Abstract
We recently demonstrated that the Gla domain-dependent interaction of protein C with endothelial protein C receptor (EPCR) leads to dissociation of the receptor from caveolin-1 and recruitment of PAR-1 to a protective signaling pathway. Thus, the activation of PAR-1 by either thrombin or PAR-1 agonist peptide elicited a barrier-protective response if endothelial cells were preincubated with protein C. In this study, we examined whether other vitamin K-dependent coagulation protease zymogens can modulate PAR-dependent signaling responses in endothelial cells. We discovered that the activation of both PAR-1 and PAR-2 in endothelial cells pretreated with factor FX (FX)-S195A, but not other procoagulant protease zymogens, also results in initiation of protective intracellular responses. Interestingly, similar to protein C, FX interaction with endothelial cells leads to dissociation of EPCR from caveolin-1 and recruitment of PAR-1 to a protective pathway. Further studies revealed that, FX activated by factor VIIa on tissue factor bearing endothelial cells also initiates protective signaling responses through the activation of PAR-2 independent of EPCR mobilization. All results could be recapitulated by the receptor agonist peptides to both PAR-1 and PAR-2. These results suggest that a cross-talk between EPCR and an unknown FX/FXa receptor, which does not require interaction with the Gla domain of FX, recruits PAR-1 to protective signaling pathways in endothelial cells.
Keywords: Blood Coagulation Factors, Endothelium, G-protein Coupled Receptors (GPCR), Receptor Regulation, Thrombin, Activated Protein C, EPCR, Factor X, Protease-activated Receptors
Introduction
Factor X (FX)3 is the zymogen for the tissue factor (TF)-factor VIIa (FVIIa) complex in the extrinsic pathway following activation to factor Xa (FXa) that binds to factor Va on negatively charged membrane surfaces in the presence of calcium to activate prothrombin to thrombin (1–3). Thrombin cleaves fibrinogen to fibrin to form blood clots at the sites of vascular injury (1–3). Thrombin also initiates the anticoagulant pathway when it binds to vascular thrombomodulin to activate protein C to activated protein C (APC) (4). The activation of protein C by the thrombin-thrombomodulin complex is accelerated when the anticoagulant zymogen is bound to endothelial protein C receptor (EPCR) (5). APC down-regulates thrombin generation by degrading procoagulant cofactors Va and VIIIa by limited proteolysis (6, 7). In addition to its anticoagulant activity, APC has been demonstrated to initiate cytoprotective and anti-inflammatory responses when it remains associated with EPCR via its N-terminal γ-carboxyglutamic acid (Gla) domain to activate protease-activated receptor 1 (PAR-1) on endothelial cells (8–12). Paradoxically, the activation of PAR-1 by thrombin is known to evoke proinflammatory responses in endothelial cells (13, 14). We recently demonstrated that when EPCR on endothelial cells is occupied by either protein C or APC, the activation of PAR-1 by thrombin also leads to initiation of protective cellular responses and down-regulation of the same proinflammatory molecules that are thought to be targets for the protective effects of APC (15, 16). Thus, in a series of studies, we showed that both EPCR and PAR-1 are colocalized within lipid rafts and that the unbound EPCR exists in association with caveolin-1 in endothelial cells (15–17). Interestingly, we discovered that the occupancy of EPCR by the Gla domain of protein C/APC leads to dissociation of EPCR from caveolin-1 and recruitment of PAR-1 to protective pathways such that the activation of the receptor by either APC or thrombin elicits essentially identical signaling responses in endothelial cells (15, 16). The physiological significance of these observations will require further investigation in in vivo settings.
It has become apparent in recent years that in addition to thrombin and APC, other coagulation proteases can also activate protease-activated receptors (PAR-1, PAR-2, and PAR-4) to initiate both proinflammatory and anti-inflammatory responses (12, 13, 18). For instance, FVIIa, by itself or in complex with TF, has been reported to initiate proinflammatory responses through the cleavage of PAR-2 on endothelial and other cell types (19). PAR-2 appears not to be activated by thrombin, however, FXa is thought to activate this receptor (13, 20). Furthermore, it has been demonstrated that the TF-FVIIa complex can indirectly signal through both PAR-1 and PAR-2 by activating FX to FXa on endothelial cells (18–20). In a recent report, it was demonstrated that FXa, through the activation of both PAR-1 and PAR-2 can initiate a potent barrier-protective effect in response to proinflammatory stimuli in activated human umbilical vein endothelial cells (HUVECs) (20). Other studies have assigned a proinflammatory role for FXa in similar in vitro cellular models (21–23). The basis for the discrepancy in results between different studies is not known.
In light of the observation that the occupancy of EPCR by the Gla domain of protein C can change the PAR-1-dependent signaling specificity of thrombin from a barrier-disruptive to a barrier-protective effect (15), we undertook the current study to determine whether the interaction of other coagulation zymogens with endothelial cell surface receptors can play a similar role in modulating the signaling specificity of PARs. We discovered that, in addition to protein C, the preincubation of endothelial cells with FX, but not with prothrombin, FVII, or FIX, also switches the signaling specificity of both PAR-1 and PAR-2 from a barrier-disruptive to a barrier-protective effect. Interestingly, in the case of signaling via PAR-1, the mechanism of this effect was found to be also mediated through membrane mobilization of EPCR because pre-treatment of endothelial cells with FX-S195A led to dissociation of EPCR from caveolin-1, without a requirement for the occupancy of EPCR. The Gla domain of FX is not involved in this process because the Gla domainless mutant of FX also elicited the same response. Further studies revealed that FXa, whether added exogenously to HUVECs as a protease, or activated from FX by picomolar concentrations of FVIIa on cells expressing TF, initiated a potent barrier-protective response in activated endothelial cells through the activation of PAR-2.
EXPERIMENTAL PROCEDURES
Construction, Expression, and Purification of Recombinant Proteins
Construction and expression of the Ser-195 → Ala (FX-S195A) (chymotrypsinogen numbering (24)) and the Gla domainless mutant of FX (GDFX) in human embryonic kidney (HEK293) cells has been described (25, 26). Expression of protein C and its S195A mutant (PC-S195A) has been described (15). A protein C mutant in the Gla domainless form (GDPC) in which 6 residues (Leu-83 to Leu-88) of the loop connecting EGF1 to the EGF2 domain of FX (GDPC/FX6aa) was expressed in the same expression system and purified to homogeneity as described (27). The construction and expression of an FX mutant lacking both Gla and EGF1 domains (E2FX) has been described (25). The E2FX mutant containing Ala substitutions for residues Arg-86 and Lys-87 (E2FX-RK/AA) was expressed using the same vector system. The cDNA fragment coding for human TF lacking its cytoplasmic domain (dc-TF) (28) was subcloned to HindIII and XbaI restriction enzymes sites of the pcDNA3.1 expression vector (Invitrogen) for transient transfection to endothelial cells.
