Abstract
Anesthetic agents can induce a paradox activation and sensitization of nociceptive sensory neurons and, thus, potentially facilitate pain processing. Here we identify distinct molecular mechanisms that mediate an activation of sensory neurons by 2,6-diisopropylphenol (propofol), a commonly used intravenous anesthetic known to elicit intense pain upon injection. Clinically relevant concentrations of propofol activated the recombinant transient receptor potential (TRP) receptors TRPA1 and TRPV1 heterologously expressed in HEK293t cells. In dorsal root ganglion (DRG) neurons, propofol-induced activation correlated better to expression of TRPA1 than of TRPV1. However, pretreatment with the protein kinase C activator 4β-phorbol 12-myristate 13-acetate (PMA) resulted in a significantly sensitized propofol-induced activation of TRPV1 in DRG neurons as well as in HEK293t cells. Pharmacological and genetic silencing of both TRPA1 and TRPV1 only partially abrogated propofol-induced responses in DRG neurons. The remaining propofol-induced activation was abolished by the selective γ-aminobutyric acid, type A (GABAA) receptor antagonist picrotoxin. Propofol but not GABA evokes a release of calcitonin gene-related peptide, a key component of neurogenic inflammation, from isolated peripheral nerves of wild-type but not TRPV1 and TRPA1-deficient mice. Moreover, propofol but not GABA induced an intense pain upon intracutaneous injection. As both the release of calcitonin gene-related peptide and injection pain by propofol seem to be independent of GABAA receptors, our data identify TRPV1 and TRPA1 as key molecules for propofol-induced excitation of sensory neurons. This study warrants further investigations into the role of anesthetics to induce nociceptor sensitization and to foster postoperative pain.
Keywords: GABA Receptors, Medicinal Chemistry, Nerve, Neuropeptide, TRP Channels, Anesthetic, Nociceptor, Pain
Introduction
The intravenous general anesthetic propofol3 (2,6-diisopropylphenol) has become one of the most widely used anesthetics. Due to a short context-sensitive half-time, propofol is used for sedations as well as for total intravenous anesthesia during surgery. A drawback of propofol, however, is its high potential to induce intense burning pain upon injection. Depending on the concentration (0.5–2%), the galenic formulation, and co-medication, 24–90% of all patients receiving propofol experience injection pain (1). It was hypothesized that propofol might indirectly or directly interact with sensory nerve fibers located in the venous adventitia (2–4). A recent study claims that the irritant receptor TRPA1, which is expressed in nociceptive sensory neurons, is the predominant molecular entity mediating activation of peripheral nerve endings by general anesthetics (5). Also, TRPA1 was found to mediate propofol-induced pain behavior induced by intranasal propofol and flexor reflex response upon intra-arterial propofol (5). TRPA1 is an excitatory cation channel that is activated by pungent or irritating substances such as acrolein, mustard oil, and formalin (6). TRPA1 has also been demonstrated to be involved in the development of increased pain sensitivity after inflammation and nerve injury (7–10). Another excitatory ion channel of the transient receptor potential family is the capsaicin receptor TRPV1. TRPV1 is activated by noxious stimuli such as capsaicin, protons, and noxious heat (11), and a substantial fraction of TRPV1-expressing neurons also express TRPA1 (6). TRPV1 is required for the development of thermal hyperalgesia and acts in concert with TRPA1 to produce bradykinin-induced hyperalgesia (9, 12–14). We have previously demonstrated that both TRPV1 and TRPA1 are activated by local anesthetics, and a recent report showed sensitizing effects of volatile anesthetics on TRPV1 (15, 16). However, there is conflicting evidence for an activation of TRPV1 by propofol (5, 17). It is conceivable that activation and sensitization of both TRPA1 and TRPV1 by anesthetics might promote the development of postoperative inflammation and pain. As both pain upon injection and postoperative pain constitute relevant side effects of even minor surgical procedures (18), the present study aims to gain a more complete understanding of molecular mechanisms underlying activation of sensory neurons by propofol.
EXPERIMENTAL PROCEDURES
Animals
Animal care and treatment were in accordance with the guidelines of the International Association for the Study of Pain (19). Adult Wistar rats (150–200 g) and adult C57BL/6, TRPV1−/−, and TRPA1−/− as well as TRPV1−/−TRPA1−/− double knock-out mice were used. Breeding pairs of TRPV1 and TRPA1 knock-out mice were obtained from Dr. John Davis (13) and Dr. David Corey (20) and continuously backcrossed to C57BL/6. Double knock-out animals were generated in our animal facility by cross-mating knockouts of both strains. All animals were genotyped by previously reported primers.
Cell Culture
Animals were killed in pure CO2 atmosphere. Dorsal root ganglia (DRGs) of all lumbar and the first two thoracic segments of the spinal column were excised and transferred into Dulbecco's modified Eagle's medium (DMEM) solution containing 50 μg/ml gentamicin (Sigma). The ganglia were treated with 1 mg/ml collagenase and 0.1 mg/ml protease for 30 min (both from Sigma) and subsequently dissociated using a fire-polished silicone-coated Pasteur pipette. The cells were plated on poly-l-lysine-coated (200 μg/ml, Sigma) coverslips and cultured in TNB 100 cell culture medium supplemented with TNB 100 lipid-protein complex, 100 μg/ml streptomycin, penicillin (all from Biochrom, Berlin), and 200 μg/ml glutamine (Invitrogen) at 37 °C and 5% CO2. For patch clamp experiments the medium was supplemented with nerve growth factor (mouse NGF 2.5S, 100 ng/ml; Alomone Labs, Tel Aviv, Israel). Electrophysiological recordings or Ca2+-imaging were performed within 20–30 h of dissociation.
Mutagenesis and Heterologous Expression
Mutagenesis of human and mouse TRPA1 was performed as described previously (21, 22). Human TRPA1 cDNA was obtained from Dr. Paul Heppenstall (EMBL, Monterotondo, Italy), constructs of mouse and human TRPA1 were obtained from Dr. Ardem Patapoutian (The Scripps Research Institute, La Jolla, CA), and all other cDNAs were obtained from Dr. David Julius (University of California, San Francisco). All constructs were confirmed by DNA sequencing. HEK293t cells were transfected as described previously (23). Briefly, HEK293t cells were transfected with plasmids of rat TRPV1 (1 μg), rat, mouse, or human TRPA1 (5 μg), rat TRPV2, TRPV3, TRPV4 (2 μg), or rat TRPM8 (2 μg) along with a reporter plasmid (CD8-pih3m; 1 μg) by the calcium phosphate precipitation method. After incubation for 12–15 h, the cells were replated in 35-mm culture dishes and used for experiments within 2–3 days. Transfection-positive cells were identified by immunobeads (anti-CD8 Dynabeads; Dynal Biotech).
Calcium Imaging
Cells grown on coverslips were loaded with 5 μm fura-2 AM and 0.02% pluronic F-127 (both from Invitrogen) for 30 min, placed into a custom-made chamber, and mounted on a Zeiss Axiovert inverse microscope with a 40× NeoFluar objective. Cells were continuously superfused with extracellular fluid (145 mm NaCl, 5 mm KCl, 1.25 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 10 mm Hepes) or test solutions, applied through a gravity-driven multi-channel common outlet superfusion system (24). Cells were illuminated with a 75-watt xenon arc lamp and a monochromator alternating between 340 and 380 nm of wavelength (Photon Technology International). Images were acquired at 1/s with 200-ms exposure time using a CCD camera, controlled by Image Master software (PTI, Birmingham, NJ). Fluorescence background was continuously recorded and subtracted before calculation of fluorescence ratios. All experimental protocols were preprogrammed. Stimulation responses were quantified as the area under the curve of the fluorescence ratio during the application period; 10 s before application was used as the reference period. Absolute increases in calcium concentration were calculated (25). An increase of the intracellular calcium concentration of at least 50 nm during the application period was considered as activation. For chemicals tested by co-application at the second of three repetitive stimuli, the mean of the first and third response was used as the reference. At the end of all protocols a 10-s stimulus of KCl 60 mm was applied as the control and normalization reference for comparison between different genotypes.
Patch Clamp Recordings
Whole cell voltage clamp was performed on small diameter DRG neurons and transfected HEK293t cells. Membrane currents were acquired with an Axopatch 200B amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA), low-passed at 1 kHz, and sampled at 2 kHz. Electrodes were pulled from borosilicate glass tubes (TW150F-3; World Precision Instruments, Berlin) and heat-polished to give a resistance of 1.5–2.0 megaohms. The standard external solution contained 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 10 mm HEPES, 10 mm glucose (pH 7.4 adjusted with tetramethylammonium hydroxide). In Ca2+-free solutions, CaCl2 was replaced by 5 mm EGTA. The internal solution contained 140 mm KCl, 2 mm MgCl2, 5 mm EGTA, and 10 mm HEPES (pH 7.4 adjusted with KOH). If not otherwise noted, cells were held at −60 mV. All experiments were performed at room temperature. Solutions were applied with a gravity-driven polytetrafluorethylene-glass multiple-barrel perfusion system. The pCLAMP 8.1 software (Axon Instruments) was used for acquisition and off-line analysis.
