Abstract
In this study, we examined the effects that antifreeze proteins have on the supercooling and ice-nucleating abilities of aqueous solutions. Very little information on such nucleation currently exists. Using an automated lag time apparatus and a new analysis, we show several dilution series of Type I antifreeze proteins. Our results indicate that, above a concentration of ∼8 mg/ml, ice nucleation is enhanced rather than hindered. We discuss this unexpected result and present a new hypothesis outlining three components of polar fish blood that we believe affect its solution properties in certain situations.
Keywords: Blood, Glycoprotein, Peptide Conformation, Peptide Interactions, Plasma, Protein Chemistry, Antifreeze, Heterogeneous, Ice, Nucleation
Introduction
In Antarctic and Arctic fishes, ice crystals suspended in the salt water column are ingested into the hypo-osmotic intestinal tract, where antifreeze molecules inhibit them from growing. Depending on the fish species, these antifreeze molecules may be peptides (AFPs)2 or glycopeptides (AFGPs), for which the kinetic effects on the growth of ice are well documented (1–3). These molecules depress the ice growth temperature, sometimes called the non-equilibrium freezing point, more than the colligative (equilibrium) melting point and so produce a thermal hysteresis of magnitude up to ∼1.2 °C. In fish, there are at least five distinct types of AFP and AFGP known, all with differing primary sequences and tertiary structures. In this study, we typically use the term AFP to encompass both AFP and AFGP. Many freeze-tolerant and freeze-avoiding insects also produce AFP molecules, but they are usually referred to as thermal hysteresis proteins.
In addition to inhibiting the growth of macroscopic ice crystals in the fish's intestinal tract, it is speculated that an important function of AFP activity is also to depress the rate or efficiency of ice nucleation in the supercooled blood, tissues and other body fluids. To our knowledge, we exhibit here rigorous data on this effect for the first time.
Inoculation of ice in the bloodstream is, in some cases, seasonal, and details are not accurately known for either Arctic or Antarctic species. Throughout the life of the fish, >40 years in some cases, volumes of blood are supercooled by as much as 1 °C. This provides a driving force for ice nucleation. There are, however, conflicting reports on the effect that AFPs have on the ability of solutions to supercool, i.e. on the heterogeneous nucleation temperature (Thet). We summarize some of those reports here.
Parody-Morreale et al. (4) reported that AFGP molecules from the Antarctic notothenioid Dissostichus mawsoni inhibit the ice-nucleating activity of membrane vesicles from the bacterium Erwinia hebicola. Using a drop-freezing assay to characterize the populations of ice nucleators, they reported saturation at high AFGP concentrations and concluded that AFGPs bind to and inhibit ice-nucleating proteins. Similar experiments have shown that insect thermal hysteresis proteins also have the ability to inhibit certain ice-nucleating proteins (5).
Conversely, Franks et al. (6) found that AFGP is not able to depress the homogeneous nucleation temperature any more than polyvinylpyrrolidone, a synthetic polymer lacking hysteretic activity. This suggests that the AFGP molecules cannot inhibit the embryonic clusters and exhibit hysteretic activity only when macroscopic ice is present and the Kelvin effect (7) is invoked. Holt (8) found that AFGP reduces the nucleation temperature of tap water but enhances the nucleation activity of bacterial nucleators. At low concentrations, Type III AFP reduces the nucleation temperature of both tap water and the bacterial nucleator. At high concentrations, Type III AFP enhances the nucleation temperature of the bacterial nucleator.
Wilson and Leader (9) found that the addition of AFGP molecules to solutions containing ice-nucleating proteins lowers Thet by ∼2 °C. They hypothesized that the AFGP molecules mask likely nucleation sites and reduce the temperature at which nucleation occurs.
Alternatively, Zachariassen et al. (10) argued that thermal hysteresis protein molecules bind to ice nuclei at the subcritical size and poison ice growth, thus rendering the system effectively supercooled. Using a nanoliter osmometer, they observed that, as the ice fraction was reduced to the critical nucleus size, the thermal hysteresis might be as much as 25 °C, which makes the insects in question appear to be supercooled.
