Abstract
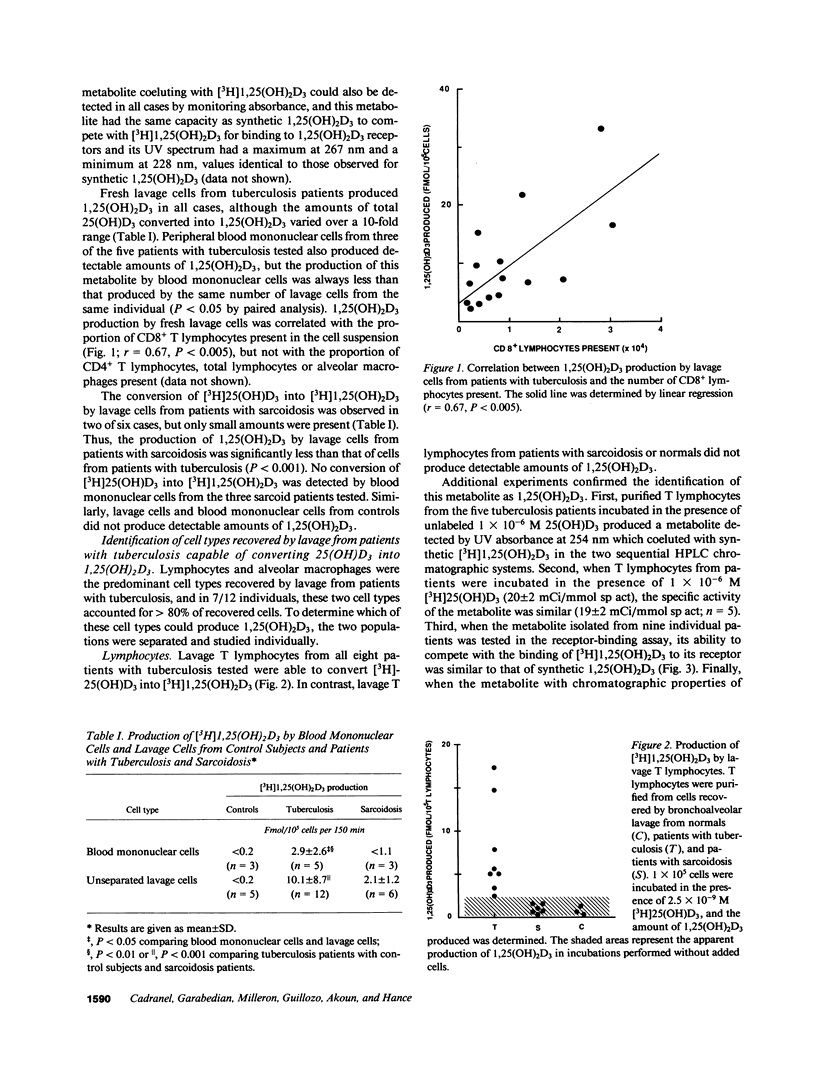
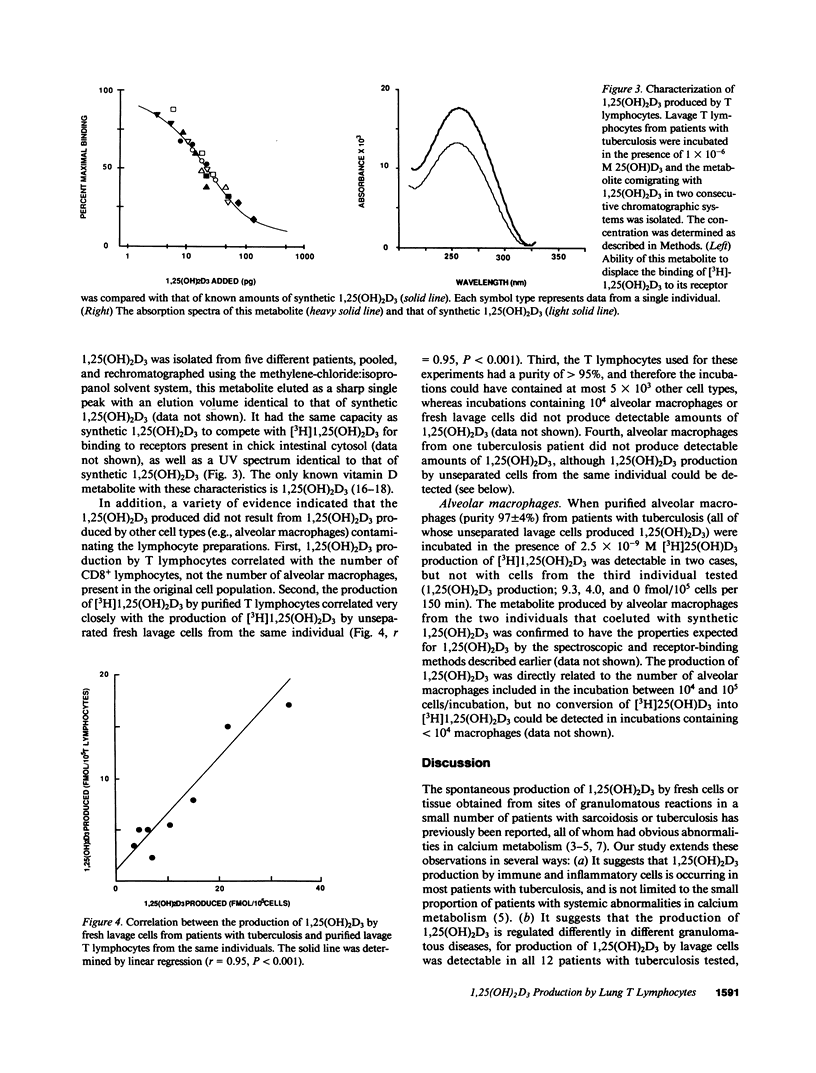
To compare extra-renal 1,25(OH)2D3 production in different types of granulomatous disease, and to identify the cell types responsible, we have evaluated the conversion of 25(OH)D3 in 1,25(OH)2D3 by uncultured cells recovered by bronchoalveolar lavage and blood mononuclear cells from normocalcemic patients with sarcoidosis and tuberculosis. 1,25(OH)2D3 was produced both by lavage cells (12/12 tuberculosis patients, 2/6 sarcoidosis patients) and blood mononuclear cells (3/5 tuberculosis patients, 0/3 sarcoidosis patients) from patients but not controls, but significantly greater amounts were produced by lavage cells from tuberculosis patients than those of sarcoidosis patients (P less than 0.001). 1,25(OH)2D3 production by lavage cells from tuberculosis patients correlated with the number of CD8+ T lymphocytes present but not other cell types. T lymphocytes appeared to be an important source of 1,25(OH)2D3 production, since purified T lymphocytes from all patients with tuberculosis produced 1,25(OH)2D3, and 1,25(OH)2D3 production by these cells correlated closely with that produced by unseparated lavage cells. Because 1,25(OH)2D3 can improve the capacity of macrophages to kill mycobacteria, our results support the conclusion that macrophage-lymphocyte interactions, mediated at least in part by 1,25(OH)2D3, may be an important component of a successful antituberculous immune response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Gacad M. A. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985 Apr 1;161(4):755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. S., Modlin R. L., Diz M. M., Barnes P. F. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab. 1989 Aug;69(2):457–460. doi: 10.1210/jcem-69-2-457. [DOI] [PubMed] [Google Scholar]
- Adams J. S., Sharma O. P., Gacad M. A., Singer F. R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983 Nov;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. S., Singer F. R., Gacad M. A., Sharma O. P., Hayes M. J., Vouros P., Holick M. F. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 1985 May;60(5):960–966. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- Breslau N. A., McGuire J. L., Zerwekh J. E., Frenkel E. P., Pak C. Y. Hypercalcemia associated with increased serum calcitriol levels in three patients with lymphoma. Ann Intern Med. 1984 Jan;100(1):1–6. doi: 10.7326/0003-4819-100-1-1. [DOI] [PubMed] [Google Scholar]
- Cadranel J., Hance A. J., Milleron B., Paillard F., Akoun G. M., Garabedian M. Vitamin D metabolism in tuberculosis. Production of 1,25(OH)2D3 by cells recovered by bronchoalveolar lavage and the role of this metabolite in calcium homeostasis. Am Rev Respir Dis. 1988 Oct;138(4):984–989. doi: 10.1164/ajrccm/138.4.984. [DOI] [PubMed] [Google Scholar]