Release of Calcitonin Gene-related Peptide (CGRP)
C57BL/6 mice of either sex with an average weight of about 20 g were used. The sciatic nerve or the skin of both hind paws distal to the knee was excised. The preparations were fixed to acrylic rods by surgical threads and placed for 30 min in a thermostatic shaking bath at 32 °C, filled with carbogen-gassed (95% O2, 5% CO2) synthetic interstitial fluid containing 108 mm NaCl, 3.5 mm KCl, 26 mm NaHCO3, 1.7 mm NaH2PO4, 9.6 mm sodium gluconate, 7.6 mm sucrose, 5.6 mm glucose, 1.5 mm CaCl2, and 0.7 mm MgSO4 (26). In each experiment, the preparations were first incubated in test tubes containing control synthetic interstitial fluid for two consecutive 5-min periods to determine basal CGRP release. During the third 5-min incubation period, the preparations were chemically stimulated in test solutions; the fourth and fifth 5-min periods were for post-stimulation control. CGRP content of the incubation fluid (120 μl for sciatic nerves, 500 μl for hind paw skin) was measured using a commercial EIA (bertin pharma, Montigny, France) as described in detail (27). CGRP concentrations were determined photometrically using a microplate reader (Dynatech). Blank samples of synthetic interstitial fluid and all stimulation solutions were measured; only the propofol vehicle, a triglyceride emulsion, interfered with the CGRP assay. A similar 70% recovery rate was measured in samples spiked with 50, 100, and 200 pg/ml CGRP, which allowed correction for this error.
Psychophysics
10 mm GABA was diluted in isotonic saline; the GABA solution and isotonic saline were titrated to pH 7.4 and sterile-filtered before injection. All subjects were intracutaneously injected with 10 mm GABA and isotonic saline (50 μl each) with a 27-gauge needle into separate spots in the center of the volar forearm. Both injections were performed in a double-blinded fashion at an interval of 15 min between injections. In a similar design, all subjects were injected with Propofol 1%® and its carrier Lipofundin®, again double-blinded at 15-min intervals. Pain was assessed on a numerical rating scale (0–10) at 1-min intervals for a period of 10 min.
Chemicals
Propofol (Sigma) was dissolved in dimethyl sulfoxide to a stock solution of 50 mm or 1 m and diluted in physiological buffer upon use. Clinical colloidal solutions of propofol were obtained from Braun (1 and 2% Propofol-Lipuro®, Melsungen, Germany) and from AstraZeneca (Disoprivan®, 1% propofol, Wedel, Germany); they could only be used diluted (1/1000–1/10) in physiological buffers, because the original solutions do not contain any essential salts or sugars. Capsaicin, GABA, capsazepine, acrolein, carvacrol, picrotoxin, muscimol, and baclofen were obtained from Sigma, PMA was from Calbiochem-Novabiochem, HC-030031 was from Enamine (Kiev, Ukraine), and BCTC was from Enzo Life Sciences (Lörrach). 10 mm capsaicin and 1 mm PMA were dissolved in ethanol, 10 mm capsazepine in dimethyl sulfoxide.
Data Analysis
Two data groups containing at least 10 samples were compared with dependent or independent samples t tests. Multiple groups and repeated measurements were compared by analysis of variance and LSD post hoc tests. CGRP release experiments were performed using both hind paw skin flaps of the same animal and compared using the Wilcoxon matched pairs test. Association of variables was tested with the product-momentum correlation. Statistical analysis was performed using Statistica 7 (Statsoft, Tulsa, OK), dose-response curves were fitted by the Hill equation in Origin 7.5 (OriginLab, Northhampton, MA). Data are presented as the mean ± S.E.; p < 0.05 was considered significant.
RESULTS
Propofol Induces an Increase in [Ca2+]i in Cultured DRG Neurons
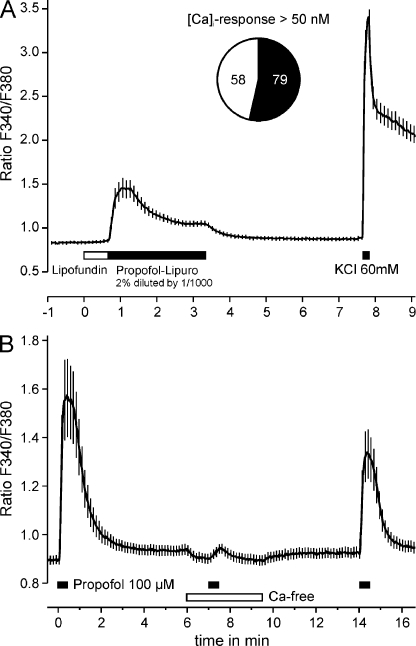
To investigate excitatory effects of propofol on sensory neurons, we first applied clinically used formulations of propofol to cultured DRG neurons from adult C57BL/6 mice. A 1000-fold diluted solution of 2% Propofol-Lipuro® (112 μm) evoked a reversible increase in intracellular calcium [Ca2+]i in 79 of 137 neurons when applied for 3 min (Fig. 1A). Notably, this response showed a transient component of less than 60 s and a sustained component that persisted as long as propofol was applied. Lipofundin® (1000-fold diluted), the carrier for propofol in Propofol-Lipuro®, induced no increase in [Ca2+]i. In contrast, a robust increase in [Ca2+]i was also induced by 100 μm propofol dissolved in external solution containing 0.2% dimethyl sulfoxide (Fig. 1B). In the absence of extracellular calcium, this increase in [Ca2+]i was completely abrogated (<4% of control, n = 30, p < 0.001, t test, Fig. 1B). A third application of propofol in the presence of extracellular calcium, however, elicited a reduced response, 57 ± 14% in size of the first. Thus, propofol apparently evokes an influx of extracellular calcium in a large subpopulation of DRG neurons.
FIGURE 1.
Propofol evokes an increase in [Ca2+]i in cultured DRG neurons from C57BL/6 mice. A, a 1000-fold diluted (112 μm) clinical propofol solution (Propofol-Lipuro® 2%) evoked an increase in [Ca2+]i in more than half of all neurons investigated. Note the persistent elevation of [Ca2+]i throughout the 3-min application of propofol. No effect was observed of the 1000-fold diluted carrier solution (Lipofundin®) applied before propofol. B, propofol-evoked increase in [Ca2+]i is due to an influx of extracellular calcium. Propofol (100 μm) in aqueous solution was applied for 30 s in intervals of 5 min. The second application of propofol was performed in calcium-free extracellular solution. The final 60 mm potassium application is not shown in this and further panels. Data are presented as the mean ± S.E. of all neurons tested.
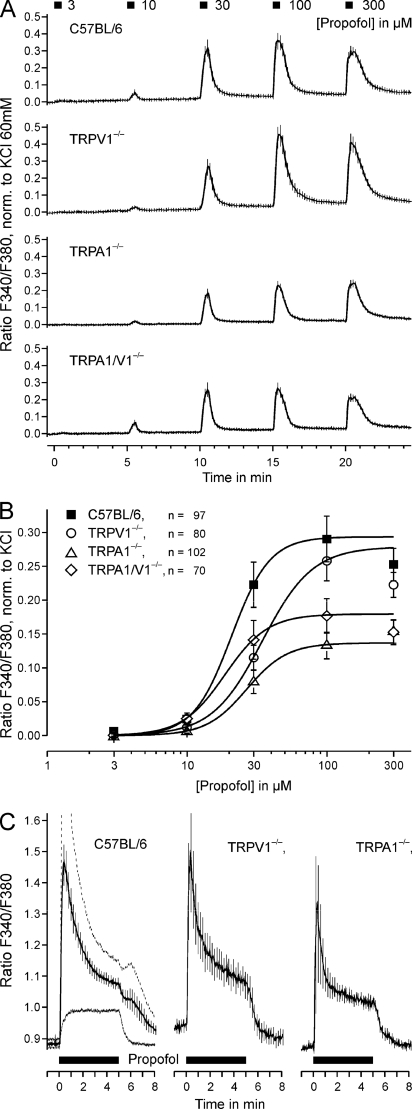
As demonstrated in Fig. 2, A and B, a 30-s application of propofol in concentrations of 10 μm or greater repeatedly induced an increase in [Ca2+]i in a concentration-dependent manner (p < 0.001 each, n = 89, t test-dependent samples, Fig. 2A). The corresponding EC50 value for wild-type DRG neurons was 21 ± 1 μm (Fig. 2B). As TRPV1 and TRPA1 were considered to be the main candidates mediating this propofol-induced increase in [Ca2+]i, concentration-dependent activation by propofol was also investigated in DRG neurons of mutant mice lacking TRPV1, TRPA1, or both receptors (Fig. 2A). Surprisingly, similar concentration-dependent responses were observed in these DRG neurons (p < 0.001 for ≥10 μm propofol, n = 80, 102, and 70 for the respective genotypes, t test-dependent samples). However, the efficacy of propofol was significantly reduced in neurons from knock-out mice as compared with wild-type (analysis of variance F(3,298) = 6.7, TRPV1−/− p = 0.036, n = 80; TRPA1−/− p < 0.001, n = 102; TRPV1/A1−/− p = 0.003, n = 70; LSD post hoc tests, Fig. 2B). Although TRPA1−/− neurons generated significantly smaller propofol-induced responses as compared with TRPV1−/− neurons (p = 0.039, LSD post-hoc test), no significant differences were observed between neurons derived from double-knock-out animals and the individual knock-out animals.
FIGURE 2.
