Abstract
LOV domains function as blue light-sensing modules in various photoreceptors in plants, fungi, algae, and bacteria. A LOV/LOV protein (LLP) has been found from Arabidopsis thaliana (AtLLP) as a two LOV domain-containing protein. However, its function remains unknown. We isolated cDNA clones coding for an LLP homolog from tomato (Solanum lycopersicum) and two homologs from the moss Physcomitrella patens. The tomato LLP (SlLLP) contains two LOV domains (LOV1 and LOV2 domains), as in AtLLP. Most of the amino acids required for association with chromophore are conserved in both LOV domains, except that the amino acid at the position equivalent to the cysteine essential for cysteinyl adduct formation is glycine in the LOV1 domain as in AtLLP. When expressed in Escherichia coli, SlLLP binds FMN and undergoes a self-contained photocycle upon irradiation of blue light. Analyses using mutant SlLLPs revealed that SlLLP binds FMN in both LOV domains, although the LOV1 domain does not show spectral changes on irradiation. However, when Gly66 in the LOV1 domain, which is located at the position equivalent to the essential cysteine of LOV domains, is replaced by cysteine, the mutated LOV1 domain shows light-induced spectral changes. In addition, all four LOV domains of P. patens LLPs (PpLLP1 and PpLLP2) show the typical features of LOV domains, including the reactive cysteine in each. This study shows that plants have a new LOV domain-containing protein family with the typical biochemical and photochemical properties of other LOV domain-containing proteins such as the phototropins.
Keywords: Flavoproteins, FMN, Photoreceptors, Plant, Protein Domains, LOV Domains
Introduction
Four photoreceptor families have been found from plants including phytochrome, cryptochrome, phototropin, and ADO/FKF/LKP/ZTL family proteins. Among them, phototropin and ADO/FKF/LKP/ZTL use a common light-sensing domain, LOV domain (1). The LOV domain was found as the light-sensing domain of the phototropin (PHOT1 and PHOT2) (2–4) and has been well characterized (1, 5–7). LOV domain-containing proteins are distributed not only in plants but also in many other organisms. For example, WC-1 and VVD are found in the ascomycete fungus Neurospora crassa (8), aureochrome in the alga Vaucheria frigida (9), and bacterial histidine kinases in the animal pathogenic bacterium Brucella abortus (10) and in the stalked bacterium Caulobacter crescentus (11). These proteins have all been shown to function as photoreceptors.
Six genes encoding LOV domain-containing proteins are found in the genome of Arabidopsis thaliana. Among them, two encode phototropins, and three encode the ADO/FKF/LKP/ZTL family proteins, proteins involved in circadian clock and photoperiod-dependent flowering functions (12–15). All of the LOV domains of phototropins and ADO/FKF/LKP/ZTL family proteins are shown to bind FMN (2, 4, 15, 16). In addition to those above, there exists a unique LOV domain-containing protein, referred to as PAS/LOV protein (PLP),2 which contains a PAS domain followed by a LOV domain (1, 17).
Ogura et al. (18) screened for proteins interacting with A. thaliana PLP using the yeast two-hybrid system. They isolated VTC2 (vitamin C defective 2), VTC2L (VTC2-like), and BLH10A and BLH10B (BEL1-like homeodomain 10 A and B proteins) as interacting proteins in yeast cells. The molecular interactions between PLP and VTC2L, BLH10A, or BLH10B in yeast were dependent on blue light irradiation, suggesting that PLP may function as a photoreceptor (18). Blue light treatment diminished the interaction. However, the flavin binding and photochemical properties of the A. thaliana PLP are unclear because the recombinant protein expressed in Escherichia coli did not show absorption spectra of flavin-binding proteins (18).
In this study, we isolate cDNAs coding for PLP homologs from tomato (Solanum lycopersicum) and the moss Physcomitrella patens, and phylogenetic analysis shows that the PAS domains of the homologs, even the PAS domain of A. thaliana PLP, are closely related to the LOV domain. Thus, we propose that the isolated LOV domain-containing protein should be referred to as LOV/LOV protein (LLP), instead of PLP. Here, we analyze chromophore binding and photochemical properties of LLP from S. lycopersicum using wild-type and mutant proteins expressed in E. coli. We also examine both LOV domains of both PpLLPs for FMN binding and photochemical properties.
EXPERIMENTAL PROCEDURES
Cloning and Sequencing of SlLLP cDNA
To obtain tomato LLP cDNA, RT-PCR was performed using total RNA from flowers of tomato (S. lycopersicum cv. Micro-Tom). The primers and DNA polymerase used were LLP-S-F and LLP-S-R (supplemental Table S1) and KOD-Plus (Toyobo, Tsuruga, Japan), respectively. The PCR products were inserted into the pGEM-T easy vector (Promega, Madison, WI) after treatment with ExTaq polymerase (Takara, Otsu, Japan) to add dT at the 3′ ends. Two clones were sequenced, and the complete match of the nucleotide sequences was verified between them. The obtained nucleotide sequence has been submitted to GenBank/EMBL/DDBJ Data Bank with accession number AB576162.
Expression of Recombinant SlLLP in E. coli
For protein expression of SlLLP, cDNA of SlLLP was amplified by PCR using the two primers LLP-F and LLP-R (supplemental Table S1). The PCR product was digested with XhoI and NotI and cloned into the XhoI-NotI site of pThioHisA plasmid (Invitrogen), and the resulting plasmid was named pThio-SlLLP. The SlLLP recombinant protein is expressed as a fusion protein with His-Patch thioredoxin at the N terminus and His6 at the C terminus. E. coli JM109 having pRARE2LysS (Novagen, Madison, WI) that supplies tRNAs for rare codons to enhance the expression of eukaryotic proteins was used for the host cell.