Human plasma proteins including factors VII/VIIa, X/Xa, IX, and prothrombin were purchased from Hematologic Technologies, Inc. (Essex Junction, VT). The non-blocking anti-PAR-1 (S-19) and function-blocking anti-PAR-1 (H-111) and anti-PAR-2 (SAM-11) antibodies, and siRNA specific for sphingosine 1-phosphate receptor 1 (S1P1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The function-blocking EPCR antibody (clone RCR-252) was purchased from Cell Sciences (Canton, MA). The antibody concentrations in the blocking assays were 25 μg/ml in all experiments. Anti-caveolin-1 antibody was purchased from BD Biosciences (San Jose, CA). Pertussis toxin (PTX) and methyl-β-cyclodextrin (MβCD) were purchased from Sigma. Tumor necrosis factor-α (TNF-α) was purchased from R&D Systems (Minneapolis, MN). Thrombin receptor agonist peptides (TRAP) specific for the activation of PAR-1 (TFLLRN) and PAR-2 (SLIGKV) were purchased from Bachem Bioscience (Torrance, CA). The anti-NF-κB p65 antibody detecting the endogenous levels of total NF-κB p65 and the anti-phospho-NF-κB p65 antibody detecting the activated Ser-536-phosphorylated form of NF-κB p65 were purchased from Cell Signaling Technology (Danvers, MA). The kits for monitoring the activation of RhoA and Rac1 GTPases were obtained from Cytoskeleton (Denver, CO).
Permeability Assays
The barrier permeability of primary HUVECs (purchased from Cambrex Bio Science Inc., Charles City, IA) or transformed HUVECs (EA.hy926 cells, kindly provided by Dr. C. Edgell from University of North Carolina at Chapel Hill, NC) in response to thrombin (5 nm for 10 min), following treatment with either APC or FXa (20 nm, 3 h), was quantitated by spectrophotometric measurement of the flux of Evan's blue-bound albumin across the cell monolayers using a modified 2-compartment chamber model as described (15). For the function-blocking antibody treatments of the monolayers, medium was removed and antibodies were added for 30 min in serum-free medium followed by analysis of the permeability as described (15). Results are expressed as mean ± S.E. and all experiments were repeated at least three times.
The same assay was employed to evaluate the effect of thrombin (2 nm for 3 h) or TRAP (0–1 mm for 3 h) on permeability of endothelial cells pretreated with near physiological concentrations of vitamin K-dependent coagulation protease zymogens (protein C, prothrombin, FVII, FIX, and FX) as well as FX-S195A and PC-S195A (50 nm) for 15 min. The permeability assays were also carried out in the presence of either PTX (100 ng/ml, 16 h) or siRNA for S1P1, EPCR, PAR-1, and PAR-2 (0.2 μg/ml, 30 min) as described (15, 29). EA.hy926 cell permeability in response to TNF-α was carried out using the same methods except that instead of 5 nm thrombin for 10 min, TNF-α (10 ng/ml for 18 h) was used to induce permeability. This permeability assay was also used to analyze the effect of FVIIa (10 pm) and the zymogen FX (175 nm) on EA.hy926 cells transiently transfected with the dc-TF construct. This assay was also used to monitor the signaling effect of thrombin in response to TNF-α on cells pretreated with GDFX, E2FX, E2FX-RK/AA, and GDPC-FX6aa derivatives (50 nm each for 30 min).
Analysis of Expression of Cell Adhesion Molecules
The expression of vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin on EA.hy926 was determined by a whole cell ELISA as described (29, 30). Briefly, confluent monolayers were treated for 4 h with 20 nm APC, thrombin ± PC-S195A, thrombin ± FX-S195A, or TRAP (100 μm) ± FX-S195A (50–175 nm) followed by stimulation with TNF-α (10 ng/ml for 4 h). Following removal of the medium and washing of cells with PBS, they were fixed by adding 50 μl of 1% paraformaldehyde for 15 min at room temperature. Mouse anti-human monoclonal antibodies to VCAM-1, ICAM-1, and E-selectin (Chemicon, Temecula, CA) and peroxidase-conjugated anti-mouse IgG antibody (Sigma) were used to measure the expression of cell adhesion molecules as described (29, 30). All measurements were performed in triplicate wells.
Adhesion Assay
Freshly isolated neutrophil adherence to EA.hy926 cells was evaluated by fluorescent labeling of neutrophils as described (29, 30). Briefly, freshly isolated peripheral blood neutrophils were labeled with 5 μm Vybrant DiD (Molecular Probes) for 20 min at 37 °C in phenol red-free RPMI containing 5% fetal bovine serum. Following twice washing of neutrophils (1.5 × 106/ml, 200 μl/well), they were resuspended in the adhesion medium (RPMI containing 2% fetal bovine serum and 20 mm HEPES) and added to confluent monolayers of EA.hy926 cells in 96-well plates, which were treated for 4 h with 20 nm of either APC or thrombin ± FX-S195A zymogen (175 nm) followed by TNF-α (10 ng/ml for 4 h). In blocking experiments, the monolayers were preincubated for 30 min at 37 °C with appropriate function-blocking antibodies. The fluorescence of labeled cells was measured (total signal) using a fluorescence microplate reader (Molecular Device). After incubation for 60 min at 37 °C, the non-adherent cells were removed by washing four times with pre-warmed RPMI and fluorescent signals of adherent cells were measured by the same methods. The percentage of adherent leukocytes was calculated by the formula: % adherence = (adherent signal/total signal) × 100, as described (31). All measurements were performed in triplicate wells. The same procedures were employed to monitor the binding of the monocytic THP-1 cells to TNF-α-stimulated endothelial cells.
Measuring RhoA and Rac1 Activation
EA.hy926 cells were grown to confluence in 6-well culture dishes and starved overnight in the serum-free medium. Determination of RhoA and Rac1 activations (RhoA-GTP or Rac1-GTP) was monitored using commercially available kits. Briefly, conditioned cells (see figure legends) were lysed using a cell lysis buffer provided by the manufacturer. After determining the protein concentration by a Bio-Rad assay, 50 μg of lysates were saved for the Western quantitation of the total RhoA. The remaining sample was incubated with 20 μg of the GST fusion protein RBD (rhotekin Rho-binding domain), which is bound to the colored glutathione-Sepharose beads, at 4 °C with rotation for 1 h. The RBD protein motif binds specifically to the active GTP-bound form of RhoA. Beads were washed, resuspended in loading buffer, and proteins were separated on 12% SDS-PAGE followed by transferring to a PVDF membrane and Western blotting using an anti-RhoA monoclonal antibody as described by the manufacturer. The same procedures were employed to monitor Rac1 activation except that appropriate cellular lysates were incubated with GST-PAK PBD (Rac1 effector protein, p21 activated kinase 1) beads that bind specifically to the active GTP-bound form of Rac1. The total and activated Rac1 were detected by Western blotting using an anti-Rac1 monoclonal antibody provided in the kit as described (16).
Measuring NF-κB Activation
EA.hy926 cells were grown to confluence in 6-well culture dishes and subjected to starvation overnight in the serum-free medium. The NF-κB pathway activation in the conditioned cell lysates (treated with different PAR-1 agonists and activated by TNF-α as described under figure legends) was monitored by Western blotting employing two antibodies that are specific for either NF-κB p65 (as an index of total cellular NF-κB p65) or its phosphorylated form (as an index of the NF-κB pathway activation) as described (16).