TRPA1 and TRPV1 are not required for propofol-evoked calcium increase in DRG neurons. A, shown is concentration-dependent activation by propofol of DRG neurons from wild-type C57BL/6 mice and mutants deficient of TRPV1, TRPA1, or both receptors. Applications lasted for 30 s and were applied at intervals of 5 min. Fluorescence ratios of all tested neurons were normalized to the individual response to 60 mm external potassium and averaged. B, concentration-response curves of propofol-evoked responses in DRG neurons derived from wild-type C57BL/6, TRPV1−/−, TRPA1−/−, and TRPV1/A1−/− mice. Curves were fitted to the Hill equation. Note the retained propofol effect even in the double mutants. C, propofol-evoked responses display similar kinetics in neurons from C57BL/6, TRPV1−/−, and TRPA1−/− animals. Propofol (100 μm) was applied for 300 s, and data are presented as the mean ± S.E. of the fluorescence ratio. For C57BL/6 neurons, two separate means are shown for neurons with a calcium increase above (n = 61, stippled line) and below (n = 51, continuous line) 50 nm in the first minute. Note the different activation time constants of 11 and 23 s of the calcium increase. In neurons from both TRPV1−/− and TRPA1−/− mice the calcium increase was also sustained over the application period but afterward returned to base line. Data are presented as the mean ± S.E. of all neurons tested.
We also explored whether the functional properties of propofol-induced responses in DRG neurons are altered in TRPV1−/− and TRPA1−/− neurons. The activation and acute adaptation or desensitization was studied by the application of 100 μm propofol for 300 s. As demonstrated in Fig. 2C, the increase in calcium showed transient and sustained components in wild-type neurons as well as in neurons lacking TRPV1 or TRPA1. Notably, such a sustained component was not observed with other TRPV1 and TRPA1 agonists (data not shown), suggesting the involvement of another mechanism for propofol-induced activation of DRG neurons. Therefore, the excitatory effect of propofol was further examined by a combined genetic and pharmacological approach.
TRPV1, TRPA1, and GABAA Receptors Mediate the Propofol-induced [Ca2+]i Rise in DRG Neurons
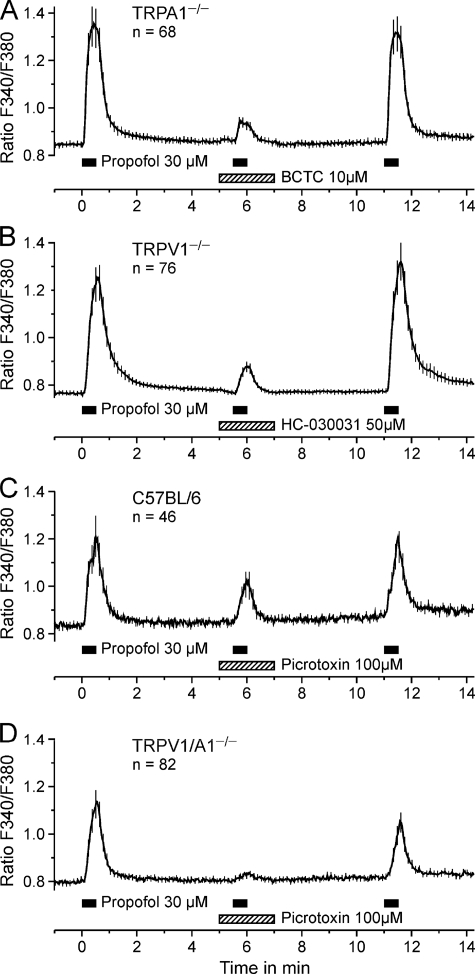
As displayed in Fig. 3, A–D, three repetitive applications of 30 μm propofol were applied to wild-type C57BL/6, TRPV1−/−, TRPA1−/−, and TRPV1/A1−/− neurons. In neurons from TRPA1−/− animals, the second response to propofol in presence of the TRPV1 antagonist BCTC (10 μm) was largely reduced (p < 0.001, n = 68, t test, Fig. 3A). In neurons from TRPV1−/− animals, the TRPA1 antagonist HC-030031 (50 μm) strongly reduced the response to propofol (p < 0.001, n = 76, t test; Fig. 3B). Both BCTC and HC-030031 did not increase calcium per se, and the propofol responses after washout of the antagonists were not different from the first one (p = 0.54 and p = 0.82, t test). HC-030031 was recently reported to be a selective blocker of TRPA1 (8). However, we found that HC-030031 (50 μm) moderately inhibited calcium increases induced by high external potassium (analysis of variance F(1,139) = 11.4, p < 0.001). In wild-type neurons, we observed an inhibition by 28% at 15 μm and by 42% at 50 μm HC-030031 (both p < 0.001, n = 23, LSD post hoc tests, Supplement 1A). Importantly, HC-030031 also inhibited potassium-induced calcium increases in neurons from TRPA1 knock-out animals (inhibition by 10% at 15 μm and by 11% at 50 μm, p = 0.009 and p = 0.020, n = 26, LSD post hoc tests, Supplement 1B), although the inhibition was less concentration-dependent and pronounced as compared with neurons from wild-type animals (p < 0.001, LSD post hoc test). Therefore, the TRPA1-related inhibition by HC-030031 of the propofol response could be overestimated. BCTC, in contrast to HC030031, did not reduce calcium influx evoked by high external potassium (data not shown).
FIGURE 3.
Selective blockers of TRPA1, TRPV1, and GABAA receptors reduce propofol-evoked responses in DRG neurons. A–D, propofol 30 μm was applied for 30 s at intervals of 5 min. As indicated in the figures, the respective blocker was co-applied with the second propofol application. A, in TRPA1−/− neurons, the TRPV1-blocker BCTC reversibly reduced the response to propofol. B, in TRPV1−/− neurons the TRPA1-blocker HC-030031 reduced the response to propofol (however, see Supplement 1). C and D, the GABAA receptor blocker picrotoxin (100 μm) reduced responses to propofol in C57BL/6 neurons (C) and completely blocked responses in neurons from TRPV1/A1−/− mice (D). Data are presented as the mean ± S.E. of all neurons tested.
Activation of GABAA receptors by propofol in the central nervous system is well known and putatively contributes to the hypnotic effects of propofol (28, 29). As GABAA receptors are expressed in primary sensory neurons, their activation was considered a possible mechanism mediating the residual, TRPA1/TRPV1-independent action of propofol in DRG neurons. Using the same protocol as for the TRP channels, the contribution of GABAA receptors was investigated using the non-competitive GABAA receptor antagonist picrotoxin. In neurons from wild-type C57BL/6 mice, 100 μm picrotoxin reduced the response to propofol by 48% (p = 0.019, n = 46, t test, Fig. 3C). In neurons from TRPV1/A1 double knock-out mice, the response was reduced by 83% (p < 0.001, n = 82, t test, Fig. 3D), and the remaining calcium increase in the presence of propofol and picrotoxin was minimal (3.3 ± 0.6 nm). Thus, GABAA receptors indeed appear to mediate propofol-induced activation of sensory neurons.
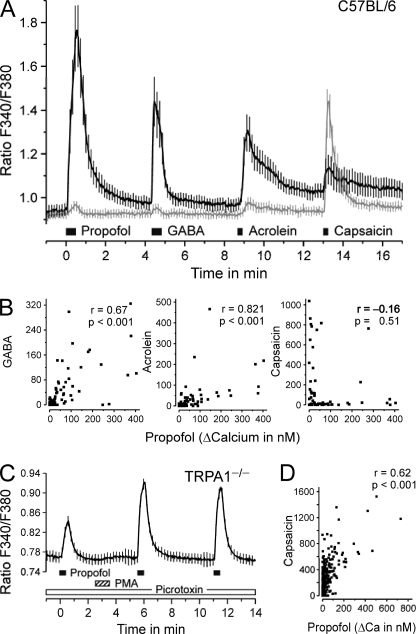
We next aimed at analyzing the relative contributions of TRPV1, TRPA1, and GABAA receptors to the effect of propofol on DRG neurons. To this end, the sensitivity of wild-type DRG neurons to propofol (30 μm), GABA (10 μm), capsaicin (100 nm), and the TRPA1 agonist acrolein (10 μm) was consecutively investigated (Fig. 4A). The responsiveness to propofol was strongly correlated to the magnitude of responses evoked by GABA (r = 0.67, p < 0.001, n = 83). A similar correlation with propofol was found for responses evoked by acrolein (r = 0.82, p < 0.001) but not by capsaicin (r = −0.16, p = 0.51, Fig. 4B). However, there was also a significant negative correlation between GABA and capsaicin responses in wild-type neurons (r = −0.23, p = 0.04), which may mask the propofol-capsaicin correlation. In fact, a significant positive correlation between propofol and capsaicin (100 nm) resulted if both TRPA1 and GABAA receptors were genetically and pharmacologically silenced (r = 0.62, p < 0.001, n = 502, Fig. 4D). In the presence of picrotoxin (100 μm), 18% of all investigated TRPA1−/− neurons (n = 502) still responded to propofol (50 μm). This fraction increased to 38% after conditioning treatment with the protein kinase C (PKC) activator PMA (100 nm for 60 s). Furthermore, the fraction of propofol-sensitive neurons, which also responded to capsaicin, increased from 45 to 93% after treatment with PMA. Synchronously, the propofol-induced calcium increase was augmented to 306% of the original response (p < 0.001, t test, Fig. 4C). GABA (10 μm) was completely ineffective under these experimental conditions (Fig. 4D).
FIGURE 4.