Purification of Recombinant SlLLP
The transformants, JM109 cells harboring pThio-SlLLP and pRARE2LysS, were grown overnight at 37 °C in Luria-Bertani medium (30 ml) supplemented with ampicillin (100 μg ml−1) and chloramphenicol (30 μg ml−1). The overnight-cultured cells (20 ml in LB medium) were added to M9 medium (1 liter) supplemented with ampicillin (100 μg ml−1) and chloramphenicol (30 μg ml−1) and were grown at 25 °C to an optical density (600 nm) of ∼0.35. Protein expression was carried out for 20 h at 20 or 25 °C in the presence of 1.5 mm isopropyl β-d-thiogalactopyranoside. The cells were harvested by centrifugation, frozen at −80 °C, thawed at 25 °C, and then resuspended in 20 ml of a disruption solution (20 mm Tris-HCl, pH 8.0, 0.5 m NaCl, 5 mm 2-mercaptoethanol, 20 mm imidazole) with 0.2% (w/v) Tween 60. After sonication for disruption, the cell extract was centrifuged at 35,000 × g for 30 min at 4 °C. The supernatant was loaded onto a nickel-Sepharose 6 Fast Flow column (1 ml of bed volume; GE Healthcare). The column was washed twice with 10 ml of the disruption solution with 0.2% (w/v) Tween 60, twice with 10 ml of the disruption solution, and then once with 5 ml of the disruption solution with 75 mm imidazole. The protein was eluted with 3 ml of the disruption solution with 200 mm imidazole. The eluate was added to 9 ml of a buffer solution (20 mm Tris-HCl, pH 8.0) and loaded onto a HiTrapQ column (GE Healthcare). The column was washed with the buffer solution with 0.15 m NaCl, and then the protein was eluted with a linear NaCl gradient (0.15–0.8 m).
Site-directed Mutagenesis
Mutant versions of pThio-SlLLP were generated by PCR-based site-directed mutagenesis (19). Mismatch primers used for the PCR are listed in supplemental Table S1. The resulting PCR products were mixed and used as templates for next PCR using LLP-F and LLP-R primers. PCRs were performed with KOD-Plus DNA polymerase (Toyobo). The resulting PCR products were cloned into pThioHisA (Invitrogen). pThio-SlLLP-C295A, -R67D, and -R296D were used for protein expression of SlLLP-C295A, -R67D, and -R296D, respectively. To express SlLLP-R67D/R296D, the 5.2- and 0.4-kb MunI-XbaI fragments of pThio-SlLLP-R67D and pThio-SlLLP-R296D, respectively, were ligated. The resulting pThio-SlLLP-R67D/R296D was used for SlLLP-R67D/R296D expression. To express SlLLP-G66C/R296D, the pThio-SlLLP-G66C/R296D plasmid was constructed using mismatch primers (supplemental Table S1) and pThio-SlLLP-R296D as a template. PCR and cloning into the pThioHisA plasmid were performed as described above.
Expression and Purification of Recombinant PpLLP1 and PpLLP2 and Each LOV Domain in E. coli
For protein expression, cDNAs were amplified by PCR using the two primers listed in supplemental Table S1. The PCR products were digested with EcoRI and XhoI and cloned into the EcoRI-XhoI site of pGEX-6P-1 plasmid (GE Healthcare), and the resulting plasmids were introduced into E. coli Rosetta2 (DE3) pLysS (Novagen, Madison, WI). The transformants were grown overnight at 37 °C in Luria-Bertani medium (10 ml) supplemented with ampicillin (100 μg ml−1) and chloramphenicol (30 μg ml−1). The overnight-cultured cells (4 ml in LB medium) were added to LB medium (0.5 liter) supplemented with ampicillin (100 μg ml−1) and chloramphenicol (30 μg ml−1) and were grown at 25 °C to an optical density (600 nm) of ∼0.5. Protein expression was carried out for 20 h at 20 °C in the presence of 0.5 mm isopropyl β-d-thiogalactopyranoside. The cells were harvested by centrifugation, frozen at −80 °C, thawed at 25 °C, and then resuspended in 25 ml of a disruption solution (20 mm Tris-HCl, pH 8.0, 5% (w/v) glycerol, 0.1 m NaCl, 10 mm 2-mercaptoethanol, 1 mm EDTA) with 0.2% (w/v) Triton X-100. After sonication for disruption, the cell extract was centrifuged at 35,000 × g for 30 min at 4 °C. The supernatant was loaded onto a glutathione-Sepharose 6 column (0.7 ml of bed volume; GE Healthcare). The column was washed twice with 10 ml of the disruption solution with 0.2% (w/v) Triton X-100 and twice with 10 ml of the disruption solution. The proteins were eluted with the disruption solution with 10 mm glutathione. The nucleotide sequences have been submitted to the GenBank/EMBL/DDBJ Data Bank with accession numbers AB576160 (PpLLP1) and AB576161 (PpLLP2).
Spectral Analysis
Absorption spectra for recombinant proteins were obtained using a spectrophotometer (Multispec-1500; Shimadzu, Kyoto, Japan). For light-induced absorption changes, the samples were irradiated with blue light for 10 s at 600 μmol m−2 s−1, and the spectra were recorded at 25 °C at regular intervals as described in the figure legends. For light-induced absorption changes of SlLLP-R67D, the sample was centrifuged briefly to remove any aggregates from the protein solution before measuring spectra. A high power blue LED (LXHL-LB3C; Phillips Lumileds, CA) was used as a light source for the light irradiation. The fluence rates were measured using a quantum sensor (LI-250A; LI-COR, Lincoln, NE).
