Abstract
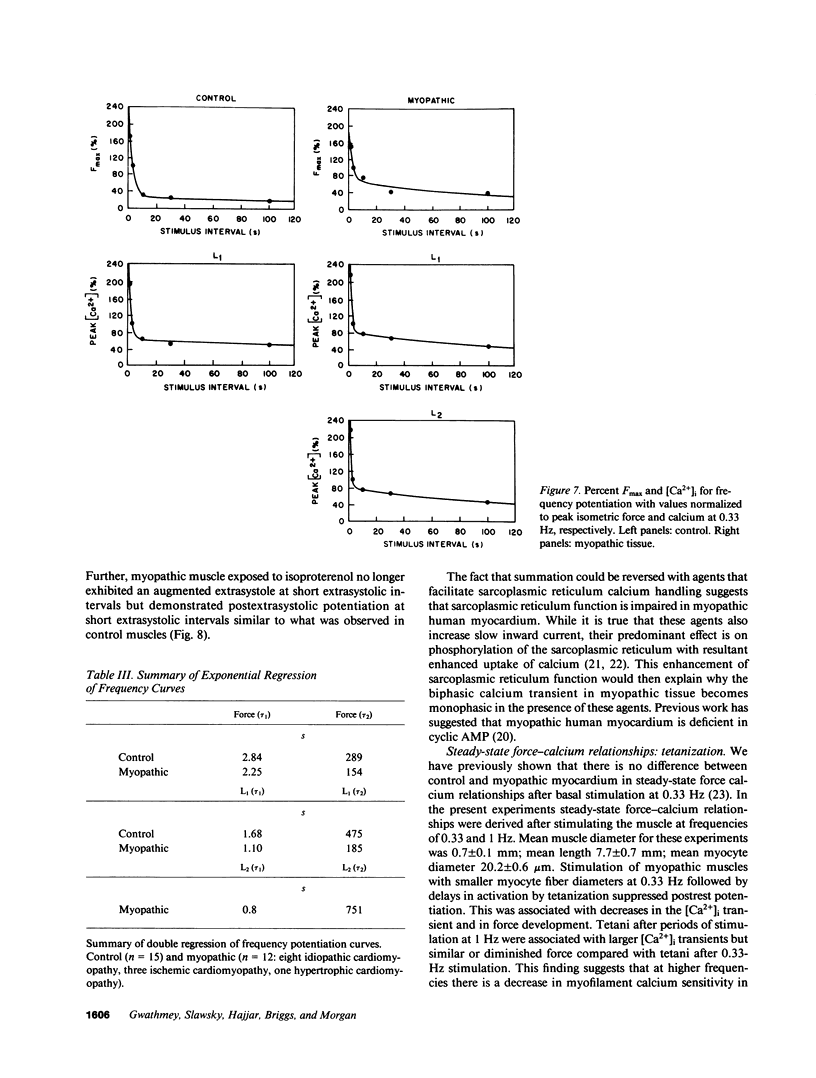
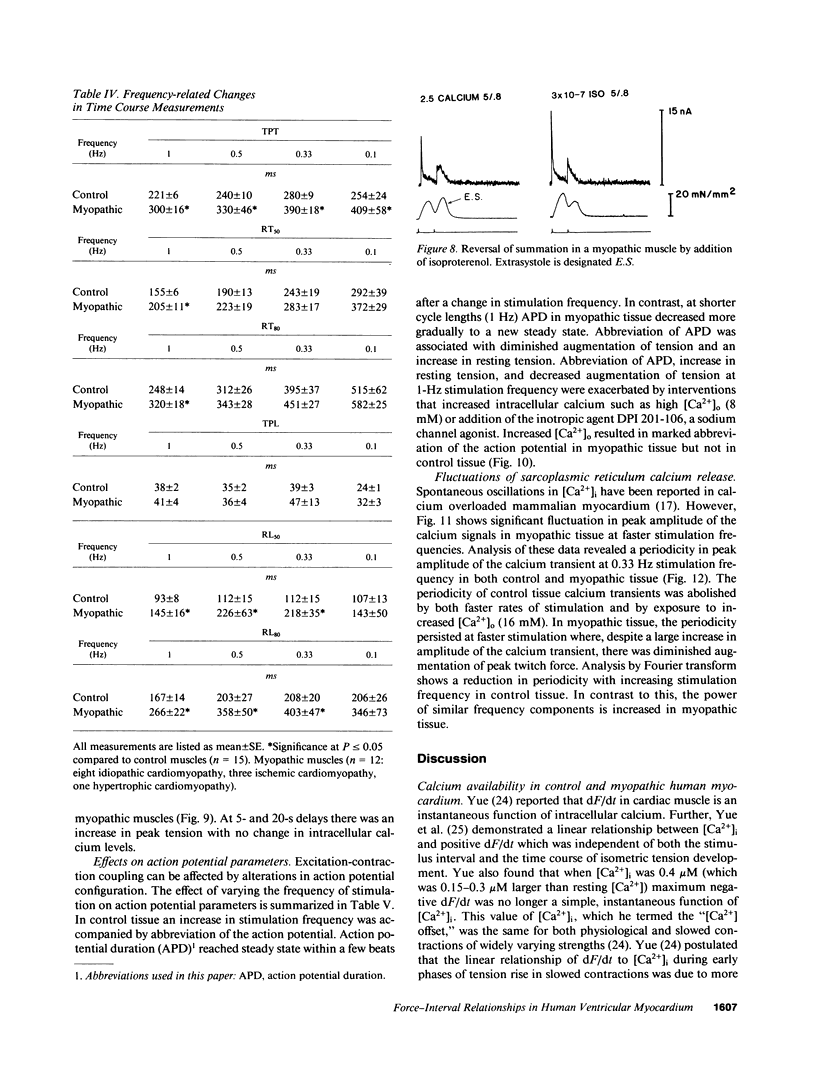
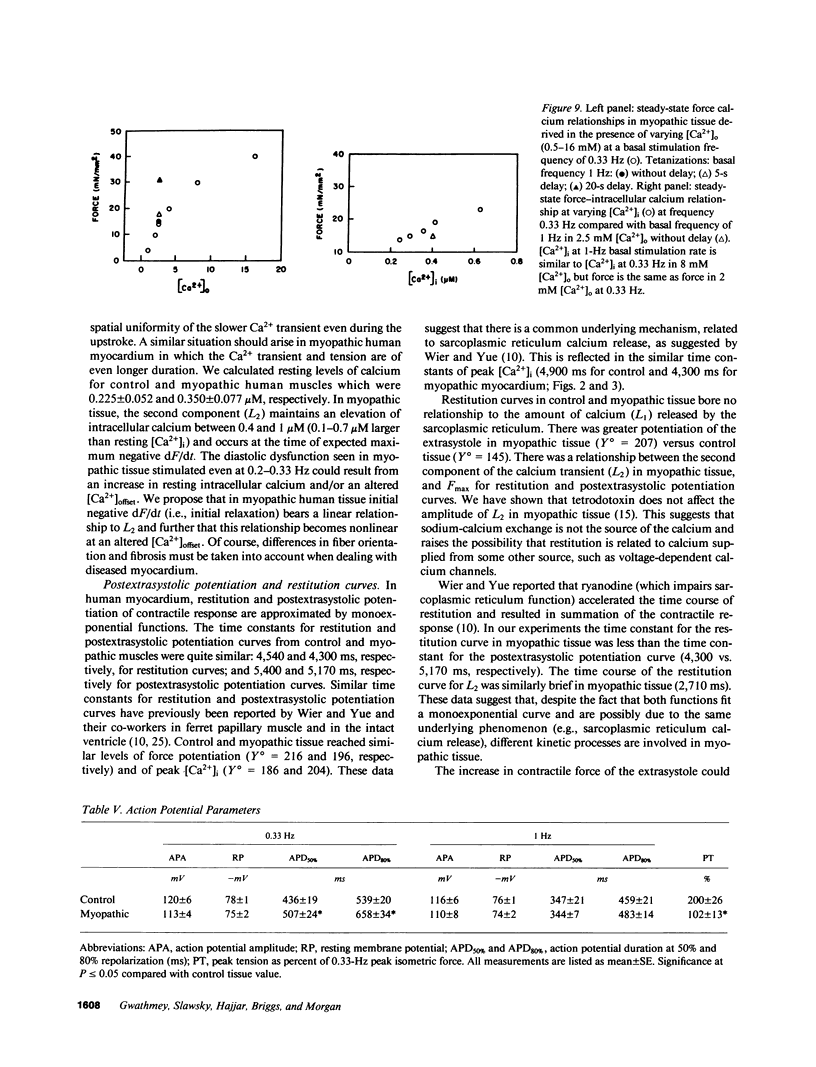
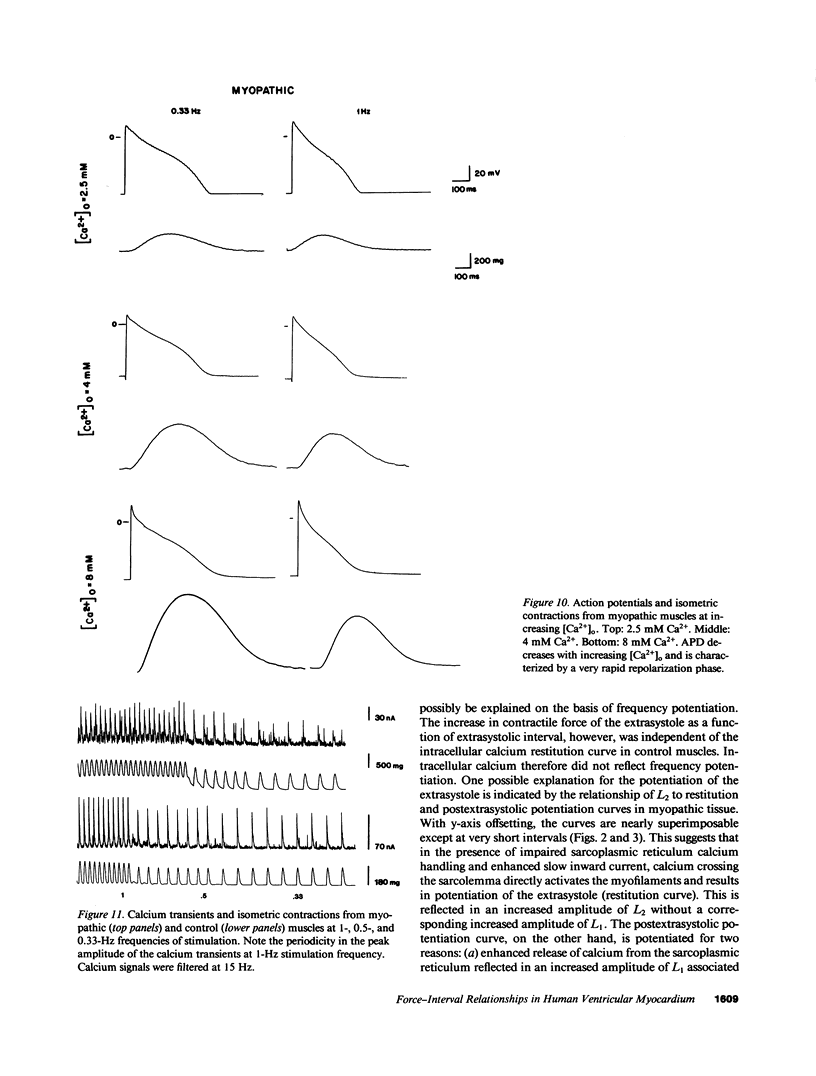
Experiments were performed in human working myocardium to investigate the relationship of intracellular calcium handling and availability to alterations in the strength of contraction produced by changes in stimulation rate and pattern. Both control and myopathic muscles exhibited potentiation of peak isometric force during the postextrasystolic contraction which was associated with an increase in the peak intracellular calcium transient. Frequency-related force potentiation was attenuated in myopathic muscles compared to controls. This occurred despite an increase in resting intracellular calcium and in the peak amplitude of the calcium transient as detected with aequorin. Therefore, abnormalities in contractile function of myopathic muscles during frequency-related force potentiation are not due to decreased availability of intracellular calcium, but more likely reflect differences in myofibrillar calcium responsiveness. Sarcolemmal calcium influx may also contribute to frequency-related changes in contractile force in myopathic muscles as suggested by a decrease in action potential duration with increasing stimulation frequency which is associated with fluctuations in peak calcium transient amplitude.
