achieved through endocytotic or apoptotic pathways (5, 28). For example, Aβ overexpression resulted in AMPA receptor endocytosis and depression of synaptic transmission (5). On the other hand, activation of cysteine proteases called caspases by Aβ results in impairment in CA1 hippocampal long term potentiation (29), possibly by suppressing AMPA receptor trafficking (28), although the mechanisms are not well understood. These studies support the idea that oligomeric Aβ disrupts learning by interfering with AMPA receptor synaptic delivery.
Activation of BDNF and AMPA receptor synaptic incorporation during in vitro classical conditioning requires a number of signal transduction molecules. As we reported earlier (25), conditioning involves the early activation of PKA, CaMKII, CaMKIV, and CREB followed by expression of mature BDNF and activation of ERK to trigger AMPA receptor synaptic delivery. Here, we observed that oligomeric Aβ treatment significantly inhibited the phosphorylation of many of these key signal transduction elements, namely CaMKII (Thr-286), CaMKIV (Thr-196), CREB (Ser-133), and ERK (Thr-183/185). However, the conditioning-induced increase in phosphorylation levels of PKA (Thr-197) and PKCζ/λ (Thr-410/403) remained unchanged. In agreement with these findings, Aβ application to hippocampal slices inhibited activity-dependent phosphorylation of CaMKII (Thr-286) and GluR1 (Ser-831) at the CaMKII-dependent site, but the phosphorylation state of GluR1 (Ser-845) at the PKA site was unchanged (30). Townsend et al. (31), using pharmacological stimulation of hippocampal cell cultures treated with soluble Aβ, also reported disrupted activation of CaMKII (Thr-286), ERK (Thr-202/Tyr-204), and Akt/PKB (Ser-473), whereas PKA and PKC (Ser-660; their antibody recognized multiple isoforms of PKC but not λ or ζ) showed normal activation. Recently, Gu et al. (4) reported that the phosphorylation level of CaMKII (Thr-286) was significantly reduced in synaptosomal fractions from APP transgenic mice. There is disagreement concerning the effect of oligomeric Aβ treatment on the activity of ERK. Rat hippocampal slice cultures exposed to 1 mm Aβ oligomers for 10–24 h induced ERK activation accompanied by cell death (32). Furthermore, hippocampal slices exposed to 100 nm Aβ oligomers increased ERK activity after 5 min, but long term exposure led to a later peak in ERK activation after 8 h followed by down-regulation (33), suggesting that different Aβ preparations, concentration, and treatment protocols contribute to differential effects on ERK activation. Significantly, exposure of brain stem preparations to oligomeric Aβ had no negative impact on phosphorylation levels of PKA (Thr-197) or PKCζ/λ (Thr-410/403), although conditioning was suppressed. Both of these sites are targeted by PDK-1. We confirmed that the conditioning-related increase in expression of phosphorylated PDK-1 at Ser-241 was not significantly affected by oligomeric Aβ treatment. It was reported that phosphorylation of Akt/PKB (Ser-473), another PDK-1-dependent kinase, was inhibited by Aβ in mouse neurons, but the site examined was not the PDK-1-sensitive site (31). Interestingly, a Chinese medicinal herb (oleander) was reported to have neuroprotective effects on cortical cell cultures from Aβ toxicity and acts to inhibit caspase-3 activity and stimulate phosphorylation of PDK-1 (34). During in vitro classical conditioning, activation of PDK-1 is the earliest identified phosphorylation event following paired CS-US stimulation and is key for downstream activation of PKA and PKC. However, activation of these kinases alone is not sufficient for AMPA receptor synaptic delivery and acquisition of conditioning. Other steps in this signal transduction cascade are clearly required, possibly the combined activation of PKA with CaMKII.