Other Analytical Procedures
Protein concentrations were determined by the method of Bradford as described in the instructions accompanying the Bio-Rad protein assay kit with γ-globulin as a standard. To determine the concentration of FMN associated with SlLLP, purified protein samples were denatured by treatment with 1% SDS, and the concentrations of the released FMN were calculated by using a series of FMN standards as described by Christie et al. (2).
RESULTS
Cloning of cDNAs Coding for LLP from S. lycopersicum and P. patens and Sequence Homology
To examine whether A. thaliana PLP (AtPLP) binds flavin as chromophore and shows a LOV domain photocycle, we tried to produce recombinant protein of AtPLP in E. coli. However, it was difficult to find optimal conditions to express a sufficient amount of the recombinant protein because of its high insolubility and low protein expression level, as was the case in another study (18). Thus, we decided to try to express homologs of AtPLP in E. coli.
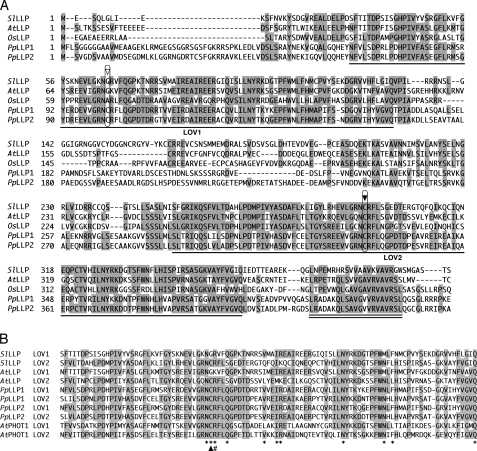
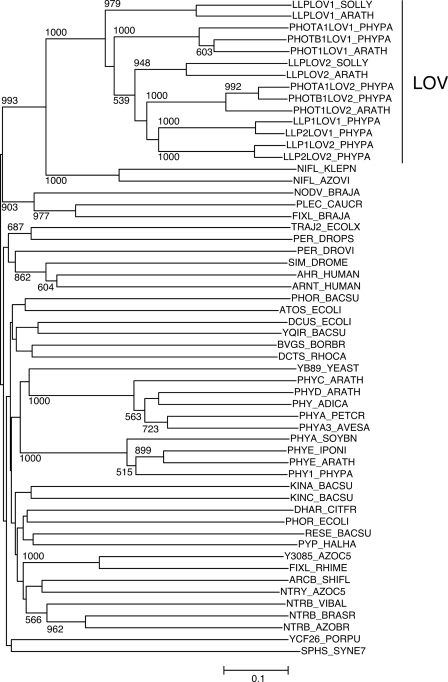
Using the amino acid sequence of AtPLP as a query in a BLAST search on a tomato (S. lycopersicum) EST database (MiBASE, Kazusa DNA Research Institute) and the P. patens genome database (JGI), we found one homolog from S. lycopersicum and two homologs from P. patens. The cDNAs coding for the homologs were obtained by RT-PCR and sequenced. The deduced amino acid sequences are shown in Fig. 1A and aligned with AtPLP and the homologous sequence in Oryza sativa (rice), which is from the rice genome database. Approximately 100 amino acids in N- and C-terminal regions showed high similarity (Fig. 1A, underlining). Phylogenetic analysis of those amino acid sequences with various PAS domain sequences clearly showed that all of the sequences including the PAS domain of AtPLP were clustered with LOV domains of phototropins (Fig. 2), as is the case with a former phylogenetic analysis (20). Thus, we propose that the isolated LOV domain-containing protein and AtPLP should be referred to as LLP, instead of PLP. The S. lycopersicum homolog is named SlLLP, and the two P. patens homologs are named PpLLP1 and PpLLP2, and the N-terminal and the C-terminal LOV domains are named LOV1 and LOV2 domains, respectively (Fig. 1A). A highly conserved short region was found in the C terminus (Fig. 1A, double underlining).
FIGURE 1.
Amino acid alignments of LLPs. A, alignment of the amino acid sequences of full-length LLPs of S. lycopersicum, A. thaliana, O. sativa, and P. patens. Amino acid residues identical in more than three sequences are shaded. Gaps introduced for good alignment are indicated by dashes. The numbers are the amino acid positions for each amino acid sequence. Single lines indicate LOV1 and LOV2 domains. The highly conserved cysteines in the LOV2 domains are indicated by the solid arrowhead. The corresponding amino acids in the LOV1 domains are indicated by the open arrowhead. A highly conserved C-terminal region is indicated by a double line. B, alignment of LOV domains of LLPs with LOV domains of A. thaliana PHOT1. Amino acid residues involved in association with FMN, as deduced from the crystal structure of a phototropin LOV domain (21), are indicated by asterisks. The highly conserved cysteines in LOV domains are indicated by a solid arrowhead. Arginines that we replaced with aspartates in this study are indicated by #.
FIGURE 2.
Phylogenetic tree of LOV domain sequences of LLPs with PAS domain superfamily sequences. The tree was constructed by the NJ method after ClustalX alignment of the amino acid sequences of LOV domains of LLPs and PAS domains. Bootstrap values in the NJ analysis were carried out based on 1,000 replications using ClustalX. Bootstrap values higher than 50% are shown for each clade. The scale indicates 0.1 nucleotide substitutions/site. PAS domain sequences were collected from the PF00989 family of the Pfam protein families database (28). LOV domain sequences were used from phototropin 1 (PHOT1) of A. thaliana (ARATH), phototropin A1 and B1 (PHOTA1 and PHOTB1) of P. patens (PHYPA), and LLPs of S. lycopersicum (SOLLY), A. thaliana (ARATH), and P. patens (PHYPA).