DRG neurons of C57BL/6 mice respond to GABA and TRP agonists. A, mean calcium levels of neurons above (black) and below (gray) 50 nm calcium increase upon stimulation with propofol. B, the calcium increase evoked by propofol (30 μm) is correlated with the responses to GABA (10 μm) and acrolein (10 μm) but not to capsaicin (100 nm). The panels provide the product-momentum correlation coefficient r. C, propofol (50 μm) activated TRPA1−/− neurons in the presence of picrotoxin (100 μm) is shown. PMA (100 nm) sensitized the response to subsequent propofol stimulations, and the sensitized propofol response is correlated to the response evoked by capsaicin (100 nm) (D). The diameter of propofol-responsive neurons was 22.6 ± 0.7 μm, similar to all tested neurons in this protocol (23.5 ± 0.7 μm) and neurons responsive to GABA (24.2 ± 0.8 μm) or acrolein (23.6 ± 0.8 μm); only capsaicin-sensitive neurons were smaller compared with the other groups (19.7 ± 0.7 μm, p ≤ 0.02 for all comparisons, t tests). Data are presented as the mean ± S.E. of all neurons tested.
Propofol Evokes Inward Currents in Mouse DRG Neurons That Are Mediated by TRPV1, TRPA1, and GABAA Receptors
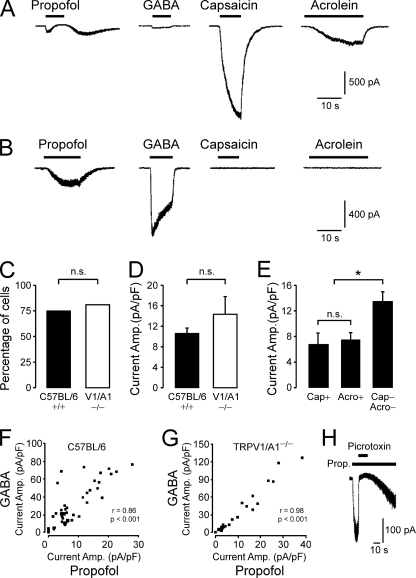
To corroborate the data obtained by calcium imaging and to study the effects of propofol in more detail, whole-cell voltage clamp was performed on DRG neurons from wild-type C57BL/6 and TRPV1/A1−/− mice. DRG neurons were characterized due to their sensitivity to propofol (300 μm), GABA (50 μm), capsaicin (1 μm), and acrolein (50 μm). The relatively high concentration of propofol was applied to obtain comparable responses. As demonstrated in Fig. 5, A and B, DRG neurons generated propofol-induced inward currents with various amplitudes and heterogeneous kinetic properties. In wild-type neurons, 300 μm propofol evoked inward currents in 75% (44/59) of all neurons tested with a mean current amplitude of 10.6 ± 1.1 pA/pF. Strikingly, both the prevalence (82%, 22/27) and the mean amplitude (14.3 ± 3.5 pA/pF) of propofol-induced currents were not different in TRPV1/A1−/− neurons as compared with wild type (p = 0.49 and p = 0.23, t test, Fig. 5, C and D). Only 7 (16%) of all propofol-sensitive wild-type neurons were also capsaicin-sensitive; these neurons generated small propofol-induced currents (6.8 ± 1.9 pA/pF). Capsaicin-induced currents were observed in 32% (19/59) of all wild-type neurons tested. Thus, the majority (12, 63%) of these neurons did not respond to propofol. Similarly, 36.4% (16/44) of all propofol-sensitive wild-type neurons were acrolein-sensitive and generated small propofol-induced currents (7.4 ± 1.3 pA/pF, p = 0.74, t test, Fig. 5E). In contrast to capsaicin-sensitive neurons, the majority of the acrolein-sensitive neurons (31%, 18/59) also generated propofol-induced currents (89%, 16/18). As demonstrated in Fig. 5A, acrolein-sensitive neurons typically generated propofol-induced currents with a resurging current after the application of propofol. Furthermore, 55% (24/44) of all propofol-sensitive wild-type neurons did not display sensitivity to capsaicin or acrolein but generated significantly larger propofol-induced currents than neurons that were sensitive to capsaicin or acrolein (13.4 ± 1.6 pA/pF, p = 0.002, t test, Fig. 5E), suggesting a negative correlation between propofol/GABA responses and the effects of the irritants. 50 μm GABA induced inward currents in 81.5% of wild type (44/54) as well as TRPV1/A1−/− neurons (22/27). All propofol-sensitive neurons also responded to GABA (wild-type 40/40, TRPV1/A1−/− 22/22). Furthermore, a strong correlation was found for peak current amplitudes evoked by propofol and GABA in neurons from both wild-type (r = 0.86, p < 0.001, Fig. 5F) and TRPV1/A1−/− animals (r = 0.98, p < 0.001, Fig. 5G). Propofol-induced inward currents in neurons derived from TRPV1/A1−/− animals were completely blocked by the GABAA receptor blocker picrotoxin (100 μm, n = 7, Fig. 5H).
FIGURE 5.
Propofol evokes inward currents in mouse DRG neurons. A and B, shown are representative current traces of DRG neurons treated with 300 μm propofol, 50 μm GABA, 1 μm capsaicin, and 50 μm acrolein. Cells were held at −60 mV, and each substance was applied for 10–30 s at intervals of 2 min. A, small and transient propofol-evoked currents with a resurgent current after application of propofol were commonly observed in neurons generating small GABA-evoked currents but large capsaicin and acrolein-evoked currents. B, large propofol-evoked currents were observed in neurons generating large GABA-evoked currents but no capsaicin-and acrolein-evoked currents. C and D, the percentage (C) of DRG neurons generating propofol-evoked (>30 pA) currents and their current amplitudes (D) were not significantly different in neurons derived from wild-type C57BL/6 and TRPV1/A1 double-knock-out mice. * denominates p < 0.05; p < 0.001; n.s., denominates a non-significant finding. E, in wild-type DRG neurons, amplitudes of propofol-evoked currents were larger in capsaicin and acrolein-insensitive neurons compared with capsaicin or acrolein-sensitive neurons. F and G, in both wild-type C57BL/6 (F) and TRPV1/A1 knock-out (G) neurons, a strong correlation was found for peak amplitudes of currents evoked by GABA and propofol. Peak current amplitudes evoked by propofol and GABA were plotted for each cell. The panels show the product-momentum correlation coefficient. H, the propofol (Prop.)-evoked (300 μm) inward currents in neurons derived from a TRPV1/A1 double-knock-out mouse were completely and reversibly blocked by the GABA antagonist picrotoxin (100 μm).
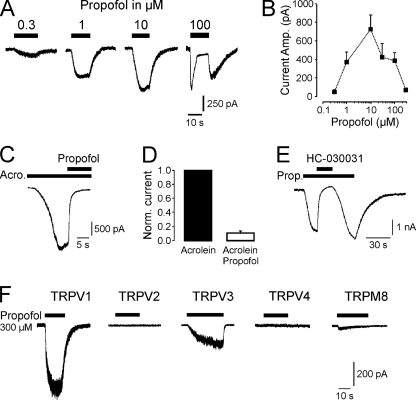
Propofol Activates and Blocks TRPA1
It was recently suggested that TRPA1 is the only TRP receptor in DRG neurons that is activated by propofol (5). We found that rat TRPA1 expressed in HEK293t cells is indeed activated by propofol in a concentration-dependent and reversible manner (Fig. 6A). Propofol induced inward currents in TRPA1-HEK293t cells at 0.3 μm and higher concentrations, thus, displaying a comparable potency on TRPA1 as has been described for GABAA receptors (30). At concentrations above 10 μm, propofol consistently activated currents that appeared to inactivate during stimulation (Fig. 6B). These currents were associated with resurging currents after termination of propofol (see 100 μm propofol in Fig. 6A). This phenomenon has been reported previously (5), and a possible mechanism might be a propofol-induced pore-block of TRPA1 in the open state. To test this hypothesis, propofol was co-applied when an acrolein-induced inward current had reached steady state (Fig. 6C). Indeed, 30 μm propofol blocked acrolein-induced currents by 89.2 ± 2.8%, n = 7 (p = 0.018 Wilcoxon, Fig. 6, C and D). Fig. 6 further demonstrates that propofol-induced currents in TRPA1-HEK293t cells were completely blocked by the TRPA1 antagonist HC-030031 (100 μm, n = 5, Fig. 6E). We also examined the effect of propofol on other TRP receptors supposed to be functionally expressed in sensory neurons or to have an impact on peripheral nociception. As demonstrated by representative current traces in Fig. 6F, 300 μm propofol activated robust inward currents in HEK293t transiently expressing rat TRPV1 and TRPV3. TRPM8-HEK293t cells produced minimal propofol-induced currents (<50 pA, n = 8), no response was observed in TRPV2, TRPV4, or non-transfected HEK293t cells (n = 9–14 for each receptor type). In the case of TRPV3, current amplitudes regularly increased upon repeated application of propofol (Supplement 2). This sensitization upon repeated stimulation is a typical feature of TRPV3 (31).
FIGURE 6.
Propofol activates and blocks TRPA1. A, shown are representative current traces of propofol-evoked inward currents. Increasing concentrations of propofol were applied for 10–15 s on different HEK293t cells expressing TRPA1 (Vm −60 mV). To prevent desensitization, only one concentration was tested on each cell. B, shown is a dose-response curve for propofol-evoked activation of TRPA1. Each concentration was tested on 6–10 cells. C and D, propofol blocks acrolein-evoked currents in HEK293t-TRPA1 cells. C, propofol (30 μm) was co-applied with 50 μm acrolein after the acrolein-evoked current had reached a steady state. D, block by propofol was calculated on normalized currents activated by 50 μm acrolein alone and in combination with 30 μm propofol. E, the TRPA1 antagonist HC-030031 (100 μm) completely blocked propofol (Prop.)-evoked currents in HEK293t-TRPA1 cells. HC-030031 was co-applied with 10 μm propofol after the propofol-evoked current had reached a steady state. F, propofol activates TRPV1 and TRPV3 but not TRPV2, TRPV4, and TRPM8. Representative current traces are shown of each TRP subunit treated with propofol. The effect of 300 μm propofol was examined on HEK293t cells expressing TRPV1, TRPV2, TRPV3, TRPV4, and TRPM8. Cells were held at −60 mV. Note the small (< 20 pA) current observed in some TRPM8-expressing cells, probably reflecting a weak activation by propofol.