There have also been several studies in which AFPs have been added to cryopreservation solutions in attempt to enhance vitrification abilities (11). Chang et al. (12) found a increase in vitrification when thermal hysteresis protein from the mealworm Tenebrio molitor was added to glycerol solutions. Sutton and Pegg (13) speculated that this result is due to impurities in the crude extract.
Du et al. (14) studied ice nucleation inhibition in microsized water droplets with added Type III AFP molecules. Using a relatively small number of samples and concentrations of AFP of up to 0.25% by weight, they reported inhibition of nucleation efficiency. However, their analysis and explanation relied heavily on classical nucleation theory (as designed for the gas-liquid system). The associated capillarity approximation is not readily transferrable to the liquid-solid system. Du et al. (14) attributed any observed inhibition of nucleation to interaction between AFP molecules and both ice nuclei and foreign (dust) particles. Following the work outlined in Ref. 14, the same group (15) reported on the effects of Type III AFP on the nucleation characteristics of nanosized particles. They discussed the self-aggregation of AFP molecules and reported on dynamic light scattering measurements showing that Type III AFP molecules appear to aggregate at concentrations of 2.5 mg/ml and above. Subsequently, they reported (16) on surface tension measurements that also showed that 2.5 mg/ml is the critical aggregation concentration and that self-aggregates may effect ice nucleation We discuss this further below.
In 2002, using an early version of an automatic lag time apparatus (ALTA) (17), we gathered statistical data on the heterogeneous nucleation properties of serum from the Antarctic notothenioid Pagothenia borchgrevinki. We observed an unusual range of nucleation temperatures but were unable to verify our results.3 We believed that the range of nucleation temperatures was attributed to molecules or particles, other than AFGP, acting as nucleation sites.
In 2005, we repeated the 2002 experiments using a partially purified AFGP from the Antarctic notothenioid D. mawsoni.3 Again, we expected that the nucleation efficiency and Thet of a solution in a given container would lower in the presence of the AFGP molecules. We found instead that AFGP enhanced nucleation above a certain concentration, and again, we could not validate these results. The counterintuitive nature of the results of these previous studies therefore initiated this work.
Our current methods allow us to accurately determine the Thet of aqueous solutions in an automated way. The measurements described below were designed to determine whether, and by how much, type I AFP molecules alter Thet compared with a control. In summary, we performed several experiments, each of which consisted of at least 300 cycles, in which a single (presumably) unchanging sample was cooled, supercooled, nucleated (at a temperature well below its equilibrium melting and non-equilibrium freezing points), and then warmed until all of the ice was melted. The average Thet was recorded for each set of 300 cycles. In this way, a dilution series was carried out for various concentrations of AFP in NaCl, including zero AFP.
EXPERIMENTAL PROCEDURES
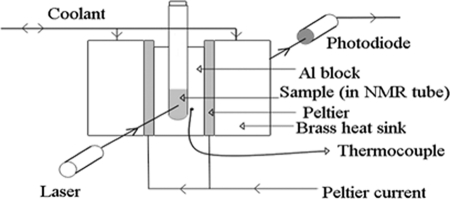
ALTA has been described in detail previously (17–19). It consists of a cooling block with dimensions of ∼5 × 5 × 1 cm that holds a 200-μl sample in a glass tube. Two Peltier devices are used to accurately control the heating and cooling rate of the block using a custom-designed computer program. A white light-emitting diode shines light through the sample block and sample tube, and a photodiode detector measures the intensity of light passing through the sample (Fig. 1). When ice is formed, the tube becomes suddenly more opaque. By monitoring the change in intensity of the light received by the photodiode and using an electronic comparator, it is possible to detect the ice formation point. The entire system is automated.
FIGURE 1.
Experimental arrangement of ALTA. Liquid samples in a glass tube are repeatedly cooled until frozen, warmed to +15 °C, and then cooled again.