Fig. 1B compares the amino acid sequences of the LOV1 and LOV2 domains of LLPs with those of the LOV domains of A. thaliana phototropin 1 (Fig. 1B). Most of the conserved amino acids that are associated with flavin binding (21) are conserved in both LOV domains of LLPs (asterisks in Fig. 1B). However, the amino acids at the position of the cysteine that is essential for photoproduct formation in LOV domains are different in the LOV1 domains of A. thaliana (glycine), O. sativa (alanine), and S. lycopersicum (glycine) LLPs (Fig. 1, A, open arrowhead, and B, arrowhead).
FMN Binding of SlLLP
To characterize the properties of flavin binding and photocycle of SlLLP, a fusion protein of SlLLP with His-Patch thioredoxin at the N terminus and His6 at the C terminus was expressed in E. coli. The His-Patch thioredoxin and His6 were fused to facilitate the purification of the fusion protein by affinity chromatography on a nickel-agarose column.
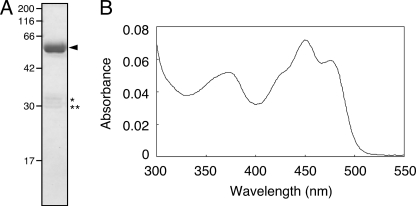
The SlLLP was purified to near homogeneity by affinity chromatography and anion exchange chromatography (Fig. 3A, arrowhead). The estimated molecular mass (56.4 kDa) by SDS-PAGE was in agreement with the theoretical values (58.8 kDa) from the amino acid sequences. The 56.4-kDa polypeptide was recognized by both anti-thioredoxin and anti-His6 antibodies (data not shown). Minor lower molecular mass polypeptides (32.9 and 30.0 kDa; Fig. 3A, asterisks) likely result from SlLLP degradation because the polypeptides were recognized by either anti-thioredoxin or anti-His6 antibodies, respectively, but not both (data not shown).
FIGURE 3.
Purification and absorption spectrum of SlLLP. A, SDS-PAGE was carried out using a 12% polyacrylamide gel. The gel was stained with Coomassie Brilliant Blue R250. The arrowhead indicates the position of SlLLP. The molecular sizes (in kDa) are indicated on the left side. The asterisks indicate degradation products of SlLLP. B, absorption spectrum of SlLLP.
The purified SlLLP exhibited features of LOV domains described previously for phototropin and other LOV domain-containing proteins (2, 4, 15), with absorption peaks in the UV-A and blue regions of the spectra and prominent vibrational bands in the blue (Fig. 3B). The flavin associated with SlLLP was identified as FMN by thin layer chromatography, according to its mobility against standard FAD, FMN, and riboflavin samples (supplemental Fig. S1). The molar ratio of FMN to protein was 0.37 for SlLLP (supplemental Table S2), although it was expected that the molar ratio will become ∼2 because SlLLP contains two LOV domains.
FMN-Cysteinyl Adduct Formation of SlLLP upon Light Irradiation
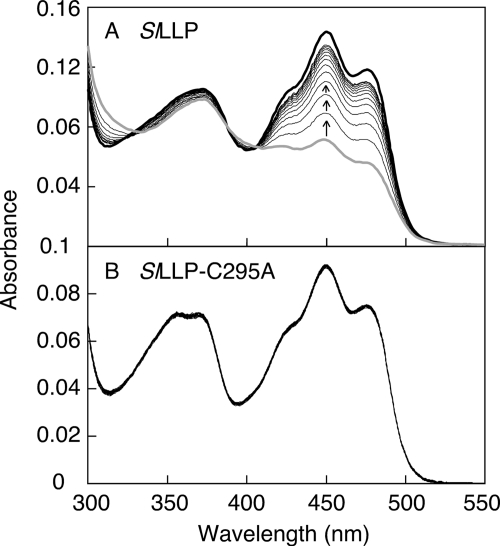
Irradiation of SlLLP with strong blue light induced a decrease of absorption in the blue region of flavin absorption (∼450 nm). During a subsequent dark incubation, the blue absorption was restored to the initial state (Fig. 4A). Three isosbestic points present at 322, 388, and 405 nm suggest that the light-induced absorption change is the consequence of FMN-cysteinyl adduct formation (3), not that of the photoreduction that occurs in many flavoproteins. A mutant SlLLP (SlLLP-C295A) was investigated because Cys295 in LOV2 domain (Fig. 1A, arrowhead) is likely the residue to form the FMN-cysteinyl adduct. The SlLLP-C295A did not exhibit any absorption change on irradiation with strong blue light (Fig. 4B). These properties indicate that an FMN-cysteinyl adduct was formed upon irradiation with blue light in SlLLP, as is the case with the LOV domain of phototropin, and Cys295 in the LOV2 domain of SlLLP is the site of FMN-cysteinyl adduct formation.
FIGURE 4.
Light-induced absorption change and dark recovery of SlLLP and SlLLP-C295A. SlLLP (A) and SlLLP-C295A (B) were irradiated with high intensity blue light (600 μmol m−2 s−1) for 10 s. The uppermost spectra (black heavy lines) and the lowermost spectra (gray heavy lines) at 450 nm represent the initial dark spectra and the spectra immediately after irradiation, respectively, of SlLLP and SlLLP-C295A. All of the spectra of SlLLP-C295A are the same because there was no absorption change on irradiation. After the onset of irradiation, the spectra were recorded at 30-min intervals (total time, 360 min) for SlLLP and 20-s intervals (total time, 160 s) for SlLLP-C295A. The arrows indicate the absorption changes with time in the subsequent dark incubation.