Immunoprecipitation, SDS-PAGE, and Western Blotting
Total cellular proteins were extracted by sonication with PBS containing 25 μm proteosome inhibitor MG132 (Sigma) and complete protease inhibitor mixture (Roche Applied Science) as described (15, 16). Lysates were combined with 3 μg of each specific antibody (H-111 for PAR-1, SAM-11 for PAR-2, EPR11-A for effective cell protease receptor 1, RCR-252 for EPCR, and anti-caveolin-1) and incubated for 2 h at 4 °C. Immunoprecipitates were collected with protein A/G-agarose (Santa Cruz, CA), fractionated on a 10% SDS-PAGE, transferred to membranes, and subjected to Western blotting with appropriate primary and horseradish peroxidase-conjugated secondary antibodies as described (16). The immunoreactive protein bands were visualized by SuperSignal West Pico (Pierce, Rockford, IL).
RESULTS
FX Switches the PAR-1- and PAR-2-dependent Signaling Specificity in Endothelial Cells
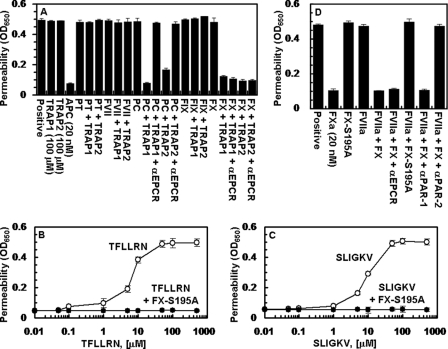
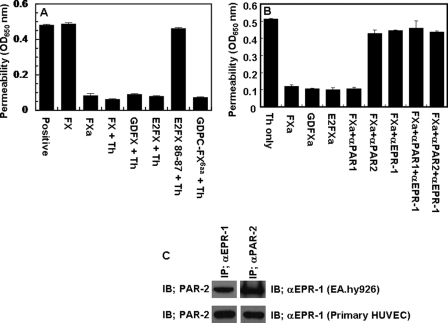
We previously demonstrated that the occupancy of EPCR by protein C switches the PAR-1-dependent signaling specificity of thrombin from a permeability enhancing to a barrier-protective effect in endothelial cells (15, 29). To determine whether the interaction of other vitamin K-dependent coagulation protease zymogens influences the PAR-1- and PAR-2-dependent signaling specificity of coagulation proteases, we pre-treated EA.hy926 cells with near physiological concentrations of protein C, prothrombin, factors VII, IX, and X before activating the receptors with TRAP highly specific for either PAR-1 (TFLLRN) or PAR-2 (SLIGKV) (32). We discovered that in addition to changing the signaling specificity of PAR-1, protein C also changed the PAR-2 signaling specificity in an endothelial cell hyperpermeability assay (Fig. 1A). Unexpectedly, we discovered that FX also exhibited a similar property. Thus both PAR-1 and PAR-2 activation elicited barrier-protective effects when endothelial cells were treated with either protein C or FX (Fig. 1A). Further studies with catalytically inactive FX-S195 mutant zymogen supported a protective role for FX in endothelial cells. As shown in Fig. 1, B and C, both TFLLRN and SLIGKV enhanced the permeability of endothelial cells in a concentration-dependent manner with maximal effects reaching saturation at ∼50 μm TRAP. However, FX-S195A switched the signaling specificity of both PAR agonist peptides so that neither agonist could elicit a barrier-disruptive effect. These results suggest that, similar to the protective EPCR-protein C system, the activation of both PAR-1 and PAR-2 initiate protective responses if endothelial cells were pretreated with the zymogen FX. Because FXa is known to activate PAR-2, these results indicated that FXa should also exhibit a barrier-protective activity in this assay. Consistent with this proposition, FXa elicited a potent barrier-protective activity in response to thrombin in endothelial cells whether it was added as a protease to cells or activated by 10 pm FVIIa on cells transiently transfected with a construct expressing a TF derivative lacking the cytoplasmic domain of the cofactor (Fig. 1D). The barrier-protective activity of FXa is mediated through the activation of PAR-2 because the function-blocking anti-PAR-2, but not anti-PAR-1 antibody inhibited this effect (Fig. 1D). It should be noted that transient transfection of endothelial cells with the TF construct was required to monitor PAR-2-dependent FX signaling because in the absence of transfection no significant amount of FXa was generated to detect any activity (data not shown).
FIGURE 1.
Effect of receptor occupancy by vitamin K-dependent coagulation protease zymogens on PAR-1- and PAR-2-dependent cellular responses. A, EA.hy926 cells were pretreated for 30 min with buffer control or near physiological concentrations of protein C (PC, 80 nm), prothrombin (PT, 1.39 μm), FVII (10 nm), FIX (90 nm), and FX (175 nm) in the absence and presence of receptor agonist peptides specific for either PAR-1 (TFLLRN) or PAR-2 (SLIGKV) (100 μm, 3 h) before inducing the permeability with thrombin (5 nm for 10 min). When function-blocking anti-EPCR antibody (αEPCR, 25 μg/ml) was present, cells were incubated with the antibody for 30 min before pre-treatment with zymogens. APC (20 nm) was used as a control for its known cytoprotective activity. B, the concentration dependence of activation of endothelial cells by the PAR-1 agonist peptide (TFLLRN, 3 h) before (○) or after (●) treatment with FX-S195A (50 nm for 30 min). C, the same as B except that the PAR-2 agonist peptide (SLIGKV) was used for activation. D, the same as A except that the TF-transfected endothelial cells were pretreated with buffer control (positive) or with the indicated proteins (FXa, 20 nm; FX-S195A, 50 nm; FVIIa, 10 pm; FX, 175 nm) before inducing permeability with thrombin (5 nm, 10 min). In the presence of function-blocking anti-PAR-1 and anti-PAR-2 antibodies (25 μg/ml), cells were first preincubated with antibodies for 30 min. All results are means ± S.D. of three different experiments.