In these experiments, the sample was cooled at a constant rate of 0.2 °C s−1 until ice formation was detected. The temperature was measured inside the block, close to the tube, to avoid having the PT100 inside the solution. This caused a time lag between the block and sample itself, but previous measurements have shown this to be unimportant because it is constant over the entire run, i.e. it tracks the temperature profile of the sample very closely.
Only the temperature was logged, and as soon as freezing was detected, the current to the Peltier devices was reversed, and the temperature was ramped back up to approximately +15 °C, where the ice crystals were melted, and the light intensity received by the detector returned to its previous value. The sample typically spent ∼110 s above the melting point to ensure that it had completely melted. However, if the ice was not melted completely, it was not possible to supercool the solution on the next run. Although we are actually measuring detection of the ice growth process and not the nucleation event per se, we have also found that, with a 200-μl water sample supercooled by, for example, 14 °C, the freeze event is very rapid compared with the time spent supercooled and need not be taken into consideration because the time offset is again constant between runs and is <0.5 s.
All water used was distilled, de-ionized, and filtered and had a conductivity of 17 megohms·cm or higher. To ensure full dissolution of the lyophilized AFP, we used aqueous solutions of NaCl (reagent-grade from Fischer) at either 300 or 500 mm to make up the starting solution for each dilution series. AFP was from A/F Protein Canada 2000 Inc. and was Type I from winter flounder (Pleuronectes americanus) lyophilized and made up to either 32 or 40 mg/ml at the start of each dilution series. Type I AFPs are alanine-rich with a molecular mass of between 3300 and 4500 Da and an α-helix conformation. The purity of this commercially available protein is unknown.
RESULTS
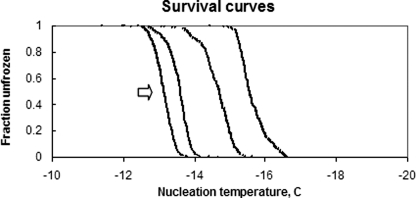
The nucleation temperatures for multiple cycles of one experiment were plotted as a histogram referred to a “Manhattan” (17) and are shown in Fig. 2. By sorting the nucleation temperatures from each Manhattan plot and plotting the fraction of runs unfrozen as a function of temperature, referred to as a “survival curve” (Fig. 3), the inherent spread of nucleation temperatures of any given sample can be easily analyzed. It can be seen in the left trace, for example, that, in all cases, the sample was still unfrozen at −12 °C but was always frozen by −14 °C. This statistical distribution is reproducible and is a fundamental property of the solution. Fig. 3 shows four of the survival curves for one of the dilution series concentrations. From left to right, the concentrations were 2, 4, 16, and 8 mg/ml (dissolved in 500 mm NaCl).
FIGURE 2.
Typical Manhattan plot. In this case, the plot is for AFP at 0.5 mg/ml dissolved in 500 mm NaCl, frozen, and thawed ∼630 times. The inherent stochastic nature of heterogeneous nucleation is evident.
FIGURE 3.
Plots showing some of the survival curves for one of the dilution series. From left to right, the concentrations were 2, 4, 16, and 8 mg/ml (dissolved in 500 mm NaCl). Clearly, 8 mg/ml AFP suppressed nucleation more efficiently than either lower or higher concentrations.
We have previously shown that the 10–90 width of these asymptotic survival curves is very close to the full width at half-height of the first derivative of the data (17), which is the nucleation probability distribution. Also, we have previously defined the Thet of any given sample and tube as the 50% level (i.e. T50%), as indicated by the arrow in Fig. 3 (18).
The above survival curves illustrate the need for an ALTA-type measure of Thet. If the measurements are done on the same sample only a few times or indeed on different samples, the results are just as likely to include two cold values as they are to include a cold and a hot value. Below, we now quote only the T50% for each AFP concentration with the understanding that it represents the T50% of such a survival curve. It should also be noted that previous work has shown that the slope of survival curves can be remarkably similar, regardless of the solution type (20).