Expression of BDNF has been shown to be significantly reduced in AD and mildly cognitively impaired patients (12–16). Recently, the BDNF gene delivered into amyloid-transgenic mice was found to reverse synapse loss, result in partial recovery of synaptic markers, and restore learning and memory (35). BDNF also plays a critical role in regulating expression and synaptic delivery of AMPA receptor subunits (6–10). Therefore, the reduction in BDNF levels associated with AD and its link to AMPA receptor trafficking may be an underlying mechanism for deficits in learning and memory. What leads to the Aβ-induced decline in BDNF protein? There is a diverse set of extracellular proteolytic enzymes, such as matrix metalloproteinases, insulin-degrading enzyme, and plasmin, that have been shown to degrade aggregated forms of Aβ (26, 36, 37). Our study revealed that an extracellularly secreted tolloid-like metalloproteinase, tTLLs, degrades oligomeric Aβ, especially the dimers and trimers, into small fragments in in vitro assays. In normal conditioning, tTLLs mRNA is transiently expressed in the early stages of conditioning and functions to convert the precursor proBDNF into mature BDNF, which is required for AMPA receptor synaptic delivery (10). In the presence of oligomeric Aβ, tTLLs is still expressed but appears to preferentially cleave Aβ instead of proBDNF, resulting in reduced levels of BDNF. Consistent with this finding, brain stem preparations incubated in recombinant tTLLs alone expressed high levels of BDNF protein. However, the addition of Aβ to the medium significantly attenuated BDNF expression. Therefore, oligomeric Aβ may compete with proBDNF as a target for cleavage, thereby reducing BDNF protein expression. An alternative to alterations in proteolytic processing as an explanation for reduced BDNF protein is that Aβ affects transcriptional control. Using human neuroblastoma SH-SY5Y cells, oligomeric Aβ resulted in decreased levels of specific BDNF mRNA transcripts (15). In our preparation, the overall level of proBDNF protein did not change after Aβ treatment, but we did not detect individual mRNA transcripts to determine whether they are selectively regulated during learning or by Aβ. In humans and rodents, there are a number of identified transcripts produced by the BDNF gene controlled by separate promoters (38) that have the ability to produce mature BDNF. There are also multiple transcripts of BDNF mRNA in turtle.3 The details of how these are independently regulated in learning and affected by Aβ require further study.
This work was supported, in whole or in part, by National Institutes of Health Grants NS051187 and P20 RR015567, which is designated as a Center of Biomedical Research Excellence (to J. K.).
Z. Zheng, B. Sabirzhanov, and J. Keifer, unpublished data.
- AD
- Alzheimer disease
- Aβ
- amyloid-β
- AβO
- oligomeric Aβ
- AβF
- fibrillar Aβ
- AβS
- scrambled Aβ
- APP
- amyloid precursor protein
- BDNF
- brain-derived neurotrophic factor
- CR
- conditioned response
- CREB
- cAMP-response element-binding protein
- tTLLs
- turtle tolloid-like protein
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- DMSO
- dimethyl sulfoxide
- p
- phosphorylated
- CS
- conditioned stimulus
- US
- unconditioned stimulus
- t
- total.
REFERENCES
- 1.Shankar G. M., Walsh D. M. (2009) Mol. Neurodegener. 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang E. H., Savage M. J., Flood D. G., Thomas J. M., Levy R. B., Mahadomrongkul V., Shirao T., Aoki C., Huerta P. T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3410–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida C. G., Tampellini D., Takahashi R. H., Greengard P., Lin M. T., Snyder E. M., Gouras G. K. (2005) Neurobiol. Dis. 20, 187–198 [DOI] [PubMed] [Google Scholar]
- 4.Gu Z., Liu W., Yan Z. (2009) J. Biol. Chem. 284, 10639–10649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. (2006) Neuron 52, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldeira M. V., Melo C. V., Pereira D. B., Carvalho R., Correia S. S., Backos D. S., Carvalho A. L., Esteban J. A., Duarte C. B. (2007) J. Biol. Chem. 282, 12619–12628 [DOI] [PubMed] [Google Scholar]
- 7.Nakata H., Nakamura S. (2007) FEBS Lett. 581, 2047–2054 [DOI] [PubMed] [Google Scholar]