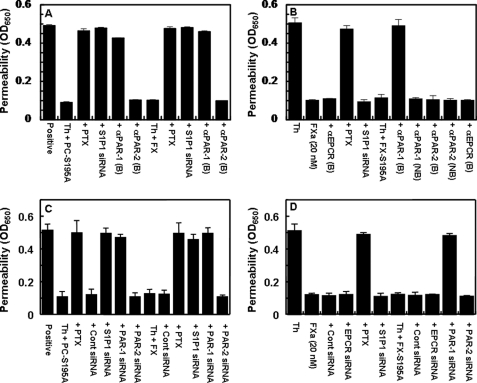
In addition to signaling through PAR-2, the results presented above with the PAR-1 agonist peptide predict that the zymogen FX can also elicit a protective signaling response indirectly through switching the PAR-1-dependent signaling specificity of thrombin, as has been observed for protein C (15). Indeed, thrombin through the activation of PAR-1 elicited a barrier-protective response on endothelial cells pre-treated with FX and/or FX-S195A (Fig. 2A, shown for FX only). Similar to the protein C-mediated protective effect, the protective effect of FX was sensitive to PTX (Fig. 2A). It is known that among G-proteins, only the signaling function of Gi/o is sensitive to PTX (33). Thus, these results suggest that the signaling response of FX may be mediated through coupling of PAR-1 to a Gi/o protein. Similar to the protective effect of protein C, a receptor cross-talk with S1P1 is required for the protective effect of FX through PAR-1 because siRNA for S1P1 inhibited this effect (Fig. 2A). Interestingly, whereas the protective effect of the protease, FXa, through the activation of PAR-2 was also sensitive to PTX, nevertheless, siRNA for S1P1 did not inhibit the PAR-2-dependent protective signaling effect of FXa, suggesting that a receptor cross-talk with S1P1 is not required for the protective signaling mechanism observed with PAR-2 activation (Fig. 2B). In contrast to the FXa-mediated PAR-2-dependent protective effect, the activity of zymogen FX/FX-S195A is mediated indirectly through activation of PAR-1 by thrombin because the function-blocking anti-PAR-1, but not anti-PAR-2 antibody inhibited this effect (Fig. 2B). The PAR-1-dependent protective effect of FX/FX-S195A is not mediated through zymogen interaction with EPCR, because the function-blocking anti-EPCR antibody did not inhibit the activity (Fig. 2B). To ensure that the FX-mediated PAR-1-dependent protective effect of thrombin is not a peculiar property of the transformed endothelial cells (EA.hy926), the same experiments of Fig. 2, A and B, were also conducted using primary HUVECs (Fig. 2, C and D), which yielded essentially identical results. Furthermore, the PAR-1-dependent protective effect of thrombin in the FX pretreated primary HUVECs was confirmed by the siRNA approach using specific siRNA for EPCR, S1P1, PAR-1, and PAR-2. Similar to results observed with the blocking antibodies for the receptors, siRNA for both PAR1 and S1P1, but not siRNA for either EPCR or PAR-2, blocked the protective activity of thrombin on FX/FX-S195A-pretreated primary HUVECs (Fig. 2, C and D).
FIGURE 2.
Protein C and FX switch the PAR-1-dependent signaling specificity of thrombin through the transactivation of S1P1. A, EA.hy926 cells were pretreated with buffer control or PC-S195A and FX (50 nm each, 30 min) and thrombin (2 nm, 3 h) before inducing the permeability with thrombin (5 nm, 10 min). In the presence of siRNA or PTX, cells were transiently transfected with siRNA for S1P1 (0.2 μg, 3 h) or PTX (100 ng/ml, 16 h) before treatment with zymogens. In the presence of the indicated blocking antibodies, cells were pretreated with anti-PAR-1 and anti-PAR-2 antibodies (25 μg/ml for 30 min). B, the same as A except that the signaling activity of FXa (20 nm) or thrombin (2 nm) or thrombin (2 nm) + FX-S195A (50 nm) in the absence and presence of blocking and non-blocking antibodies to the indicated receptors were compared. C and D, the same as A and B except that instead of EA.hy926 cells, primary HUVECs, and instead of blocking antibodies, siRNA specific for the receptors (EPCR, PAR-1, and PAR-2, 0.2 μg, 3 h) were used in the experiments. All results are means ± S.D. of three different experiments.
FX Inhibits Thrombin-mediated Leukocyte Adhesion to Endothelial Cells
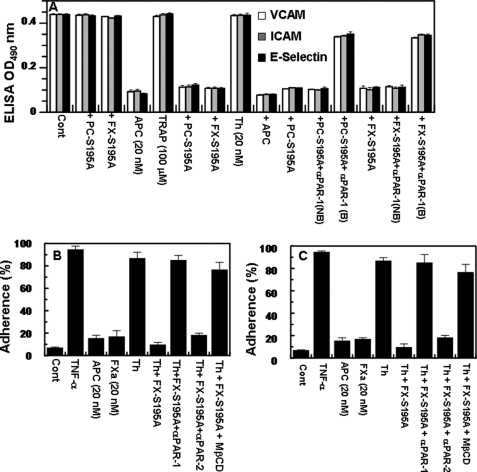
We previously demonstrated that the occupancy of EPCR by protein C suppresses the TNF-α- or thrombin-mediated interaction of neutrophils with endothelial cells by inhibiting the expression of cell surface adhesion molecules VCAM-1, ICAM-1, and E-selectin (15, 16, 29, 30). The results presented in Fig. 3A indicate that, similar to protein C-S195A, pre-treating endothelial cells with FX-S195A switched the signaling specificity of thrombin, thus conferring anti-inflammatory activities for the protease through the activation of PAR-1. This was supported by the observation that TRAP (TFLLRN) also elicited a protective response if cells were pretreated with either PC-S195A or FX-S195A (Fig. 3A). The concentration dependence of the protective effect of thrombin on S195A pre-treated cells revealed that a thrombin concentration of 10–20 nm is required for the maximal inhibition of expression of all three cell adhesion molecules and that increasing the concentration of thrombin to 50 nm eliminated the protective effect of the protease (data not shown). This is consistent with our previous results (16) and suggests that higher concentrations of thrombin may also signal through other cell surface receptor(s) that can counteract its protective effect. Consistent with a PAR-1-dependent response for both protein C and FX mutant zymogens, the function-blocking anti-PAR-1 antibody inhibited the protective effect of thrombin (Fig. 3A). A similar down-regulation of cell adhesion molecules was observed if FXa was directly used to monitor the expression of adhesion molecules. Thus, FXa (20 nm) inhibited thrombin-mediated up-regulation of all three molecules through the activation of PAR-2 as evidenced by the anti-PAR-2, but not anti-PAR-1, antibody blocking the protective effect of FXa (data not shown). Further studies were initiated to determine whether the inhibition of the expression of these adhesion molecules by thrombin in the presence of FX-S195A correlates with the inhibition of binding of neutrophils to TNF-α-stimulated EA.hy926 cells. The results presented in Fig. 3B demonstrate that, similar to APC, thrombin potently blocks the adhesion of neutrophils to TNF-α-stimulated endothelial cells by a PAR-1-dependent pathway if the cell monolayer was pretreated with FX-S195A prior to incubation with thrombin. A similar approach was employed to monitor the adhesion of the monocytic THP-1 cell line to TNF-α-stimulated EA.hy926 cells, which were pretreated with the FX-S195A zymogen. Similar to results obtained with neutrophils, thrombin inhibited adhesion of THP-1 cells to TNF-α-stimulated endothelial cells pretreated with FX-S195A (Fig. 3C). The function-blocking anti-PAR-1, but not anti-PAR-2 antibody abrogated the anti-inflammatory effect of TNF-α in FX-S195A-pretreated endothelial cells (Fig. 3, B and C). These results clearly suggest that cleavage of PAR-1 by thrombin elicits anti-inflammatory responses in endothelial cells if the cell monolayer is pretreated with the FX zymogen. It should be noted that FXa also inhibited the adhesion of leukocytes to activated endothelial cells (Fig. 3, B and C), in this case, however, the effect was mediated through PAR-2 because only anti-PAR-2 antibody inhibited this effect (data not presented). As expected, MβCD abrogated the PAR-1-dependent protective activity of thrombin on the FX-S195A-pretreated cells, suggesting that the interactions take place within the lipid rafts of endothelial cells (Fig. 3, B and C).