Effect of Imidazole on the Rate of Dark Reversion of SlLLP
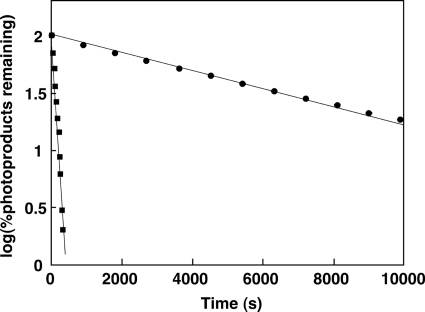
We noticed that when samples after nickel-Sepharose 6 Fast Flow column, which contain 200 mm imidazole added to elute recombinant protein from the column, were used for analyzing light-induced absorption changes, the half-life of photoproduct became very short compared with samples excluding imidazole by ion exchange chromatography. Fig. 5 shows the effect of imidazole on the rate of dark reversion of photoproduct. The half-lives of photoproduct under conditions with and without imidazole were 61.4 s and 65.4 min, respectively (Fig. 5). The rate of dark reversion became ∼64-fold faster in the presence of imidazole. The imidazole-dependent acceleration of dark reversion was also seen in cyanobacterial LOV domains (22) and the LOV2 domain of oat phototropin 1 (23).
FIGURE 5.
Effect of imidazole on dark reversion kinetics of SlLLP. The amount of photoproduct remaining after dark incubation is plotted against the duration of dark incubation in the presence (square) or the absence (circle) of 100 mm imidazole.
Analyses of FMN Binding and Photochemical Properties of the LOV1 Domain of SlLLP Using Site-directed Mutagenized Proteins
The loss of light-induced absorption changes by replacement of Cys295 in the LOV2 domain of SlLLP shows that the LOV2 domain is an FMN-binding site. However, it is unclear whether the LOV1 domain binds FMN. To investigate whether the LOV1 domain of SlLLP binds FMN, we constructed a truncated form of SlLLP consisting of the LOV1 domain alone as well as that consisting of the LOV2 domain alone. However, both truncated proteins were expressed as insoluble protein. The full-length polypeptide seems to be required for expression in a soluble form. We tried to express a full-length SlLLP with a point mutation, by which the mutant protein is considered to dissociate FMN from the LOV2 domain, so that the potential flavin binding site remaining in the mutant protein would be the LOV1 domain. A candidate position of the point mutation was Arg296, next to the indispensable cysteine for photoreaction (Fig. 1B, #), because the arginine next to the cysteine in LOV domains has been shown to interact with the phosphate group of FMN by forming a salt bridge (21, 24), and point mutations of the arginine result in a complete loss of FMN binding (3).
SlLLP-R296D
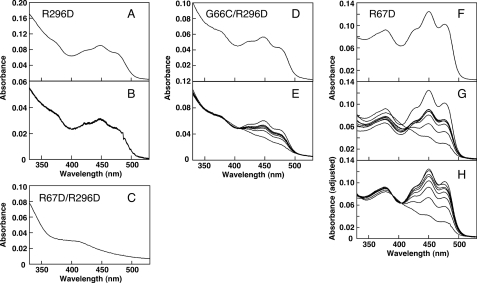
The SlLLP-R296D, in which Arg296 was replaced by aspartate, was constructed. Fig. 6A shows the absorption spectrum of the SlLLP-R296D, which was similar to those of LOV domains. However, no absorption change upon blue light irradiation was observed (Fig. 6B). Flavin released from SlLLP-R296D showed the same mobility as standard FMN in a TLC analysis. Thus, FMN is suggested to bind to the LOV1 domain, but the LOV1 domain did not undergo the typical photocycle of LOV domains on the time scale of 0.1 s, probably because the LOV1 domain contains a glycine at the position equivalent to the conserved cysteine essential for FMN-cysteinyl adduct formation in LOV domains (Fig. 1B, arrowhead).
FIGURE 6.
Absorption spectra and absorption change and recovery of SlLLP-R296D, SlLLP-R67D, and SlLLP-R67D/R296D. A, absorption spectrum of SlLLP-R296D. B, the uppermost spectrum represents the initial dark spectrum of SlLLP-R296D. After the onset of blue light irradiation (600 μmol m−2 s−1) for 10 s, the spectra were recorded at 0.1-s intervals (total time, 1 s). C, absorption spectrum of SlLLP-R67D/R296D. D, absorption spectrum of SlLLP-G66C/R296D. E, the uppermost and the bottom spectra represent the initial dark spectrum of SlLLP-G66C/R296D and the spectrum right after irradiation with blue light (600 μmol m−2 s−1, 10 s), respectively. The spectra after 85, 170, 250, 320, and 395 s (from bottom to top) are shown. F, absorption spectrum of SlLLP-R67D. G, the uppermost and the bottom spectra represent the initial dark spectrum of SlLLP-R67D and the spectrum right after irradiation with blue light (600 μmol m−2 s−1, 10 s), respectively. The spectra after 60, 120, 180, 240, 360, and 480 min (from bottom to top) are shown. H, spectra in E are normalized in each case to the value of the 405-nm isosbestic point.
SlLLP-R67D/R296D
Next, we constructed a mutant SlLLP, SlLLP-R67D/R296D, in which Arg67 in the LOV1 domain was replaced by aspartate in addition to the R296D replacement. We expected that the LOV1 domain would fail to bind FMN as a consequence of the R67D mutation because the Arg67 in the LOV1 domain is equivalent to Arg296 in the LOV2 domain (Fig. 1B, #). Fig. 6C shows the absorption spectrum of SlLLP-R67D/R296D. It lacks all but a minor absorption peak in the blue region, showing that SlLLP-R67D/R296D did not bind FMN, and the R67D replacement resulted in a loss of FMN binding. This result indicates that the LOV1 domain in SlLLP binds FMN.