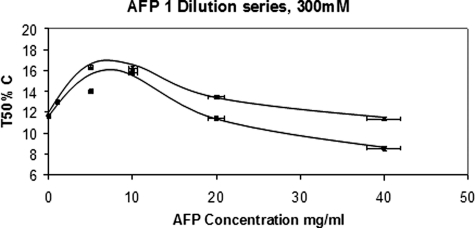
Fig. 4 shows two dilution series, both with AFP dissolved in 300 mm NaCl. The increase in levels of supercooling at ∼8 mg/ml AFP is real because the accuracy of the T50% measurement is better than 0.2 °C. It is interesting and surprising that, at higher protein concentrations, the nucleation efficiency was actually enhanced. The same glass tube was used in both series, and we observed that the T50% for water only was the same. This was expected because the heterogeneous nucleation is almost certainly occurring at the tube wall (20). Previous work with hydrophobic monolayers and a selection of various tubes has confirmed that assumption (21).
FIGURE 4.
These two dilution series show that the AFPs lowered Thet significantly at ∼8 mg/ml but that the nucleation process was actually enhanced at higher concentrations.
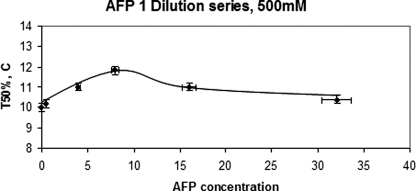
In all cases, the T50% values plotted had the melting point depression factor of the NaCl subtracted as well as another factor, often called λ. This is the level at which Thet is lowered due to the addition of solutes. We have recently determined this factor for both NaCl and glucose, and in both cases, it is 2.0 (22). This factor has been known to be 2.0 for homogeneous nucleation for some time, at least for low molecular weight solutes such as NaCl (23, 24). We also assumed complete dissociation of the NaCl. We did not subtract the melting point depression due specifically to the colligative nature of the Type I AFPs because, at 40 mg/ml, this is only 0.2 °C and even less at the lower concentrations. Factoring it in would not change the shape of the curves presented here by any significant amount. Fig. 5 shows a dilution series in which AFP was dissolved in 500 mm NaCl. A different glass tube was used, and we observed that, at zero AFP, Thet was different for that tube compared with the tube used for the data shown in Fig. 4. As mentioned above, this is because the nucleation is almost certainly occurring at some defect or “site” on the tube wall, at least at zero concentration of AFP. We also observed that the trend in nucleation efficiency was remarkably similar to that found with AFP dissolved in 300 mm NaCl (Fig. 4). At first glance, it would seem that there is an optimum concentration of AFP required to lower Thet (of ∼8 mg/ml). We discuss this possibility below.
FIGURE 5.
Another dilution series in a different glass tube in which AFP was dissolved in 500 mm NaCl. Again, at a concentration of ∼8 mg/ml, the nucleation was hindered the most.
DISCUSSION
We now introduce a new possible explanation of the above data and our earlier data on AFGP. We postulate that there are three components of polar fish blood that affect its solution properties as the temperature is changed.
We postulate a first component, denoted “S,” which includes sugars, salts, and other simple solute effects on nucleation, and the effect of these is colligative, i.e. if pure water has a T50% of approximately −11 °C in a certain glass tube, adding sucrose or other simple solutes will depress the equilibrium freezing point colligatively, and the whole nucleation survival curve will shift by a fixed amount, depending on λ. This is now well understood and not controversial (22).
We postulate a second component, denoted “I,” and hypothesize that this is the AFP (or AFGP) molecules. This component moves the T50% to colder temperatures either by masking possible nucleation sites or by binding to embryonic ice crystals or some combination of the two. This would hinder nucleation and require that any successful nucleation occur at another site and hence at a colder temperature. This component is relatively well established.