FIGURE 3.
TNF-α-mediated up-regulation of cell adhesion molecules on endothelial cells. A, the PAR-1-dependent effect of APC (20 nm, 4 h), thrombin (20 nm, 4 h), and TFLLRN (100 μm, 4 h) on the modulation of the cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in response to TNF-α (10 ng/ml, 4 h) was monitored by ELISA after treating cells with either PC-S195A (50 nm, 30 min) or FX-S195A (175 nm, 30 min) in the absence and presence of the function-blocking anti-PAR-1 antibody (25 μg/ml). B, endothelial cells were activated with TNF-α (10 ng/ml, 4 h) after treating cells with APC (20 nm, 4 h), FXa (20 nm, 4 h), and thrombin (20 nm, 4 h) ± FX-S195A (175 nm, 30 min), and the adherence of neutrophils (panel B) or THP-1 cells (panel C) was monitored as described under “Experimental Procedures.” In the presence of MβCD (10 mm, 1 h) and function-blocking anti-PAR-1 anti-PAR-2 antibodies (25 μg/ml, 30 min), cells were first incubated with these reagents. All results are means ± S.D. of three different experiments.
The Barrier-protective Effect of FX/FXa Is Mediated through Modulation of the Rho Family of GTPases
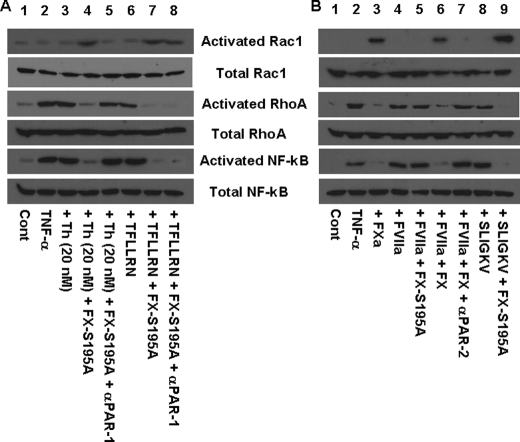
It has been previously demonstrated that the barrier-disruptive effect of thrombin and the opposite barrier-protective effect of APC are mediated through these proteases differentially modulating the cytoskeletal proteins, actin and myosin, via the activation of the Rho family of small GTPases (16, 34). Thus, the proinflammatory molecules have been shown to increase the levels of the phosphorylated myosin light chain through the activation of RhoA, thereby inducing the formation of actin stress fibers that can lead to cellular contraction and disruption of the endothelial barrier function (34, 35). On the other hand, the barrier-protective effect of APC is mediated through the APC activation of another member of the Rho family of GTPases called Rac1 whose activity is associated with the cytoskeletal remodeling that counteracts the barrier-disruptive effects of the proinflammatory molecules (34, 35). To determine whether FX through recruiting PAR-1 alters the downstream signaling specificity of thrombin, the ability of thrombin to modulate the two proteins of the Rho family of GTPases was analyzed in the TNF-α-stimulated endothelial cells in the absence and presence of FX-S195A. As presented on Fig. 4A, thrombin in the presence of FX-S195A, but not in its absence, activated Rac1 and inhibited the activation of RhoA in TNF-α-stimulated endothelial cells (lane 4). The switch in the signaling specificity of thrombin was mediated through activation of PAR-1 because the function-blocking anti-PAR-1 antibody eliminated the protective effect of thrombin (Fig. 4A, lane 5). The PAR-1-specific TRAP (TFLLRN) yielded identical results as evidenced by the FX-S195A changing the signaling specificity of the agonist peptide from activating RhoA to Rac1 activation (Fig. 4A, lanes 6 and 7) and as expected the anti-PAR-1 antibody did not block the activity of TRAP (lane 8). Consistent with the GTPase activities regulated by modulation of the NF-κB pathway, the protective activity of FX-S195A was associated with inhibition of the NF-κB pathway in TNF-α-stimulated endothelial cells (Fig. 4A, bottom two rows). Further studies indicated that FXa, whether added directly to the TNF-α-stimulated endothelial cells or generated by the picomolar concentration of FVIIa on TF bearing cells, also elicited an anti-inflammatory response (Fig. 4B, lanes 3 and 6). In this case, however, the response was mediated through activation of PAR-2 because the anti-PAR-2 antibody inhibited the effect (Fig. 4B, lane 7). Consistent with the PAR-2 specificity of the response, the PAR-2-specific TRAP also activated Rac1 in the presence of FX-S195A, however, in the absence of the zymogen mutant, activation of PAR-2 by the PAR-2 agonist peptide resulted in activation of RhoA (Fig. 4B, lanes 8 and 9). Similar to results in Fig. 4A, the protective activity of FXa is mediated through inhibition of the NF-κB pathway (Fig. 4B). These results clearly suggest that FX/Xa indirectly through PAR-1 and directly through the activation of PAR-2 elicits anti-inflammatory responses in stimulated endothelial cells through modulation of the Rho and NF-κB pathways.
FIGURE 4.
Regulation of activation of Rho GTPases and NF-κB by coagulation proteases and PAR-1 and PAR-2 agonist peptides. A, TNF-α-mediated (10 ng/ml for 1 h) activation of Rho GTPases and NF-κB before and after treatment of endothelial cells with either 20 nm thrombin ± FX-S195A (175 nm) or 100 μm TFLLRN ± FX-S195A was analyzed by Western blotting using specific antibodies as described under “Experimental Procedures.” Lane 1, not treated control; lane 2, TNF-α only; lane 3, cells incubated with 20 nm thrombin for 30 min followed by addition of TNF-α; lane 4, cells treated with FX-S195A for 30 min followed by incubation with 20 nm thrombin for 30 min and then stimulation with TNF-α; lane 5, the same as lane 4 except that the cell monolayer was incubated with anti-PAR-1 antibody (25 μg/ml for 30 min); lanes 6–8, the same as lanes 3–5 except that instead of thrombin, TFLLRN (100 μm) was used as the PAR-1 agonist (30 min). B, the same as A except that the effect of PAR-2 activation by FXa alone, FX (175 nm) activated by FVIIa (10 pm) on TF-transfected cells, or SLIGKV (100 μm for 30 min) was monitored. Lane 1, not treated control; lane 2, TNF-α only; lane 3, FXa (20 nm) + TNF-α; lane 4, FVIIa (10 pm) + TNF-α; lane 5, FVIIa (10 pm) + FX-S195A + TNF-α; lane 6, FVIIa (10 pm) + FX + TNF-α; lane 7, FVIIa (10 pm) + FX + anti-PAR-2 antibody + TNF-α; lane 8, SLIGKV + TNF-α; lane 9, SLIGKV + FX-S195A + TNF-α.