SlLLP-G66C/R296D
We constructed a mutant SlLLP, SlLLP-G66C/R296D, in which Gly66 in the LOV1 domain (Fig. 1A, open arrowhead) was replaced by cysteine in the R296D background. We expected that the G66C replacement might generate LOV domain photochemistry for the LOV1 domain because Gly66 is located at the position corresponding to the cysteine essential for photochemistry in the LOV domain. The absorption spectrum of SlLLP-G66C/R296D was similar to that of SlLLP-R296D (Fig. 6, A and D). SlLLP-G66C/R296D showed an absorption change upon blue light irradiation and a subsequent dark recovery (Fig. 6E). Three isosbestic points are present at 343, 384, and 406 nm. The light-induced absorption change is likely the consequence of FMN-cysteinyl adduct formation with the Cys66. The single G66C amino acid replacement confers photochemistry to the LOV1 domain.
SlLLP-R67D
SlLLP-R67D, in which Arg67 in the LOV1 domain was replaced by aspartate (Fig. 1B, #), showed the typical spectrum of a LOV domain (Fig. 6F). When measuring light-induced absorption changes and dark recovery of SlLLP-R67D, we observed aggregation in the protein solution. To reduce and remove the aggregation, 5% (w/v) glycerol was added to the protein solution, and the protein solution was centrifuged before measuring each time spectrum. Fig. 6G shows the absorption change upon irradiation of blue light and subsequent dark recovery of SlLLP-R67D. The absorption decrease was not restored to the initial state (Fig. 6G), probably because of the removal of the aggregated protein and the consequent decrease in the concentration of SlLLP-R67D. When each absorption spectrum was expanded to have the same absorbance at 405 nm (one of the isosbestic points of SlLLP), spectral changes of SlLLP-R67D became similar to those of wild-type SlLLP (compare Figs. 4A and 6H). The absorbances at 450 nm of SlLLP-R67D and wild-type SlLLP were decreased to 33 and 49% of those of the initial non-light-irradiated forms, respectively (Figs. 4A and 6H), showing that the relative amount of FMN involved in the light-induced absorption change in SlLLP-R67D is higher than that in wild-type SlLLP. The replacement of Arg67 resulted in dissociation of FMN bound to the LOV1 domain and increased in the relative amount of FMN able to form the FMN-cysteinyl adduct in SlLLP-R67D. This result also shows that the LOV1 domain in SlLLP binds FMN.
FMN Binding and Photochemical Properties of P. patens LLPs, PpLLP1 and PpLLP2
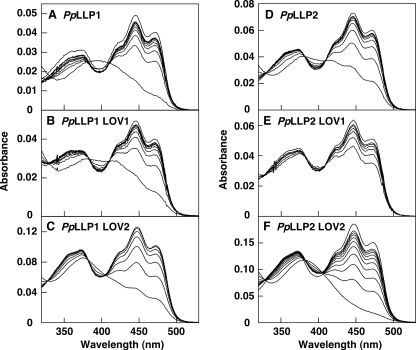
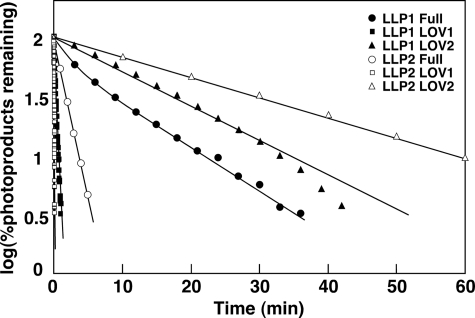
To characterize the properties of flavin binding and photocycle of PpLLP1 and PpLLP2, the full-length proteins and each of the four LOV domains were expressed in E. coli as GST fusion proteins. Full-length PpLLP1 and PpLLP2 and LOV domains of PpLLP1 (PpLLP1LOV1 and PpLLP1LOV2) and PpLLP2 (PpLLP2LOV1 and PpLLP2LOV2) were purified by affinity chromatography. FMN was detected by a thin layer chromatography from PpLLP1 and PpLLP2 (supplemental Fig. S1). Fig. 7 shows absorption spectra and spectral changes upon blue light irradiation. All of the purified recombinant proteins exhibited spectral features of LOV domain-containing protein, i.e. absorption peaks in the UV-A and blue regions, light-induced absorption change, and having three isosbestic points during the absorption change (Fig. 7). Semilogarithmic plots for photoproduct remaining versus time after a light exposure (dark incubation time) were nearly log-linear for the single LOV domain proteins (Fig. 8), indicating that regeneration of the dark form is first order. The rate constants of dark recovery for PpLLP1 LOV1 and LOV2 were 3.5 and 6.9 × 10−2 min−1, and those for PpLLP2 LOV1 and LOV2 were 33 and 3.9 × 10−2 min−1, respectively. The dark recovery of the LOV1 domain is faster than that of the LOV2 domain in both PpLLP1 and PpLLP2. The dark recovery of the full-length LLP1 was slower than that of the LLP2.
FIGURE 7.
Light-induced absorption change and dark recovery of PpLLP1 and PpLLP2. Recombinant protein of PpLLP1 (A), PpLLP2 (D), or each LOV domain (B, C, E, and F) were irradiated with high intensity blue light (600 μmol m−2 s−1) for 10 s. The uppermost spectrum in each panel at 450 nm represent the initial dark spectra. After the onset of irradiation, the spectra were recorded at 6-min intervals (A), 10-s intervals (B), 9-min intervals (C), 1-min intervals (D), 1-s intervals (E), and 10-min intervals (F).
FIGURE 8.
Reaction kinetics for the dark recovery of the light-activated full-length and LOV domain proteins from PpLLP1 and PpLLP2. The kinetic data were obtained by following the absorption changes at 450 nm in Fig. 7.