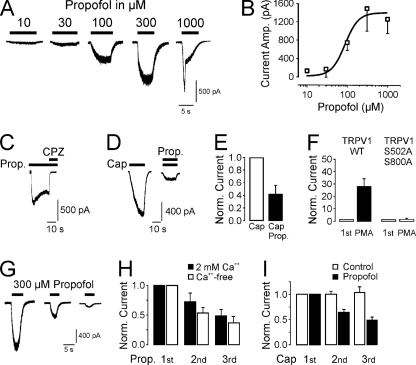
Propofol Activates and Desensitizes TRPV1
The action of propofol on TRPV1 was further investigated on the recombinant rat TRPV1 heterologously expressed in HEK293t cells. As demonstrated in Fig. 7A, propofol activated TRPV1 in a concentration-dependent manner with an EC50 of 90 ± 20 μm (n = 6–10 for each concentration, Fig. 7B). Propofol-induced inward currents were completely blocked by the competitive TRPV1 antagonist capsazepine (10 μm, 99 ± 3%, n = 10, Fig. 7C). Unlike most TRPV1 agonists, propofol did not sensitize TRPV1 when co-applied in half-maximal effective concentrations together with another TRPV1-agonist. As demonstrated in Fig. 7, D and E, co-application of capsaicin (5 nm) and propofol (100 μm) resulted in even smaller currents than application of capsaicin alone (58.4 ± 13.7% reduction, p = 0.003, n = 8, t test). The propofol sensitivity of TRPV1 was strongly enhanced by the activation of PKC by PMA, an effect that had previously proven very effective in calcium imaging experiments on DRG neurons (Fig. 4C). When the initial application of 100 μm propofol was followed by a 3-min lasting superfusion of the PKC activator PMA (1 μm), the inward currents activated by a second application of propofol displayed a 28 ± 6.8-fold increase in peak amplitudes (p = 0.001, t test, n = 8, Fig. 7F). This PMA-induced sensitization of propofol-induced inward currents was abrogated in the TRPV1 double-mutant S502A/S800A, the putative PKC phosphorylation sites of TRPV1 (1.6 ± 0.7-fold increase, p = 0.42, t test, n = 6, Fig. 7E). We also explored the ability of propofol to desensitize TRPV1. A pronounced desensitization and tachyphylaxis is a typical feature of TRPV1 when activated repeatedly. This process is induced by a rise in [Ca2+]i and can, thus, be significantly reduced by the removal of extracellular Ca2+ ions (21). When applied repeatedly, 300 μm propofol induced inward currents displaying a strong desensitization (analysis of variance, F(1,32) = 12.392, p = 0.001, Fig. 7G). The responses to subsequent propofol applications were reduced to 72.3 ± 14.2 and 48.8 ± 10.2% in the presence of extracellular calcium and not significantly different in the absence of extracellular calcium (53.7 ± 20.1%, n = 5 and 37.0 ± 24.0%, n = 7, p = 0.66, LSD post-hoc test, Fig. 7, G and H). In contrast, repeated applications of capsaicin (100 nm) in the absence of extracellular Ca2+ produced stable inward currents without any sign of desensitization (2nd application to 99.9 ± 5.5% and 3rd application to 103.4 ± 10.9%, n = 5). Intriguingly, continuous application of propofol (30 μm) during the intervals (3 min) of the capsaicin applications resulted in a cross-desensitization of the capsaicin-induced currents even in the absence of extracellular Ca2+ (2nd application to 63.8 ± 6.2% and 3rd application to 48.6 ± 6.7%, p = 0.001 and 0.012, Wilcoxon, n = 8).
FIGURE 7.
Propofol activates and desensitizes TRPV1. A, shown are traces of propofol-evoked inward currents. Increasing concentrations of propofol were applied for 10–15 s on HEK293t-TRPV1 cells (Vm −60 mV). To prevent desensitization, only one concentration was tested on each cell. B, shown is a concentration-response curve for propofol-evoked activation of TRPV1. The curve corresponds to a fit with the Hill equation. C, the TRPV1-antagonist capsazepine (CPZ, 10 μm) blocked propofol-evoked TRPV1 currents. CPZ was co-applied with propofol (100 μm) after the propofol-evoked current had reached a steady state. D and E, Propofol (Prop.) does not potentiate capsaicin (Cap)-evoked TRPV1-currents. D, capsaicin (5 nm) was applied alone and then co-applied with 100 μm propofol. E, when normalized to the current activated by capsaicin alone, co-application with propofol resulted in a reduction of the current amplitude. F, activation of PKC sensitized propofol-evoked currents in HEK293t-TRPV1 cells. After the first application of propofol (1 μm), cells were treated with the PKC activator PMA (100 nm). Whereas wild-type TRPV1 was sensitized by PMA, no sensitization was observed on the TRPV1-mutant S502A/S800A. G and H, calcium-independent desensitization of propofol-evoked TRPV1 currents is shown. G, representative traces displaying desensitization of currents activated by repeated applications of 300 μm propofol are shown. Propofol was applied for 5–10 s at intervals of 2 min (Vm −60 mV). H, when normalized to the first application, a calcium-independent desensitization was revealed. I, propofol desensitizes capsaicin-evoked currents in HEK293t-TRPV1 cells. Capsaicin (100 nm) was applied every 2 min in a calcium-free extracellular solution. Whereas no significant desensitization of capsaicin-evoked currents was observed in cells treated with control solution, a continuous application of 30 μm propofol resulted in desensitization.
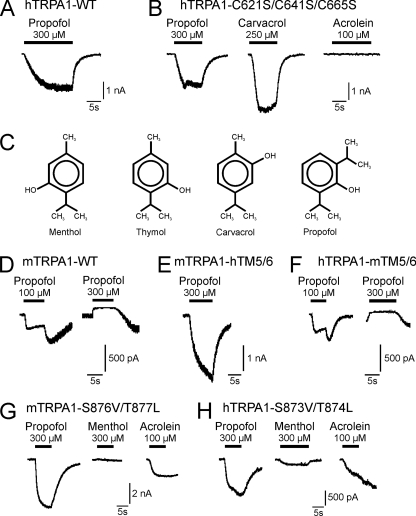
Propofol Modulates TRPA1 by Mechanisms Other Than Covalent Modification of Reactive Cysteine Residues or Interacting with Residues within TM5
Considering our data and previous reports, propofol obviously activates several TRP channels with the greatest potency for TRPA1 followed by TRPV1. The relatively slow kinetics of propofol-induced TRPA1 currents resemble the properties of currents activated by substances considered to activate TRPA1 by a covalent modification of reactive cysteine residues, i.e. acrolein and mustard oil (32, 33). Notably, the same mechanism (i.e. a covalent modification of cysteine residues) was also demonstrated to mediate a sensitization or activation of TRPV1 (37, 38). We, therefore, asked if propofol modulates TRP channels by this mechanism and first studied mutant constructs of human TRPA1 (hTRPA1) in which the responsible cysteine residues were replaced by serine (hTRPA1-C621S/C641S/C665S). As previously demonstrated, this triple mutant was completely insensitive to 100 μm acrolein (Fig. 8B). However, it still generated large inward currents after application of 300 μm propofol (697.5 ± 263.3 pA, n = 8) and the previously established TRPA1-agonist carvacrol (250 μm) (Fig. 8B). Thus, modification of cysteine residues is unlikely to be the common mechanism for propofol-induced activation of TRP channels. While performing the experiments on human TRPA1, we observed a prominent species difference between wild-type rat and human TRPA1 with regard to propofol-induced inward currents. The above-mentioned resurging currents, which were observed for rTRPA1 after termination of propofol in concentrations above 10 μm (Fig. 6A), were missing for hTRPA1 even with 300 μm propofol (Fig. 8A, n = 7 for wild-type hTRPA1). A similar species difference between rodent and human TRPA1 was recently demonstrated for activation by menthol; mouse TRPA1 (mTRPA1) is both activated and blocked by menthol, whereas hTRPA1 is only activated (22). This differential effect might be determined by the pore region including the transmembrane domain 5 (TM5). Based on comparative studies on mammalian TRPA1 and the menthol-insensitive Drosophila TRPA1, Xiao et al. (22) also identified specific residues within TM5 as being crucial for both menthol and thymol sensitivity of TRPA1. Notably, menthol, thymol, and its isomer carvacrol all display close structural similarities to propofol (Fig. 8C). Furthermore, menthol and propofol share common interaction sites for activation of GABAA receptors (39, 40). We, therefore, pursued the possibility that propofol employs the same mechanisms as menthol and thymol to activate TRPA1 and next explored mutant constructs of mouse and human TRPA1. Wild type mTRPA1 behaved similarly to rTRPA1 and displayed propofol-induced inward currents at 100 μm and inhibition at 300 μm followed by resurging currents (Fig. 8D, n = 6). The chimera mTRPA1-hTM5–6, however, in which the region TM5 through TM6 from hTRPA1 was introduced, behaved like wild-type hTRPA1 and displayed large propofol-induced currents without a resurging current after application (Fig. 8E, n = 5). Accordingly, the reverse chimera hTRPA1-mTM5–6 behaved like wild-type mTRPA1 and displayed propofol-induced currents at 100 μm and inhibition at 300 μm followed by prominent resurging currents (Fig. 8F, n = 6). We next examined if residues within TM5, which were demonstrated to be required for activation by menthol and thymol (22) are also required for propofol sensitivity of TRPA1. Surprisingly, 300 μm propofol induced large inward currents in Hek293t cells expressing the menthol-insensitive mutants mTRPA1-S876V/T877L (Fig. 8G, 1909.6 ± 808.6 pA, n = 5) and hTRPA1-S873V/T874L (Fig. 8H, 493.4 ± 162.0 pA, n = 5) (Fig. 8H). Notably, mTRPA1-S876V/T877L generated propofol-induced current lacking a resurging current after application. In contrast to propofol, we found that both mutants were insensitive to carvacrol (data not shown). Thus, despite similarities in chemical structure, propofol obviously employs other or additional mechanisms to gate TRPA1 as compared with carvacrol, thymol, and menthol.