FX Recruits PAR-1 to a Protective Pathway by Mediating the Dissociation of EPCR from Caveolin-1 in Lipid Rafts of Endothelial Cells
To understand the mechanism by which FX recruits PAR-1 to a protective signaling pathway, we immunoprecipitated the total protein extract of non-treated and FX-S195A-treated endothelial cells with anti-caveolin-1, anti-PAR-1, anti-EPCR, and anti-PAR-2 antibodies and subjected them to a 10% reducing SDS-PAGE. Western blot analyses of immunoprecipitates indicated that with the exception of PAR-2, the antibody specific for each receptor co-immunoprecipitates the other two receptors from the extract derived from non-treated endothelial cells (Fig. 5, A–C), suggesting that both EPCR and PAR-1 are associated with caveolin-1 in the same membrane microdomains. Interestingly, when endothelial cells were pretreated with FX-S195A, whereas both EPCR and PAR-1 pairs and caveolin-1 and PAR-1 pairs remained associated (Fig. 5A), EPCR and caveolin-1 were not co-immunoprecipitated by the same antibody pairs (Fig. 5, B and C), suggesting that the occupancy of an endothelial receptor by the FX zymogen leads to dissociation and/or migration of EPCR out of the caveolar compartment, a process that appears to couple PAR-1 to the Gi protein, thereby changing the specificity of the PAR-1 signaling from a proinflammatory to an anti-inflammatory response. In contrast to PAR-1, the same experimental approach did not provide any evidence of PAR-2 associating with any one of the other three receptors on either FX-S195A-treated or non-treated cells (Fig. 5, A–D).
FIGURE 5.
Immunoprecipitation and Western blotting of total cellular proteins. Total cellular proteins from non-treated and FX-S195A (175 nm, 30 min)-treated EA.hy926 cells were immunoprecipitated with anti-PAR-1 (A), anti-caveolin-1 (B), anti-EPCR (C), and anti-PAR-2 (D) antibodies, separated on SDS-PAGE and immunoblotted (IB) using the same four antibodies as described under “Experimental Procedures.”
FX-mediated Recruitment of PAR-1 to a Protective Pathway Is Independent of Gla Domain
We previously demonstrated that interaction of the Gla domain of protein C with EPCR is required for its ability to recruit PAR-1 to a protective pathway on endothelial cells (15, 16). To determine whether this is also true for FX/Xa, we compared the activities of recombinant FX zymogen derivatives lacking either Gla domain (GDFX) or both Gla and EGF1 domains (E2FX) in the hyperpermeability assay. Interestingly, both deletion derivatives of FX switched the PAR-1-dependent signaling specificity of thrombin from a permeability enhancing effect to a barrier-protective effect, suggesting that interaction of the Gla domain of FX with endothelial cells is not required for recruiting PAR-1 to a protective pathway (Fig. 6A). It has been previously reported that the 6 residues of the inter-EGF sequence Leu-83 to Leu-88 (Leu-Phe-Thr-Arg-Lys-Leu) in FXa competes with binding of the radiolabeled FXa to an endothelial cell surface (36). To determine whether interaction of the Gla-domainless FX derivatives with the same receptor accounts for its signaling activity on endothelial cells, the same experiments were conducted with a Gla-domainless protein C in which the 6 residues of the inter-EGF sequence of protein C were replaced with the corresponding residues of FX (GDPC-FX6aa). This Gla-domainless protein C mutant recruited PAR-1 to a protective pathway similar to GDFX and E2FX (Fig. 6A), suggesting that interaction of this sequence with an endothelial cell surface receptor is required for receptor mobilization by FX, and its protective signaling effect. Further studies with an E2FX mutant having Ala substitutions for the two N terminus residues, Arg-86 and Lys-87 (E2FX-RK/AA), suggested that this mutant is inactive in the permeability assay (Fig. 6A), suggesting that the two basic inter-EGF residues interact with the putative FX receptor on endothelial cells. Consistent with results presented above, both wild-type and Gla-domainless FXa derivatives exhibited a direct PAR-2-dependent protective effect on endothelial cells as evidenced by the blocking anti-PAR-2, but not anti-PAR-1 antibody reversing the protective effect of FXa derivatives (Fig. 6B). The identity of the FX/FXa receptor on endothelial cells is not known. However, several years ago, a receptor, termed effector cell protease receptor-1 (EPR-1), was reported to be the putative FXa receptor responsible for the cellular effect of the protease in endothelial cells (37). Although further studies did not confirm the identity of this receptor (38), nevertheless, our results showed that a commercially available antibody (EPR11-A obtained from Alpha Diagnostica Int. Inc., San Antonia, TX) inhibited the protective effect of FXa derivatives (Fig. 6B). Interestingly, further studies indicated that both PAR-2 and the putative FX/FXa receptor may be associated with each other because either anti-PAR-2 or anti-EPR-1 antibody co-immunoprecipitated both receptors derived from cell lysates of either transformed or primary HUVECs (Fig. 6C).
FIGURE 6.
Thrombin reverses the hyperpermeability effect of TNF-α in endothelial cells pretreated with FX derivatives. A, EA.hy926 cells were incubated with FX derivatives (50 nm each for 30 min) followed by incubation with thrombin (2 nm, 3 h) and activation of cells by TNF-α (10 ng/ml for 18 h). The permeability was monitored as described under “Experimental Procedures.” B, the same as A except that cells were incubated with FXa derivatives (20 nm) with or without preincubating cells with blocking anti-PAR-1, anti-PAR-2, and anti-EPR-1 (EPR11-A) antibodies. C, co-immunoprecipitation and Western blotting of total cellular proteins derived from transformed (EA.hy926) and primary HUVECs using anti-PAR-2 and anti-EPR-1 antibodies. IB, immunoblot. All results in A and B are means ± S.D. of three different experiments.
DISCUSSION
We have demonstrated in this study that interaction of the zymogen FX with an endothelial cell surface receptor can recruit both PAR-1 and PAR-2 to protective signaling pathways as evidenced by the specific agonist peptides to both receptors reversing the permeability enhancing effect of thrombin to a barrier-protective effect in cells pretreated with FX or FX-S195A (Fig. 1A). This property of FX is reminiscent of the signaling mechanism of protein C/APC, which also recruits PAR-1 to a cytoprotective pathway upon interaction with EPCR on endothelial cells (15, 16). The results presented in Fig. 1 further indicate that protein C also changes the specificity of the PAR-2 signaling. Thus activation of both PAR-1 and PAR-2 by coagulation proteases or by the specific receptor agonist peptides elicited only protective cellular responses as long as the specific receptor for either protein C or FX was occupied by its respective ligand. We previously demonstrated that the protective activity of protein C is mediated through the Gla domain-dependent interaction of the protein C/APC with EPCR (15, 16), an interaction that leads to dissociation of EPCR from caveolin-1 in lipid rafts of endothelial cells, thereby coupling PAR-1 to the PTX-sensitive Gi/o subfamily of G-proteins (15, 16, 29). Interestingly, it appears that FX also changes the signaling specificity of PAR-1 by a similar mechanism as evidenced by co-immunoprecipitation studies that showed that the treatment of endothelial cells with FX-S195A, similar to that of protein C-S195A, leads to dissociation of EPCR from caveolin-1. This mechanism of receptor occupancy, changing the PAR-1-dependent signaling specificity, is not shared by PAR-2 because no association between PAR-2, EPCR, or caveolin-1 was observed, suggesting that the protective effect of PAR-2 is mediated through a different mechanism. The observation that the siRNA for S1P1 inhibited the PAR-1-dependent, but not the PAR-2-dependent protective activities of both protein C and FX zymogens is consistent with this hypothesis. These results, together with the PTX sensitivity of both PAR-1 and PAR-2 effects suggest that a cross-talk between PAR-1 and S1P1, but not between PAR-2 and S1P1 is required for modulation of the G-protein coupling specificity of these receptors.