DISCUSSION
In this study, we demonstrate that LLPs are plant flavoproteins that undergo the typical photocycle of other LOV domain-containing proteins such as phototropins. Although the physiological function of LLPs remain unknown, homologous sequences have been found from A. thaliana, S. lycopersicum, O. sativa, and P. patens (as shown in Fig. 1A) and also from poplar (Populus trichocarpa), grapevine (Vitis vinifera), and the spikemoss Selaginella moellendorffii in their complete genome sequences by BLAST search (supplemental Fig. S2). When compared with amino acid sequences of LLPs from flowering plants and P. patens, not only the LOV domains but also the intervening sequences between LOV1 and LOV2 domains and the C-terminal extensions outside of LOV2 domains show significant similarities. This sequence conservation suggests that LLPs of flowering plants and P. patens are evolutionarily related. The amino acid at the position equivalent to the essential cysteine in the LOV1 domain of angiosperm LLPs is glycine or alanine, whereas that of the moss P. patens LLPs and the spikemoss S. moellendorffii LLP is cysteine (Fig. 1 and supplemental Fig. S2). The cysteine in the LOV1 domain seems to be lost in the evolution from spikemoss to angiosperm. The wide distribution implies that LLP may play a role in common physiological responses of plants.
A. thaliana LLP was first described in a review by Crosson et al. (17), referred to as PLP, and has been so far referred to as PLP in several studies (1, 18, 25). Because LOV domains are members of the large and diverse superfamily of PAS domains (2), the amino acid sequences of PAS and LOV domains resemble each other. In the case of A. thaliana LLP, the essential cysteine for photochemical reaction of LOV domains is not conserved in the LOV1, so that the N-terminal domain is recognized only as a PAS domain. However, a phylogenetic analysis clearly shows that the N-terminal domains of A. thaliana and S. lycopersicum LLPs are more closely related to LOV domains of phototropins than to PAS domains (Fig. 2). Thus, we propose that the N-terminal domains should be classified as LOV domains.
Only one amino acid replacement (G66C) restored LOV domain-like photochemistry in the LOV1 domain of SlLLP. Amino acids required for the photochemical reaction have been maintained even in the absence of the cysteine. This fact suggests that LOV1 domains of angiosperm LLPs may have lacked the cysteine during their evolution. The LOV1 domains of A. thaliana phototropin 1 and 2 are proposed to function as a dimerization site for the phototropins (26). Interestingly, the dimer formation is light-independent (26). Therefore, a photochemical function of LOV1 domains may not be necessary to allow them to undergo protein-protein interaction. Although the LOV1 domain of SlLLP does not show any light-induced spectral change, FMN binding in the LOV1 domain might be required to keep the proper three-dimensional structure for its dimerization function.
The molar ratio of FMN to protein for wild-type SlLLP was 0.37 (supplemental Table S2), although we expected that it would become ∼2 because SlLLP contains two LOV domains. In addition to SlLLP, all of the SlLLP variants and all proteins derived from PpLLP1 and PpLLP2 showed low molar ratios compared with the value expected from the number of LOV domain having the potential FMN binding in each protein (supplemental Tables S2 and S3). However, the measured ratios of the proteins containing one potential FMN-binding LOV domain become roughly half of those containing two potential domains (supplemental Tables S2 and S3). In the case of phototropins, it has been shown that the ratios are 0.6 to 1.0 for single and 1.2 to 2.1 for double LOV domain-containing proteins (2). The low ratios could be due to low affinity of LOV domains of LLPs to FMN, and FMN could be released from the LOV domains during purification. It could also be possible that the capacity of FMN biosynthesis in E. coli cells is not sufficient to provide FMN to all expressed proteins. Although the protein samples used in this study include apoprotein without FMN, properties of FMN binding and light-dependent FMN-cysteinyl adduct formation are probably not affected by the presence of the apoprotein.
Imidazole accelerates the rate of dark reversion of SlLLP (Fig. 5). The imidazole-dependent acceleration was also observed in PpLLP1 and PpLLP2 (supplemental Fig. S3). Alexandre et al. (23) have shown that, using LOV domains of phototropins, imidazole acts as base and catalyzes the dark reversion via abstraction of the proton from N5 position of the FMN-cysteinyl adduct. In LLPs, imidazole probably acts through the same mechanisms. The authors also suggest that a histidine can be involved in the tuning of the rate of dark reversion via a hydrogen bonding network from a surface-exposed histidine because of the absence of a histidine in the vicinity of FMN chromophore in Avena sativa PHOT1 LOV2 used for their experiments (23). The histidines of LLPs (His340 of SlLLP, His370 of PpLLP1, and His383 of PpLLP2), which are located at the positions equivalent to the above-mentioned surface-exposed histidine of A. sativa PHOT1 LOV2, could act as intrinsic base catalysts via a hydrogen bonding network. On the other hand, the histidine is not conserved even in the LOV domains that have been already recognized the imidazole-dependent acceleration of dark reversion: Chlamydomonas reinhardtii PHOT LOV2 (23) and cyanobacterial LOVs (All2875-LOV and Alr3170-LOV) (22). The imidazole-dependent acceleration may not be related to the presence of an intrinsic histidine involved in the dark reversion.
Ogura et al. (18) have shown that AtLLP interacts with VTC2, VTC2L, BLH10A, and BLH10B in yeast two-hybrid systems. The interactions with VTC2L, BLH10A, and BLH10B are specifically diminished by blue light irradiation in yeast cells, suggesting that LLP may function as a photoreceptor (18). The LOV2 domain of A. thaliana phototropin 2 binds to its C-terminal Ser/Thr kinase domain in the dark and inhibits the kinase activity (27). When irradiated with light, LOV2 domain dissociates from the kinase domain so that the kinase activity is activated (27). Because LLPs do not contain effector domains in themselves, unlike phototropins, binding proteins such as those mentioned above may be working as effectors.