FIGURE 8.
Potential interaction sites of propofol on TRPA1. A and B, representative current traces of propofol-evoked inward currents on wild-type hTRPA1 (A) or hTRPA1-C621S/C641S/C665S (B) are shown. Propofol (300 μm), carvacrol (250 μm), and acrolein (100 μm) were applied for 10–30 s on HEK293t cells held at Vm −60 mV. C, chemical structures of menthol (2-isopropyl-5-methylcyclohexanol), thymol (2-isopropyl-5-methylphenol), carvacrol (5-isopropyl-2-methylphenol), and propofol (2,6-diisopropylphenol) are shown. Transmembrane domain 5 is a determinant for species different activation of TRPA1 by propofol. D–F, shown are representative current traces of inward currents evoked by propofol (100 and 300 μm) on wild-type mTRPA1 (D), the chimera mTRPA1-hTM5/6 (E), and the chimera hTRPA1-mTM5/6 (F). Propofol was applied for 10–15 s on HEK293t cells held at Vm −60 mV. Propofol and menthol require distinct interactions sites to activate TRPA1. G–H, representative current traces of inward currents evoked by propofol (300 μm), menthol (300 μm) and acrolein (100 μm) on the mutant constructs mTRPA1-S876V/T877L (G) and hTRPA1-S873V/T874L (H) are shown. Substances were applied for 10–30 s on HEK293t cells held at Vm −60 mV.
Propofol Induces a TRPV1 and TRPA1 but Not GABAA-dependent Release of CGRP from Isolated Peripheral Nerves
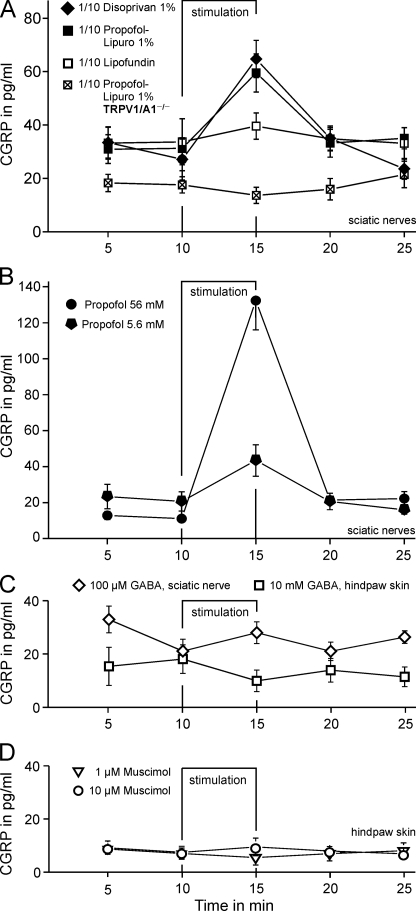
The experiments performed on cultured cells suggest that the excitatory effect of propofol is largely carried by TRPA1 and GABAA receptors, both masking a substantial contribution of TRPV1 that may at least become relevant under conditions of inflammatory sensitization. However, DRG neurons in culture are just a model of their nociceptive nerve endings that express functional TRPA1 and TRPV1 channels to mediate pain upon activation. In contrast, it is quite uncertain whether GABA is able to excite peripheral nerve fibers to induce pain as well. To address this question, we now aimed at investigating whether propofol is able to activate peripheral nerve endings and induce a release of the proinflammatory neuropeptide CGRP. Activation of nociceptive neurons leads to a Ca2+-dependent release of CGRP, which contributes to neurogenic inflammation, to peripheral sensitization of nociceptive afferents, and to central sensitization in the spinal cord (34, 35). Isolated mouse sciatic nerves were stimulated with clinically used emulsions of propofol or with Lipofundin®. 1% Propofol-Lipuro® or 1% Disoprivan®, 10-fold diluted in synthetic interstitial fluid (= 5.6 mm propofol), evoked a significant release of CGRP (both p = 0.012 and n = 8, Wilcoxon) with similar efficacies (p = 0.40, U test). In contrast, 10-fold diluted Lipofundin® did not stimulate CGRP release (Fig. 9A). In TRPV1/A1 double knock-out animals, no CGRP release was observed upon stimulation with Propofol-Lipuro® 1% (p = 0.27, n = 8, Wilcoxon). However, high external KCl (60 mm) evoked similar responses to those observed in wild types (data not shown). The diluted clinical emulsions are known to contain “free” propofol in the aqueous phase at concentrations of 5 and 6.7 μm, respectively (36). These concentrations and 100-fold higher ones of propofol in aqueous solution (using DMSO as a solubilizer) did not evoke a significant CGRP release from the isolated nerve preparation (data not shown). Propofol at 5.6 mm, the same concentration as nominally contained in both 10-fold diluted clinical emulsions, induced a significant response of about the same magnitude as diluted Propofol-Lipuro® and Disoprivan® (Fig. 9A). This response was clearly concentration-dependent as the 10-fold higher propofol concentration 56 mm (as in the undiluted original clinical emulsions) caused a 5-fold greater CGRP release (Fig. 9B).
FIGURE 9.
Propofol-stimulated release of CGRP from isolated sciatic nerves. A, Lipofundin® is the medium-chain triglyceride/long-chain triglyceride carrier solution of Propofol-Lipuro® is shown. Compared with diluted Lipofundin®, 10-fold diluted triglyceride emulsions of 1% propofol (5.6 mm in Propofol-Lipuro® and the long-chain triglyceride emulsion Disoprivan®) evoked a significant and reversible increase in CGRP release in C57BL/6 mice. This response was abrogated in nerves from TRPV1/A1−/− double knock-out mice (n = 8 sciatic nerves per group). B, propofol in aqueous solution (5.6 mm from 1 m stock in DMSO) induced about as much CGRP release as the diluted clinical emulsions of the same overall concentration, and 5-fold more CGRP was released at a 10-fold higher propofol concentration which equals the original emulsions (n = 8 sciatic nerves for both concentrations). C, GABA did not stimulate CGRP release either from sciatic nerves (n = 7) or at 100-fold higher concentration from hind paw skin of C57BL/6 mice (n = 6). D, activation of GABAA receptors by muscimol did also not increase CGRP release from hind paw skin (n = 4 each).
Surprisingly, the application of 100 μm GABA did not evoke any release of CGRP from sciatic nerves (p = 0.73, n = 7, Wilcoxon). The exposure of hind paw skin, another established model for activation of peripheral nerve endings, to 10 mm GABA also did not elicit any CGRP release (p = 0.26, n = 6, Wilcoxon, lower concentrations for GABA not shown, Fig. 9C).
Similarly, the selective GABAA receptor agonist muscimol did not elicit any CGRP release (1 and 10 μm, n = 4 each, Fig. 9D). Finally, GABA (100 μm) did not amplify the release of CGRP induced by high potassium (60 mm). Correspondingly, the selective GABAB-receptor agonist baclofen (100 μm) did not inhibit high potassium-induced release of CGRP, suggesting that GABAB receptor stimulation is unlikely to mask GABAA-evoked CGRP release (Supplement 3).
Propofol but Not GABA Induces an Intense Pain upon Intracutaneous Injection
Notably, stimulated CGRP release only covers the peptidergic subpopulation of peripheral nociceptors, and propofol-induced injection pain is a phenomenon observed in humans. Therefore, we finally employed psychophysics on volunteers (the authors of the study, n = 5) with the intention to further narrowing down the roles of TRP channels and GABAA receptors in mediating propofol-induced injection pain. 1% Propofol-Lipuro®, Lipofundin®, buffered GABA (10 mm) or isotonic saline was injected intracutaneously at separate marked sites of the volar forearm (50 μl). Drugs were applied in a double-blinded fashion and in random order at an interval of 15 min. Whereas propofol injection was reported more or less painful for several minutes by all subjects (on a numerical scale), no painful sensation was reported by anyone of the subjects after injection of Lipofundin®, GABA, or isotonic saline.
DISCUSSION
Propofol is one of the most widely used general anesthetics in clinical practice. We show that propofol activates the TRP receptors TRPA1 and TRPV1 in DRG neurons and in HEK293t cells. In addition, GABAA receptors substantially contribute to the propofol-induced response in DRG neurons as measured by both calcium imaging and whole-cell patch clamp. Propofol, but not GABA, also evokes release of CGRP from isolated peripheral nerve of wild-type but not TRPV1/A1-deficient mice. Finally, intracutaneous injection of propofol induced an intense pain in humans, an effect that could not be mimicked by GABA.