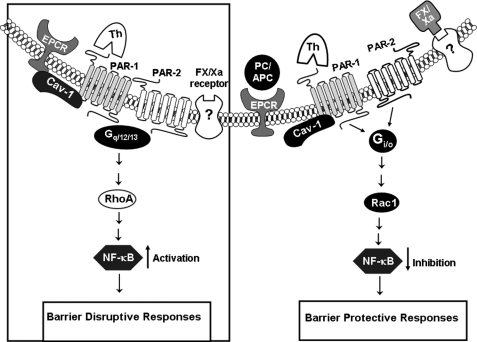
It has been previously demonstrated that FXa, via a loop connecting the EGF1 to EGF2 domain, interacts with a receptor on endothelial cells to elicit intracellular signaling responses (21, 36). The identity of this receptor is not known and the hypothesis that EPR-1 is the putative FXa receptor responsible for the cellular effect of the protease in endothelial cells (37) could not be confirmed by the failure to detect transcripts that would correspond to the sequence of EPR-1 in endothelial cells (38). Nevertheless, our mutagenesis studies with both E2FX and GDFX and the GDPC chimera, possessing the 6 inter-connecting EGF residues of FX, support the hypothesis that interaction of this sequence (in particular two basic Arg and Lys residues) with an endothelial cell surface receptor is required for the protective signaling effect of FX (Fig. 6). Further support for the existence of a FX/FXa receptor associated with PAR-2 on endothelial cells is provided by the observation that a commercially available antibody (EPR11-A) blocked the protective effect of FXa and co-immunopreciptated PAR-2 from endothelial cell lysate (Fig. 6, B and C). Thus, similar to the protein C occupancy of EPCR, the FX/FXa occupancy of this putative endothelial cell surface receptor alters the G-protein coupling specificities of both PAR-1 and PAR-2 so that the activation of both receptors by either thrombin (PAR-1) or FXa (PAR-2) elicits protective responses in the cytokine-activated endothelial cells. The observations that FXa, whether added directly to activated endothelial cells or generated from the FX zymogen activation by FVIIa on endothelial cells bearing TF, elicited a barrier-protective effect in the hyperpermeability assay, inhibited the expression of cell adhesion molecules, and impeded the interaction of leukocytes with the TNF-α-activated endothelial cells, are consistent with this hypothesis. Similar to the protective activity of the APC/protein C system, both the indirect PAR-1-dependent and direct PAR-2-dependent protective activities of FX/FXa (Fig. 4) were mediated through the activation of Rac1 and inhibition of the activation of RhoA and NF-κB pathways as evidenced by the agonist peptides to both PAR-1 and PAR-2 specifically mediating the activation of RhoA in non-treated endothelial cells, but the same peptides preferentially activating Rac1 in the FX-S195A-treated endothelial cells. A hypothetical model summarizing these results is presented in Fig. 7.
FIGURE 7.
A hypothetical model of PAR-1 and PAR-2 activation by coagulation proteases when either EPCR or an unknown FX/FXa receptor is occupied by natural ligands. In the absence of protein C/APC or FX/FXa, EPCR is associated with caveolin-1 (Cav-1) within the lipid rafts of endothelial cells (left panel in the box). In this case, thrombin appears to signal via Gq and/or G12/13, thereby enhancing cellular permeability through the activation of RhoA and eliciting proinflammatory responses through the activation of NF-κB (16). The occupancy of either EPCR by protein C (PC) or FX receptor by its ligand results in dissociation of EPCR from caveolin-1 (right panel). This process appears to be linked with the coupling of PAR-1 to Gi/o. In this case, the activation of PAR-1 by thrombin increases the barrier integrity of endothelial cells by activating Rac1 GTPase and eliciting an anti-inflammatory response through the inhibition of NF-κB (16). A similar protective effect is mediated when FXa binds to its receptor on endothelial cells. In this case, not only the activation of PAR-1 by thrombin is protective, but FXa also elicits a protective response directly through the activation of PAR-2, thereby activating Rac1 GTPase and inhibiting the NF-κB pathway. See text for more details.
The results presented above underscore the potential problems associated with in vitro studies using different cellular models to draw physiologically relevant conclusions related to the PAR-dependent signaling specificity of coagulation proteases. It is not therefore surprising that different studies using different cell lines have often reported inconsistent results (both cytoprotective and cytotoxic) for the activation of either PAR-1 or PAR-2 by coagulation proteases under different conditions. Given the importance of coagulation proteases in maintaining hemostasis, and the requirement for their rapid activation during injury and inflammation, it is likely that the co-receptor signaling system described above on endothelial cells is a safeguard mechanism to ensure that PAR-1 and PAR-2 activation by coagulation proteases would only elicit protective responses on the healthy vasculature bearing the appropriate cell surface receptors. Thus, we hypothesize that the activation of both PAR-1 and PAR-2 by coagulation proteases, under physiological conditions where the cell surface receptors (i.e. EPCR and an FX receptor) are occupied by their natural ligands, will elicit protective signaling responses by the inhibition of the NF-κB and RhoA pathways and the activation of the Rac1 pathway. In further support of this hypothesis, we recently demonstrated that occupancy of EPCR by protein C also inhibits thrombin mobilization of P-selectin from Weibel-Palade bodies in endothelial cells, thus inhibiting the interaction of leukocytes with the endothelium (39). Whether the FX receptor occupancy on endothelial cells also inhibits thrombin mobilization of P-selectin from Weibel-Palade bodies needs further investigation.
Acknowledgment
We thank Audrey Rezaie for proofreading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 101917 and HL 68571 from the NHLBI (to A. R. R.).
- FX
- factor X
- FXa
- activated factor X
- FVIIa
- activated factor VII
- TF
- tissue factor
- dc-TF
- tissue factor in which the cytoplasmic tail has been deleted
- FIX
- factor IX
- APC
- activated protein C
- EPCR
- endothelial protein C receptor
- PAR
- protease-activated receptor
- Gla
- γ-carboxyglutamic acid
- GDFX
- Gla-domainless FX in which residues 1–45 from the amino terminus of factor X have been deleted
- E2FX
- factor X mutant in which Gla and EGF-1 domains including residues 1–86 from the amino terminus have been deleted
- GDPC/FX6aa
- Gla-domainless protein C mutant in which residues 1–46 have been deleted and its 6 residues of the loop connecting the EGF-1 to EGF-2 domain have been replaced with the corresponding residues of factor X (Leu-83 to Leu-88)
- PC-S195A and FX-S195A
- protein C and factor X mutants in which the catalytic residue Ser-195 in the chymotrypsin numbering system (24) has been replaced with an Ala
- PTX
- pertussis toxin
- MβCD
- methyl-β-cyclodextrin
- S1P1
- sphingosine 1-phosphate receptor 1
- EPR-1
- effective cell protease receptor-1
- TRAP
- thrombin receptor agonist peptide
- GDPC
- Gla-domainless protein C
- HUVEC
- human umbilical vein endothelial cell
- VCAM-1
- vascular cell adhesion molecule 1
- ICAM
- intercellular adhesion molecule 1.