We postulate a third component, denoted “N,” and this is yet to be identified at the molecular level. This moves the T50% to warmer temperatures, and this is the component that accelerates nucleation. In the data presented here, as the concentration increases, eventually this component swamps the effects of the other two components. This is the least well established component. Ultimately, we plan to demonstrate the molecular nature of this component but have not yet performed a molecular level identification, hence the careful phrasing of our explanation as a hypothesis to be tested by further work. Experiments with synthetically made AFP (or AFGP; and hence free of as yet uncharacterized “impurities” or “potentiators” in naturally occurring fish blood) are one obvious further route of investigation. Using pure substances may also allow us to determine whether self-aggregation is the cause of the increased nucleation efficiency.
Historically, there has been difficulty in accurately determining the supercooling ability of solutions. Because each different solution in a different container will, by definition, have a different “most efficient nucleation site,” each solution will have a different nucleation temperature. Workers have often sought to overcome this problem by looking at hundreds of similar volume droplets from the same bulk solution and comparing some sort of average nucleation temperature with that of a different bulk solution (4). Using the ALTA technique described above eliminates this multiple nucleation site issue but does introduce another factor for consideration, namely the question of whether multiple freeze-thaw cycles on the same sample are a problem. We have previously shown that, for simple inorganic solutions, this is certainly not an issue (22), but multiple temperature cycles on a protein solution may present problems. However, when we cycled ALTA with a single Type I AFP solution and changed nothing but the temperature over, for example, 300 cycles, we observed that the survival curve held its shape. Indeed, it is the same shape as any of the inorganic solutions we have tested over many years (17–22). This is strong evidence that the constituents of the solution, including the proteins, are not in any way changing to a degree that affects either the colligative properties of the solution or any interactions the protein molecules might have with the nucleation site or with initial ice growth.
It is worth noting here that the concentration of AFP molecules in the blood of fishes containing AFP is typically between 10 and 35 mg/ml depending on the species and, with Arctic fishes, depending on the season. Our above data showing enhanced nucleation above ∼8 mg/ml are therefore difficult to explain because it must still be assumed that all antifreeze molecules act to stop ice growth in the fishes. In the intestinal tract, this is by binding to macroscopic ice crystals and retarding or stopping their growth. There would not seem to be any situation where it is advantageous to enhance ice nucleation, and it may be that, in body fluids requiring hindrance of ice nucleation, concentrations of AFP of ∼10 mg/ml are ideal and are maintained at this level. Further work is needed to probe this idea. With this idea in mind, it is also difficult to reconcile the critical aggregation concentration of 2.5 mg/ml found by Du et al. (15) for Type III AFPs.
It has recently been shown that some AFGP molecules act in concert with a second set of proteins called antifreeze-potentiating proteins. This is currently necessitating a revised assessment of the method of action of AFGPs to include the contribution of antifreeze-potentiating proteins to freezing avoidance in general (25). The existence of other proteins in the blood that interact with ice may explain our seemingly anomalous 2002 and 2005 results mentioned above. They may be the component N in those cases, and further nucleation work with AFGP- and antifreeze-potentiating protein-containing solutions is also required.
The Type I AFP used in these experiments was purified from Arctic fishes, and no similar antifreeze-potentiating protein molecules have, to our knowledge, been reported from those species. It is possible though that some molecules other than Type I AFP are present in the lyophilized powder supplied. It is assumed that the Type I AFP supplied is mainly the isoform known as HPLC6 (3300 Da), which is typically present in the fishes' blood at 10–15 mg/ml in winter (26). We look forward to seeing further measurements of Thet on many different AFP types to determine fully the cause of the nucleation enhancement above certain concentrations.
Acknowledgment
We are grateful to Professor Art DeVries for the supply of the original fish serum and AFGP and for many helpful discussions.
P. W. Wilson, A. L. DeVries, and A. D. J. Haymet, unpublished data.
- AFP
- antifreeze protein
- AFGP
- antifreeze glycoprotein
- Thet
- heterogeneous nucleation temperature
- ALTA
- automatic lag time apparatus.