Activation of TRP Channels by Propofol
TRPA1 has been reported to be responsible for the activation of nociceptors by general anesthetics, including propofol (5). TRPA1 was identified as the principal molecular determinant of propofol-induced pain in two acute animal models of pain, nocifensive behavior after nasal epithelial application and vascular pain. We confirm with the following findings that TRPA1 significantly contributes to propofol-induced activation of nociceptors; 1) propofol activated recombinant TRPA1, 2) in DRG neurons, the propofol-induced activation correlated well with activation by the TRPA1 agonist acrolein, and it could be antagonized by the TRPA1-inhibitor HC-030031, and 3) deletion of TRPA1 reduced the activation by propofol in TRPA1−/− as compared with both WT and TRPV1−/− neurons. Matta et al. (5) reported that DRG neurons from TRPA1 knock-out animals failed to respond to 100 μm propofol. In contrast, we performed several experiments indicating that propofol induces a significant activation of TRPV1; 1) propofol activated inward currents in TRPV1-transfected HEK293t cells, 2) the TRPV1 antagonists BCTC and capsazepine inhibited propofol-induced responses in HEK293t cells and DRG neurons, 3) propofol activated DRG neurons in the absence of TRPA1 and GABAA receptor contributions, and 4) propofol-induced responses were sensitized by activation of PKC. In DRG neurons the activation of TRPV1 by propofol appeared to be masked by the more obvious TRPA1 and GABAA-mediated effects. These results are reminiscent of a recent report showing that volatile anesthetics activate TRPV1 after phosphorylation through PKC (16). Thus, activation of TRPV1 by general anesthetics may be more important under conditions of inflammation due to a PKC-mediated phosphorylation of TRPV1 triggered by inflammatory mediators such as bradykinin and prostaglandins.
The activation of TRPA1 and TRPV1 by propofol might be indicative for a conserved mechanism of chemical activation. A covalent modification of cysteine residues was previously identified as a common mechanism for activation of TRPA1 and TRPV1 (32, 33, 37, 38). We found that the acrolein-insensitive mutant hTRPA1-C621S/C641S/C665S is sensitive to propofol and, thus, TRP channels most likely are not gated by propofol via covalent modification. The retained carvacrol sensitivity of hTRPA1-C621S/C641S/C665S is not surprising regarding the similar chemical structures of propofol and carvacrol. Differential effects of these substances on hTRPA1 and mTRPA1 indeed appear to be encoded by the same molecular determinants within TM5, i.e. a bimodal action on mTRPA1 with activation and block as compared with only an activation of hTRPA1. However, the previously demonstrated insensitivity of mTRPA1-S876/T877L and hTRPA1-S873V/T874L to menthol and thymol (22) did not apply for propofol, which induced large inward currents on both mutants. In contrast, we found that both mTRPA1-S876/T877L and hTRPA1-S873V/T874L were also insensitive to carvacrol. Notably, menthol has general anesthetic properties and activates GABAA receptors via interaction sites that are also known to be required for activation by propofol (39, 40). Taking into account the striking similarities between propofol and menthol in chemical structures and the mode of action on GABAA receptors, it is rather surprising that the mechanisms for activation of TRPA1 by propofol are apparently distinct from those required for activation by menthol, thymol, and carvacrol. Whereas the mechanism for propofol-induced activation of TRPA1, thus, remains to be identified, our data suggest that propofol seems to be an interesting substance for further studies into the molecular pharmacology of TRPA1 and other TRP channels.
Activation of GABAA Receptors by Propofol
Prolonged application of propofol produced a biphasic response in DRG neurons with a non-adapting component distinct from the desensitization reported for both TRPV1 and TRPA1 activation by agonists in the presence of calcium (41, 42). About half of the activation by propofol was concentration-dependently retained in TRPV1/A1 double knock-out animals, clearly in contrast with the previously reported results (5). This remaining action of propofol was highly correlated with the current or calcium influx elicited by GABA and was abrogated by the GABAA receptor antagonist picrotoxin. Furthermore, the lack of a major rightward shift of the propofol concentration-response curve in TRPA1−/− or TRPV1−/− neurons suggests a similar potency of propofol on GABAA receptors and TRP channels. Thus, our cellular data clearly suggest that GABAA receptors account for a significant proportion of the propofol sensitivity of sensory neurons. However, we also show that propofol but not GABA or the selective GABAA receptor agonist muscimol evoke a release of CGRP from sciatic nerves in a previously established model (43, 44). Similarly, GABA failed to amplify, and the selective GABAB-receptor agonist baclofen failed to inhibit high potassium-induced CGRP release from hind paw skin. Additionally, intracutaneous injection of GABA did not elicit a painful sensation in humans. Considering the existing literature on the action of GABA on peripheral sensory neurons, these results are in part controversial. In contrast to the inhibitory profile of GABA in the central nervous system, GABA is known to induce a depolarization and an increased excitability in sensory neurons from frogs (45), cats (46), rats (46–48), and humans (49). Accordingly, GABAA receptors are expressed in primary afferent neurons with a significant co-expression with nociceptive markers such as TRPV1 (50–53). Moreover, peripheral injection of GABA or the GABAA receptor agonist muscimol was reported to induce or increase pain-like behavior in rodents (54, 55). The depolarizing effect of GABA on sensory neurons is due to a high intracellular chloride concentration resulting from expression of the NKCC1 co-transporter (56). Whereas GABAA receptors appear to be expressed in the majority of DRG neurons, it was suggested that only those neurons expressing the Cav3.2/α1H T-type calcium channel are able to generate GABA-induced action potentials (57). Accordingly, a recent report from Carr et al. (49) demonstrated a GABAA receptor-mediated increase in excitability in only ∼40% of C-fibers in human peripheral nerves. Notably, the same study only reported an increased electrical excitability and not an activation of C-fibers by GABA. This notion is in good agreement with the lack of injection pain after intracutaneous injection of GABA in our study, collectively suggesting that GABA itself does not activate action potentials in human C-fibers. In addition, our data add to rather conflicting literature on the GABA effects on neuropeptide release from C-fibers comprising GABAA-mediated inhibition (59), facilitation (60, 61), and no effect (62, 63). Taken together, our data and a considerable number of previous reports do not support the possibility that GABAA receptors mediate a substantial activation of peripheral sensory neurons or a release of neuropeptides. Additionally, our data do not support the possibility that GABAB receptor stimulation masks any potential GABAA receptor evoked pronociceptive effect. However, given the complexity of GABAergic signaling in sensory neurons, a pro-nociceptive action of propofol mediated by GABAA receptors cannot be totally excluded.
Clinical Relevance and Conclusions
Our data reveal TRPA1 and TRPV1 as main mediators of propofol-induced pain and release of neuropeptides. The release of neuropeptides from peripheral and central terminals of sensory neurons induces vascular leakage and dilatation and is thought to contribute to neurogenic inflammation in the periphery and to central sensitization in the spinal dorsal horn. Although corresponding data in a clinical setting is currently lacking, it seems conceivable that propofol can cause a clinically significant sensitization of nociceptors by directly activating or sensitizing TRPA1 and TRPV1. As this condition might persist beyond the intra-operative period, it might prove relevant in the etiology of post-operative and persistent pain. If this concept was supported by clinical data, it might alter the understanding of the consequences of the intra-operative use of propofol and other general anesthetics with a similar profile. In this regard, TRPV1 and/or TRPA1 antagonists might prove to be helpful analgesic adjuncts for the prevention and the treatment of post-operative pain.
Supplementary Material
Acknowledgments
We thank Iwona Izydorczyk, Annette Kühn, Kerstin Fischer, and Rebecca Günther for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grants NA-350/3-3 and LU 728/3-1, KFO 130 (to A. L., C. N., and P. W. R.) and by the Dr. Robert Pfleger-Stiftung (to K. K.).

The on-line version of this article (available at http://www.jbc.org) contains Supplements 1–3.
- propofol
- 2,6-diisopropylphenol
- TRPA1
- transient receptor potential cation channel, subfamily A, member 1
- hTRPA1
- human TRPA1
- TRPV1
- transient receptor potential cation channel, subfamily V, member 1
- CGRP
- calcitonin gene-related peptide
- DRG
- dorsal root ganglion
- GABA
- γ-aminobutyric acid
- TM5
- transmembrane domain 5
- pF
- picofarads
- MCT/LCT
- medium-chain/long-chain triglycerides
- BCTC
- 4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide.