REFERENCES
- 1.Mann K. G., Jenny R. J., Krishnaswamy S. (1988) Annu. Rev. Biochem. 57, 915–956 [DOI] [PubMed] [Google Scholar]
- 2.Davie E. W., Fujikawa K., Kisiel W. (1991) Biochemistry 30, 10363–10370 [DOI] [PubMed] [Google Scholar]
- 3.Jackson C. M., Nemerson Y. (1980) Annu. Rev. Biochem. 49, 765–811 [DOI] [PubMed] [Google Scholar]
- 4.Esmon C. T. (1993) Thromb. Haemost. 70, 29–35 [PubMed] [Google Scholar]
- 5.Stearns-Kurosawa D. J., Kurosawa S., Mollica J. S., Ferrell G. L., Esmon C. T. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10212–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker F. J., Fay P. J. (1992) FASEB J. 6, 2561–2567 [DOI] [PubMed] [Google Scholar]
- 7.Dahlbäck B. (1991) Thromb. Haemost. 66, 49–61 [PubMed] [Google Scholar]
- 8.Fukudome K., Esmon C. T. (1995) J. Biol. Chem. 270, 5571–5577 [DOI] [PubMed] [Google Scholar]
- 9.Regan L. M., Mollica J. S., Rezaie A. R., Esmon C. T. (1997) J. Biol. Chem. 272, 26279–26284 [DOI] [PubMed] [Google Scholar]
- 10.Taylor F. B., Jr., Stearns-Kurosawa D. J., Kurosawa S., Ferrell G., Chang A. C., Laszik Z., Kosanke S., Peer G., Esmon C. T. (2000) Blood 95, 1680–1686 [PubMed] [Google Scholar]
- 11.Riewald M., Petrovan R. J., Donner A., Mueller B. M., Ruf W. (2002) Science 296, 1880–1882 [DOI] [PubMed] [Google Scholar]
- 12.Mosnier L. O., Zlokovic B. V., Griffin J. H. (2007) Blood 109, 3161–3172 [DOI] [PubMed] [Google Scholar]
- 13.Coughlin S. R. (2005) J. Thromb. Haemost. 3, 1800–1814 [DOI] [PubMed] [Google Scholar]
- 14.Ludeman M. J., Kataoka H., Srinivasan Y., Esmon N. L., Esmon C. T., Coughlin S. R. (2005) J. Biol. Chem. 280, 13122–13128 [DOI] [PubMed] [Google Scholar]
- 15.Bae J. S., Yang L., Manithody C., Rezaie A. R. (2007) Blood 110, 3909–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae J. S., Rezaie A. R. (2009) Thromb. Haemost. 101, 513–520 [PMC free article] [PubMed] [Google Scholar]
- 17.Bae J. S., Yang L., Rezaie A. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2867–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruf W., Dorfleutner A., Riewald M. (2003) J. Thromb. Haemost. 1, 1495–1503 [DOI] [PubMed] [Google Scholar]
- 19.Riewald M., Ruf W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7742–7747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feistritzer C., Lenta R., Riewald M. (2005) J. Thromb. Haemost. 3, 2798–2805 [DOI] [PubMed] [Google Scholar]
- 21.Senden N. H., Jeunhomme T. M., Heemskerk J. W., Wagenvoord R., van't Veer C., Hemker H. C., Buurman W. A. (1998) J. Immunol. 161, 4318–4324 [PubMed] [Google Scholar]
- 22.Krupiczojc M. A., Scotton C. J., Chambers R. C. (2008) Int. J. Biochem. Cell Biol. 40, 1228–1237 [DOI] [PubMed] [Google Scholar]
- 23.Borensztajn K., Stiekema J., Nijmeijer S., Reitsma P. H., Peppelenbosch M. P., Spek C. A. (2008) Am. J. Pathol. 172, 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. (1989) EMBO J. 8, 3467–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezaie A. R., Neuenschwander P. F., Morrissey J. H., Esmon C. T. (1993) J. Biol. Chem. 268, 8176–8180 [PubMed] [Google Scholar]
- 26.Rezaie A. R., Manithody C., Yang L. (2005) J. Biol. Chem. 280, 32722–32728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezaie A. R., Esmon C. T. (1992) J. Biol. Chem. 267, 26104–26109 [PubMed] [Google Scholar]
- 28.Neuenschwander P. F., Bianco-Fisher E., Rezaie A. R., Morrissey J. H. (1995) Biochemistry 34, 13988–13993 [DOI] [PubMed] [Google Scholar]
- 29.Bae J. S., Rezaie A. R. (2008) Thromb. Haemost. 100, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae J. S., Yang L., Manithody C., Rezaie A. R. (2007) J. Biol. Chem. 282, 9251–9259 [DOI] [PubMed] [Google Scholar]
- 31.Kim I., Moon S. O., Kim S. H., Kim H. J., Koh Y. S., Koh G. Y. (2001) J. Biol. Chem. 276, 7614–7620 [DOI] [PubMed] [Google Scholar]
- 32.Blackhart B. D., Emilsson K., Nguyen D., Teng W., Martelli A. J., Nystedt S., Sundelin J., Scarborough R. M. (1996) J. Biol. Chem. 271, 16466–16471 [DOI] [PubMed] [Google Scholar]
- 33.Hepler J. R., Gilman A. G. (1992) Trends Biochem. Sci. 17, 383–387 [DOI] [PubMed] [Google Scholar]
- 34.Finigan J. H., Dudek S. M., Singleton P. A., Chiang E. T., Jacobson J. R., Camp S. M., Ye S. Q., Garcia J. G. (2005) J. Biol. Chem. 280, 17286–17293 [DOI] [PubMed] [Google Scholar]
- 35.Kouklis P., Konstantoulaki M., Vogel S., Broman M., Malik A. B. (2004) Circ. Res. 94, 159–166 [DOI] [PubMed] [Google Scholar]
- 36.Ambrosini G., Plescia J., Chu K. C., High K. A., Altieri D. C. (1997) J. Biol. Chem. 272, 8340–8345 [DOI] [PubMed] [Google Scholar]
- 37.Altieri D. C. (1994) J. Biol. Chem. 269, 3139–3142 [PubMed] [Google Scholar]
- 38.Zaman G. J., Conway E. M. (2000) Blood 96, 145–148 [PubMed] [Google Scholar]
- 39.Bae J. S., Rezaie A. R. (2010) J. Thromb. Haemost. 8, 1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]