REFERENCES
- 1.DeVries A. L. (1971) Science 172, 1152–1155 [DOI] [PubMed] [Google Scholar]
- 2.Cheng C. C., DeVries A. L. (1991) in Life Under Extreme Conditions (DiPrisco G. ed) pp. 1–14, Springer-Verlag, Berlin [Google Scholar]
- 3.Ananthanarayanan V. S. (1989) Life Chem. Rep. 7, 1–32 [Google Scholar]
- 4.Parody-Morreale A., Murphy K. P., Di Cera E., Fall R., DeVries A. L., Gill S. J. (1988) Nature 333, 782–783 [DOI] [PubMed] [Google Scholar]
- 5.Wu D. W., Duman J. G., Xu L. (1991) Biochim. Biophys. Acta 1076, 416–420 [DOI] [PubMed] [Google Scholar]
- 6.Franks F., Darlington J., Schenz T., Mathias S. F., Slade L., Levine H. (1987) Nature 325, 146–147 [Google Scholar]
- 7.Wilson P. W. (1993) CryoLett. 14, 31–36 [Google Scholar]
- 8.Holt C. B. (2003) CryoLett. 24, 323–330 [Google Scholar]
- 9.Wilson P. W., Leader J. P. (1995) Biophys. J. 68, 2098–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariassen K. E., DeVries A. L., Hunt B., Kristiansen E. (2002) Cryobiology 44, 132–141 [DOI] [PubMed] [Google Scholar]
- 11.Wowk B., Leitl E., Rasch C. M., Mesbah-Karimi N., Harris S. B., Fahy G. M. (2000) Cryobiology 40, 228–236 [DOI] [PubMed] [Google Scholar]
- 12.Chang Z., Hansen T. N., Baust J. G. (1991) CryoLett. 12, 215–226 [Google Scholar]
- 13.Sutton R. L., Pegg D. E. (1993) CryoLett. 14, 13–20 [Google Scholar]
- 14.Du N., Liu X. Y., Hew C. L. (2003) J. Biol. Chem. 278, 36000–36004 [DOI] [PubMed] [Google Scholar]
- 15.Du N., Liu X. Y., Hew C. L. (2006) J. Phys. Chem. 110, 20562–20567 [DOI] [PubMed] [Google Scholar]
- 16.Liu X. Y., Du N. (2004) J. Biol. Chem. 279, 6124–6131 [DOI] [PubMed] [Google Scholar]
- 17.Barlow T. W., Haymet A. D. J. (1995) Rev. Sci. Instrum. 66, 2996–3007 [Google Scholar]
- 18.Heneghan A. F., Wilson P. W., Wang G., Haymet A. D. J. (2001) J. Chem. Phys. 115, 7599–7608 [Google Scholar]
- 19.Wilson P. W., Heneghan A. F., Haymet A. D. J. (2003) Cryobiology 46, 88–98 [DOI] [PubMed] [Google Scholar]
- 20.Heneghan A. F., Wilson P. W., Haymet A. D. J. (2002) Proc. Natl. Acad. Sci U. S. A. 99, 9631–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heneghan A. F., Moore H. J., Lee T. R., Haymet A. D. J. (2004) Chem. Phys. Lett. 385, 441–445 [Google Scholar]
- 22.Wilson P. W., Haymet A. D. J. (2009) Phys. Chem. Chem. Phys. 11, 2679–2682 [DOI] [PubMed] [Google Scholar]
- 23.Zobrist B., Marcolli C., Peter T., Koop T. (2008) J. Phys. Chem. A 112, 3965–3975 [DOI] [PubMed] [Google Scholar]
- 24.Zachariassen K. E., Kristiansen E., Pedersen S. A., Hammel H. T. (2004) Cryobiology 48, 309–321 [DOI] [PubMed] [Google Scholar]
- 25.Præbel K., Hunt B., Hunt L. H., DeVries A. L. (2009) Comp. Biochem. Physiol. A 154, 564–569 [DOI] [PubMed] [Google Scholar]
- 26.Hew C. L., Wang N. C., Yan S., Cai H., Sclater A., Fletcher G. L. (1986) Eur. J. Biochem. 15, 267–272 [DOI] [PubMed] [Google Scholar]