REFERENCES
- 1.Picard P., Tramèr M. R. (2000) Anesth. Analg. 90, 963–969 [DOI] [PubMed] [Google Scholar]
- 2.Stokes D. N., Robson N., Hutton P. (1989) Br. J. Anaesth. 62, 202–203 [DOI] [PubMed] [Google Scholar]
- 3.Klement W., Arndt J. O. (1991) Br. J. Anaesth. 67, 281–284 [DOI] [PubMed] [Google Scholar]
- 4.Doenicke A. W., Roizen M. F., Rau J., Kellermann W., Babl J. (1996) Anesth. Analg. 82, 472–474 [DOI] [PubMed] [Google Scholar]
- 5.Matta J. A., Cornett P. M., Miyares R. L., Abe K., Sahibzada N., Ahern G. P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8784–8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Story G. M., Peier A. M., Reeve A. J., Eid S. R., Mosbacher J., Hricik T. R., Earley T. J., Hergarden A. C., Andersson D. A., Hwang S. W., McIntyre P., Jegla T., Bevan S., Patapoutian A. (2003) Cell 112, 819–829 [DOI] [PubMed] [Google Scholar]
- 7.Jordt S. E., Bautista D. M., Chuang H. H., McKemy D. D., Zygmunt P. M., Högestätt E. D., Meng I. D., Julius D. (2004) Nature 427, 260–265 [DOI] [PubMed] [Google Scholar]
- 8.McNamara C. R., Mandel-Brehm J., Bautista D. M., Siemens J., Deranian K. L., Zhao M., Hayward N. J., Chong J. A., Julius D., Moran M. M., Fanger C. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bautista D. M., Jordt S. E., Nikai T., Tsuruda P. R., Read A. J., Poblete J., Yamoah E. N., Basbaum A. I., Julius D. (2006) Cell 124, 1269–1282 [DOI] [PubMed] [Google Scholar]
- 10.Obata K., Katsura H., Mizushima T., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Tominaga M., Noguchi K. (2005) J. Clin. Invest. 115, 2393–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 12.Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. (2000) Science 288, 306–313 [DOI] [PubMed] [Google Scholar]
- 13.Davis J. B., Gray J., Gunthorpe M. J., Hatcher J. P., Davey P. T., Overend P., Harries M. H., Latcham J., Clapham C., Atkinson K., Hughes S. A., Rance K., Grau E., Harper A. J., Pugh P. L., Rogers D. C., Bingham S., Randall A., Sheardown S. A. (2000) Nature 405, 183–187 [DOI] [PubMed] [Google Scholar]
- 14.Bandell M., Story G. M., Hwang S. W., Viswanath V., Eid S. R., Petrus M. J., Earley T. J., Patapoutian A. (2004) Neuron 41, 849–857 [DOI] [PubMed] [Google Scholar]
- 15.Leffler A., Fischer M. J., Rehner D., Kienel S., Kistner K., Sauer S. K., Gavva N. R., Reeh P. W., Nau C. (2008) J. Clin. Invest. 118, 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornett P. M., Matta J. A., Ahern G. P. (2008) Mol. Pharmacol. 74, 1261–1268 [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi S., Tomioka A., Sudo M., Nakamura A., Shirakura K., Takagishi K., Kohama K. (2001) Neurosci. Lett. 312, 45–49 [DOI] [PubMed] [Google Scholar]
- 18.Perkins F. M., Kehlet H. (2000) Anesthesiology 93, 1123–1133 [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M. (1983) Pain 16, 109–110 [DOI] [PubMed] [Google Scholar]
- 20.Kwan K. Y., Allchorne A. J., Vollrath M. A., Christensen A. P., Zhang D. S., Woolf C. J., Corey D. P. (2006) Neuron 50, 277–289 [DOI] [PubMed] [Google Scholar]
- 21.Mohapatra D. P., Nau C. (2003) J. Biol. Chem. 278, 50080–50090 [DOI] [PubMed] [Google Scholar]
- 22.Xiao B., Dubin A. E., Bursulaya B., Viswanath V., Jegla T. J., Patapoutian A. (2008) J. Neurosci. 28, 9640–9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohapatra D. P., Wang S. Y., Wang G. K., Nau C. (2003) Mol. Cell. Neurosci. 23, 314–324 [DOI] [PubMed] [Google Scholar]
- 24.Dittert I., Vlachová V., Knotková H., Vitásková Z., Vyklicky L., Kress M., Reeh P. W. (1998) J. Neurosci. Methods 82, 195–201 [DOI] [PubMed] [Google Scholar]
- 25.Poenie M., Tsien R. (1986) Prog. Clin. Biol. Res. 210, 53–56 [PubMed] [Google Scholar]
- 26.Bretag A. H. (1969) Life Sci. 8, 319–329 [DOI] [PubMed] [Google Scholar]
- 27.Averbeck B., Reeh P. W. (2001) Neuropharmacology 40, 416–423 [DOI] [PubMed] [Google Scholar]
- 28.Collins G. G. (1988) Br. J. Pharmacol. 95, 939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concas A., Santoro G., Serra M., Sanna E., Biggio G. (1991) Brain Res. 542, 225–232 [DOI] [PubMed] [Google Scholar]
- 30.Rudolph U., Antkowiak B. (2004) Nat. Rev. Neurosci. 5, 709–720 [DOI] [PubMed] [Google Scholar]
- 31.Benham C. D., Gunthorpe M. J., Davis J. B. (2003) Cell Calcium 33, 479–487 [DOI] [PubMed] [Google Scholar]
- 32.Hinman A., Chuang H. H., Bautista D. M., Julius D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19564–19568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macpherson L. J., Dubin A. E., Evans M. J., Marr F., Schultz P. G., Cravatt B. F., Patapoutian A. (2007) Nature 445, 541–545 [DOI] [PubMed] [Google Scholar]
- 34.Planells-Cases R., Garcìa-Sanz N., Morenilla-Palao C., Ferrer-Montiel A. (2005) Pflugers Arch. 451, 151–159 [DOI] [PubMed] [Google Scholar]
- 35.Mogil J. S., Miermeister F., Seifert F., Strasburg K., Zimmermann K., Reinold H., Austin J. S., Bernardini N., Chesler E. J., Hofmann H. A., Hordo C., Messlinger K., Nemmani K. V., Rankin A. L., Ritchie J., Siegling A., Smith S. B., Sotocinal S., Vater A., Lehto S. G., Klussmann S., Quirion R., Michaelis M., Devor M., Reeh P. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12938–12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamakage M., Iwasaki S., Satoh J., Namiki A. (2005) Anesth. Analg. 101, 385–388 [DOI] [PubMed] [Google Scholar]
- 37.Salazar H., Llorente I., Jara-Oseguera A., García-Villegas R., Munari M., Gordon S. E., Islas L. D., Rosenbaum T. (2008) Nat. Neurosci. 11, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang H. H., Lin S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20097–20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watt E. E., Betts B. A., Kotey F. O., Humbert D. J., Griffith T. N., Kelly E. W., Veneskey K. C., Gill N., Rowan K. C., Jenkins A., Hall A. C. (2008) Eur. J. Pharmacol. 590, 120–126 [DOI] [PubMed] [Google Scholar]
- 40.Zhang X. B., Jiang P., Gong N., Hu X. L., Fei D., Xiong Z. Q., Xu L., Xu T. L. (2008) PLoS. One 3, e3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koplas P. A., Rosenberg R. L., Oxford G. S. (1997) J Neurosci. 17, 3525–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y. Y., Chang R. B., Waters H. N., McKemy D. D., Liman E. R. (2008) J. Biol. Chem. 283, 32691–32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauer S. K., Reeh P. W., Bove G. M. (2001) Eur. J. Neurosci. 14, 1203–1208 [DOI] [PubMed] [Google Scholar]
- 44.Fischer M. J., Reeh P. W. (2007) Eur. J. Neurosci. 25, 3570–3575 [DOI] [PubMed] [Google Scholar]
- 45.Barker J. L., Nicoll R. A., Padjen A. (1975) J. Physiol. 245, 521–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallagher J. P., Higashi H., Nishi S. (1978) J. Physiol. 275, 263–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desarmenien M., Santangelo F., Loeffler J. P., Feltz P. (1984) Exp. Brain Res. 54, 521–528 [DOI] [PubMed] [Google Scholar]
- 48.Deschenes M., Feltz P., Lamour Y. (1976) Brain Res. 118, 486–493 [DOI] [PubMed] [Google Scholar]
- 49.Carr R. W., Sittl R., Fleckenstein J., Grafe P. (2010) PLoS. One 5, e8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuyama T., Sato M., Sato K., Araki T., Inagaki S., Takagi H., Tohyama M. (1992) Brain Res. Mol. Brain Res. 12, 335–338 [DOI] [PubMed] [Google Scholar]
- 51.Ma W., Saunders P. A., Somogyi R., Poulter M. O., Barker J. L. (1993) J. Comp. Neurol. 338, 337–359 [DOI] [PubMed] [Google Scholar]
- 52.Persohn E., Malherbe P., Richards J. G. (1991) Neuroscience 42, 497–507 [DOI] [PubMed] [Google Scholar]
- 53.Peeters P. J., Aerssens J., de Hoogt R., Stanisz A., Göhlmann H. W., Hillsley K., Meulemans A., Grundy D., Stead R. H., Coulie B. (2006) Physiol. Genomics 24, 252–263 [DOI] [PubMed] [Google Scholar]
- 54.Ault B., Hildebrand L. M. (1994) Neuropharmacology 33, 109–114 [DOI] [PubMed] [Google Scholar]
- 55.Carlton S. M., Zhou S., Coggeshall R. E. (1999) Neuroscience 93, 713–722 [DOI] [PubMed] [Google Scholar]
- 56.Sung K. W., Kirby M., McDonald M. P., Lovinger D. M., Delpire E. (2000) J. Neurosci. 20, 7531–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aptel H., Hilaire C., Pieraut S., Boukhaddaoui H., Mallié S., Valmier J., Scamps F. (2007) Mol. Cell. Neurosci. 36, 293–303 [DOI] [PubMed] [Google Scholar]
- 58.Deleted in proof
- 59.Go V. L., Yaksh T. L. (1987) J. Physiol. 391, 141–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santicioli P., Tramontana M., Del Bianco E., Maggi C. A., Geppetti P. (1991) Life Sci. 48, L69–L72 [DOI] [PubMed] [Google Scholar]
- 61.Lao L., Marvizón J. C. (2005) Neuroscience 130, 1013–1027 [DOI] [PubMed] [Google Scholar]
- 62.Malcangio M., Bowery N. G. (1993) J. Pharmacol. Exp. Ther. 266, 1490–1496 [PubMed] [Google Scholar]
- 63.Riley R. C., Trafton J. A., Chi S. I., Basbaum A. I. (2001) Neuroscience 103, 725–737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.