- Clavel S., Garabedian M., Tau C., Orgiazzi J. Synthèse extra-rénale du calcitriol dans la sarcoïdose. Presse Med. 1987 Jan 31;16(3):107–110. [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dodd R. C., Cohen M. S., Newman S. L., Gray T. K. Vitamin D metabolites change the phenotype of monoblastic U937 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7538–7541. doi: 10.1073/pnas.80.24.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetchick D. A., Bertolini D. R., Sarin P. S., Weintraub S. T., Mundy G. R., Dunn J. F. Production of 1,25-dihydroxyvitamin D3 by human T cell lymphotrophic virus-I-transformed lymphocytes. J Clin Invest. 1986 Aug;78(2):592–596. doi: 10.1172/JCI112614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Koeffler H. P., Reichel H., Bishop J. E., Norman A. W. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985 Mar 15;127(2):596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Lecossier D., Valeyre D., Loiseau A., Battesti J. P., Soler P., Hance A. J. T-lymphocytes recovered by bronchoalveolar lavage from normal subjects and patients with sarcoidosis are refractory to proliferative signals. Am Rev Respir Dis. 1988 Mar;137(3):592–599. doi: 10.1164/ajrccm/137.3.592. [DOI] [PubMed] [Google Scholar]
- Mason R. S., Frankel T., Chan Y. L., Lissner D., Posen S. Vitamin D conversion by sarcoid lymph node homogenate. Ann Intern Med. 1984 Jan;100(1):59–61. doi: 10.7326/0003-4819-100-1-59. [DOI] [PubMed] [Google Scholar]
- Mayer E., Bishop J. E., Ohnuma N., Norman A. W. Biological activity assessment of the vitamin D metabolites 1,25-dihydroxy-24-oxo-vitamin D3 and 1,23,25-trihydroxy-24-oxo-vitamin D3. Arch Biochem Biophys. 1983 Jul 15;224(2):671–676. doi: 10.1016/0003-9861(83)90254-0. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Sommerfeld J. L., Pramanik B. C., Gardner R., Sherry A. D., Partridge J. J., Uskokovic M. R., Horst R. L. 19-nor-10-ketovitamin D derivatives: unique metabolites of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3. Biochemistry. 1983 Jul 19;22(15):3636–3640. doi: 10.1021/bi00284a015. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Barbers R., Norman A. W. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 1987 Dec;65(6):1201–1209. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Norman A. W. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989 Apr 13;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- Rigby W. F. The immunobiology of vitamin D. Immunol Today. 1988 Feb;9(2):54–58. [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988 Oct;138(4):768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]