The photochemical properties of LLPs shown in this study indicate that LLP family proteins have the potential to function in light perception. As a next step, it is important to elucidate the biological function of LLPs. Making gene disruptions of P. patens and studying phenotypes of the gene disruptants may reveal their possible photoreceptor roles.
Supplementary Material
Acknowledgment
We thank Dr. Winslow Briggs (Carnegie Institution for Science) for helpful comments and critical reading of the manuscript.
This work was supported in part by the Ministry of Education, Sciences and Culture with a Grant-in-Aid for Young Scientists (B) (to M. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Figs. S1–S3.
- PLP
- PAS/LOV protein
- LLP
- LOV/LOV protein.
REFERENCES
- 1.Christie J. M. (2007) Annu. Rev. Plant Biol. 58, 21–45 [DOI] [PubMed] [Google Scholar]
- 2.Christie J. M., Salomon M., Nozue K., Wada M., Briggs W. R. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8779–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon M., Christie J. M., Knieb E., Lempert U., Briggs W. R. (2000) Biochemistry 39, 9401–9410 [DOI] [PubMed] [Google Scholar]
- 4.Kasahara M., Swartz T. E., Olney M. A., Onodera A., Mochizuki N., Fukuzawa H., Asamizu E., Tabata S., Kanegae H., Takano M., Christie J. M., Nagatani A., Briggs W. R. (2002) Plant Physiol. 129, 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kottke T., Hegemann P., Dick B., Heberle J. (2006) Biopolymers 82, 373–378 [DOI] [PubMed] [Google Scholar]
- 6.Briggs W. R. (2007) J. Biomed. Sci. 14, 499–504 [DOI] [PubMed] [Google Scholar]
- 7.Tokutomi S., Matsuoka D., Zikihara K. (2008) Biochim. Biophys. Acta 1784, 133–142 [DOI] [PubMed] [Google Scholar]
- 8.Schwerdtfeger C., Linden H. (2003) EMBO J. 22, 4846–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi F., Yamagata D., Ishikawa M., Fukamatsu Y., Ogura Y., Kasahara M., Kiyosue T., Kikuyama M., Wada M., Kataoka H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19625–19630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz T. E., Tseng T. S., Frederickson M. A., Paris G., Comerci D. J., Rajashekara G., Kim J. G., Mudgett M. B., Splitter G. A., Ugalde R. A., Goldbaum F. A., Briggs W. R., Bogomolni R. A. (2007) Science 317, 1090–1093 [DOI] [PubMed] [Google Scholar]
- 11.Purcell E. B., Siegal-Gaskins D., Rawling D. C., Fiebig A., Crosson S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18241–18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers D. E., Schultz T. F., Milnamow M., Kay S. A. (2000) Cell 101, 319–329 [DOI] [PubMed] [Google Scholar]
- 13.Nelson D. C., Lasswell J., Rogg L. E., Cohen M. A., Bartel B. (2000) Cell 101, 331–340 [DOI] [PubMed] [Google Scholar]
- 14.Schultz T. F., Kiyosue T., Yanovsky M., Wada M., Kay S. A. (2001) Plant Cell 13, 2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imaizumi T., Tran H. G., Swartz T. E., Briggs W. R., Kay S. A. (2003) Nature 426, 302–306 [DOI] [PubMed] [Google Scholar]
- 16.Sakai T., Kagawa T., Kasahara M., Swartz T. E., Christie J. M., Briggs W. R., Wada M., Okada K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosson S., Rajagopal S., Moffat K. (2003) Biochemistry 42, 2–10 [DOI] [PubMed] [Google Scholar]
- 18.Ogura Y., Komatsu A., Zikihara K., Nanjo T., Tokutomi S., Wada M., Kiyosue T. (2008) J. Plant Res. 121, 97–105 [DOI] [PubMed] [Google Scholar]
- 19.Higuchi R., Krummel B., Saiki R. K. (1988) Nucleic. Acids Res. 16, 7351–7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa M., Takahashi F., Nozaki H., Nagasato C., Motomura T., Kataoka H. (2009) Planta 230, 543–552 [DOI] [PubMed] [Google Scholar]
- 21.Crosson S., Moffat K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2995–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narikawa R., Zikihara K., Okajima K., Ochiai Y., Katayama M., Shichida Y., Tokutomi S., Ikeuchi M. (2006) Photochem. Photobiol. 82, 1627–1633 [DOI] [PubMed] [Google Scholar]
- 23.Alexandre M. T., Arents J. C., van Grondelle R., Hellingwerf K. J., Kennis J. T. (2007) Biochemistry 46, 3129–3137 [DOI] [PubMed] [Google Scholar]
- 24.Fedorov R., Schlichting I., Hartmann E., Domratcheva T., Fuhrmann M., Hegemann P. (2003) Biophys. J. 84, 2474–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogura Y., Tokutomi S., Wada M., Kiyosue T. (2008) Plant Signal Behav. 3, 966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsura H., Zikihara K., Okajima K., Yoshihara S., Tokutomi S. (2009) FEBS Lett. 583, 526–530 [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka D., Tokutomi S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13337–13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn R. D., Mistry J., Tate J., Coggill P., Heger A., Pollington J. E., Gavin O. L., Gunasekaran P., Ceric G., Forslund K., Holm L., Sonnhammer E. L., Eddy S. R., Bateman A. (2010) Nucleic Acids Res. 38, D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.