- 8.Li W., Keifer J. (2008) Neuroscience 155, 686–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Keifer J. (2009) Neurobiol. Learn. Mem. 91, 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keifer J., Sabirzhanov B. E., Zheng Z., Li W., Clark T. G. (2009) J. Neurosci. 29, 14956–14964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itami C., Kimura F., Kohno T., Matsuoka M., Ichikawa M., Tsumoto T., Nakamura S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13069–13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor B., Young D., Yan Q., Faull R. L., Synek B., Dragunow M. (1997) Brain Res. Mol. Brain Res. 49, 71–81 [DOI] [PubMed] [Google Scholar]
- 13.Holsinger R. M., Schnarr J., Henry P., Castelo V. T., Fahnestock M. (2000) Brain. Res. Mol. Brain Res. 76, 347–354 [DOI] [PubMed] [Google Scholar]
- 14.Peng S., Wuu J., Mufson E. J., Fahnestock M. (2005) J. Neurochem. 93, 1412–1421 [DOI] [PubMed] [Google Scholar]
- 15.Garzon D. J., Fahnestock M. (2007) J. Neurosci. 27, 2628–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapia-Arancibia L., Aliaga E., Silhol M., Arancibia S. (2008) Brain Res. Rev. 59, 201–220 [DOI] [PubMed] [Google Scholar]
- 17.Komulainen P., Pedersen M., Hänninen T., Bruunsgaard H., Lakka T. A., Kivipelto M., Hassinen M., Rauramaa T. H., Pedersen B. K., Rauramaa R. (2008) Neurobiol. Learn. Mem. 90, 596–603 [DOI] [PubMed] [Google Scholar]
- 18.Anderson C. W., Keifer J. (1999) J. Neurophysiol. 81, 1242–1250 [DOI] [PubMed] [Google Scholar]
- 19.Keifer J., Armstrong K. E., Houk J. C. (1995) J. Neurosci. 15, 5036–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keifer J. (2001) J. Neurosci. 21, 2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlgren K. N., Manelli A. M., Stine W. B., Jr., Baker L. K., Krafft G. A., LaDu M. J. (2002) J. Biol. Chem. 277, 32046–32053 [DOI] [PubMed] [Google Scholar]
- 22.Trommer B. L., Shah C., Yun S. H., Gamkrelidze G., Pasternak E. S., Stine W. B., Manelli A., Sullivan P., Pasternak J. F., LaDu M. J. (2005) Neurobiol. Dis. 18, 75–82 [DOI] [PubMed] [Google Scholar]
- 23.Mokin M., Keifer J. (2006) J. Neurosci. Methods 157, 218–224 [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z., Keifer J. (2008) Neuroscience 156, 872–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Z., Keifer J. (2009) J. Neurophysiol. 101, 2539–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan P., Hu X., Song H., Yin K., Bateman R. J., Cirrito J. R., Xiao Q., Hsu F. F., Turk J. W., Xu J., Hsu C. Y., Holtzman D. M., Lee J. M. (2006) J. Biol. Chem. 281, 24566–24574 [DOI] [PubMed] [Google Scholar]
- 27.Mokin M., Zheng Z., Keifer J. (2007) J. Neurophysiol. 98, 1278–1286 [DOI] [PubMed] [Google Scholar]
- 28.Lu C., Wang Y., Furukawa K., Fu W., Ouyang X., Mattson M. P. (2006) J. Neurochem. 97, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 29.Minogue A. M., Schmid A. W., Fogarty M. P., Moore A. C., Campbell V. A., Herron C. E., Lynch M. A. (2003) J. Biol. Chem. 278, 27971–27980 [DOI] [PubMed] [Google Scholar]
- 30.Zhao D., Watson J. B., Xie C. W. (2004) J. Neurophysiol. 92, 2853–2858 [DOI] [PubMed] [Google Scholar]
- 31.Townsend M., Mehta T., Selkoe D. J. (2007) J. Biol. Chem. 282, 33305–33312 [DOI] [PubMed] [Google Scholar]
- 32.Chong Y. H., Shin Y. J., Lee E. O., Kayed R., Glabe C. G., Tenner A. J. (2006) J. Biol. Chem. 281, 20315–20325 [DOI] [PubMed] [Google Scholar]
- 33.Bell K. A., O'Riordan K. J., Sweatt J. D., Dineley K. T. (2004) J. Neurochem. 91, 349–361 [DOI] [PubMed] [Google Scholar]
- 34.Yu M. S., Lai S. W., Lin K. F., Fang J. N., Yuen W. H., Chang R. C. (2004) Int. J. Mol. Med. 14, 917–924 [PubMed] [Google Scholar]
- 35.Nagahara A. H., Merrill D. A., Coppola G., Tsukada S., Schroeder B. E., Shaked G. M., Wang L., Blesch A., Kim A., Conner J. M., Rockenstein E., Chao M. V., Koo E. H., Geschwind D., Masliah E., Chiba A. A., Tuszynski M. H. (2009) Nat. Med. 15, 331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker H. M., Kihiko M., Caldwell J. N., Wright S., Kawarabayashi T., Price D., Walker D., Scheff S., McGillis J. P., Rydel R. E., Estus S. (2000) J. Neurosci. 20, 3937–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Strooper B. (2010) Physiol. Rev. 90, 465–494 [DOI] [PubMed] [Google Scholar]
- 38.Pruunsild P., Kazantseva A., Aid T., Palm K., Timmusk T. (2007) Genomics 90, 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]