Full text
PDF
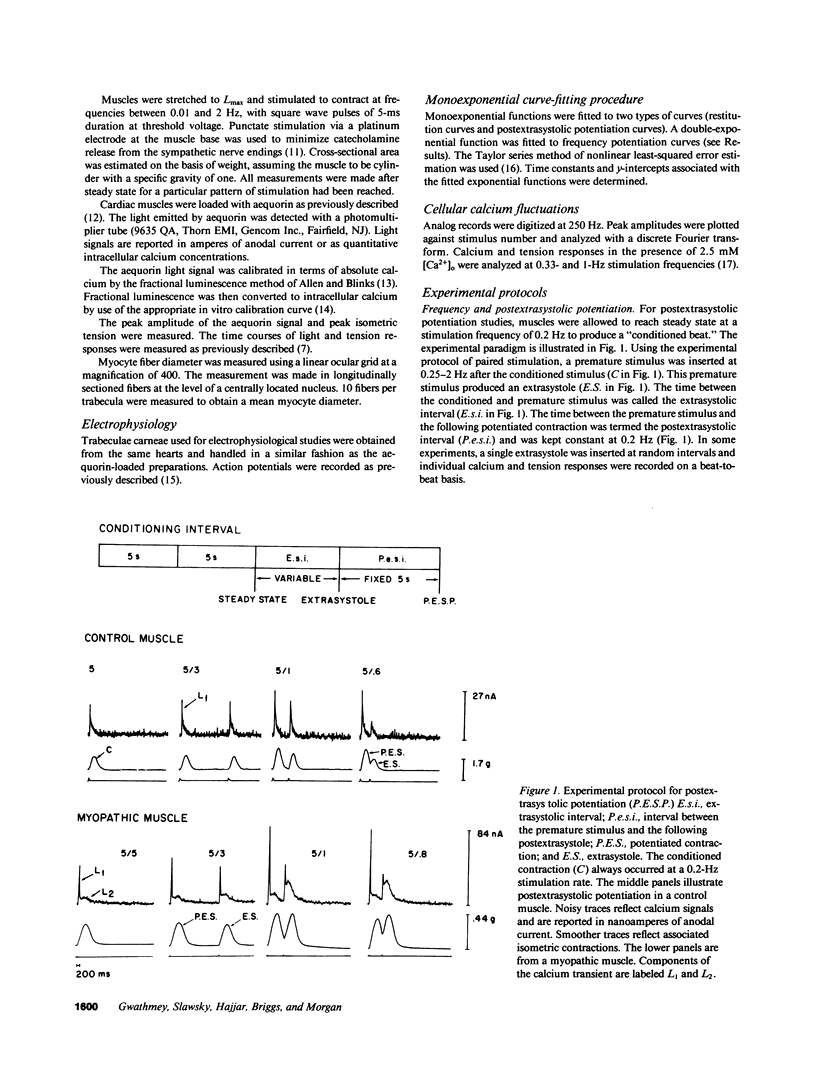

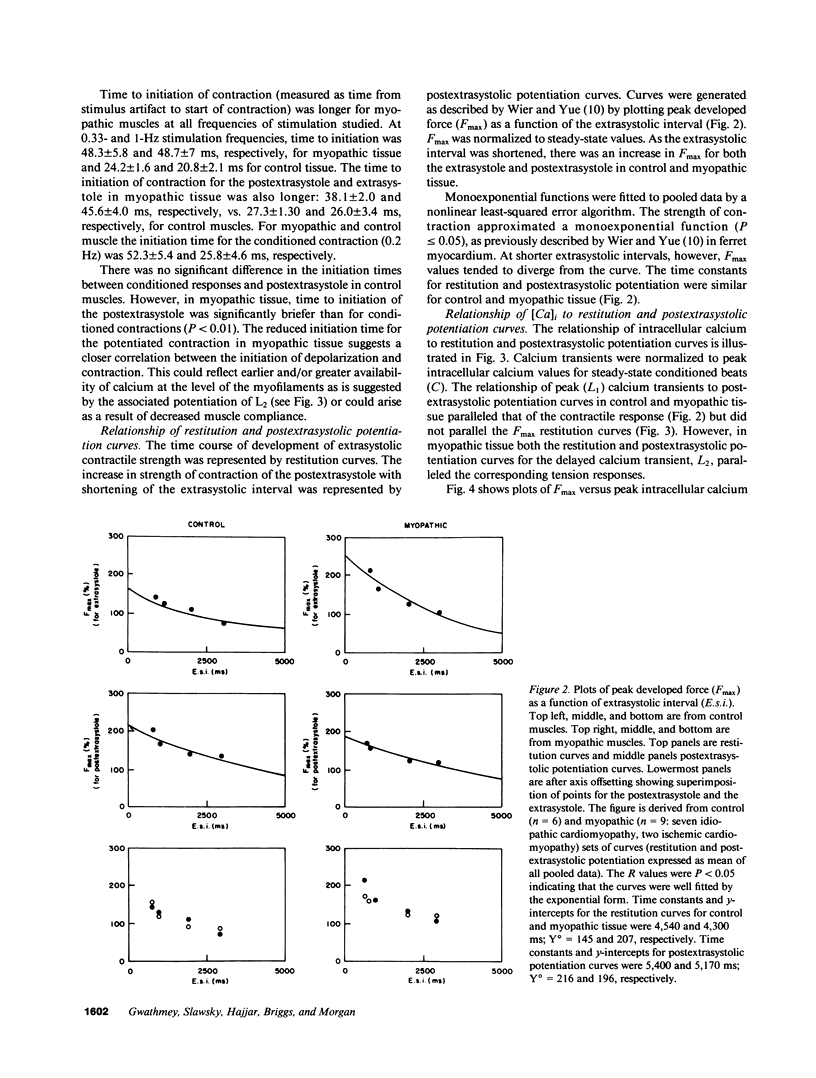
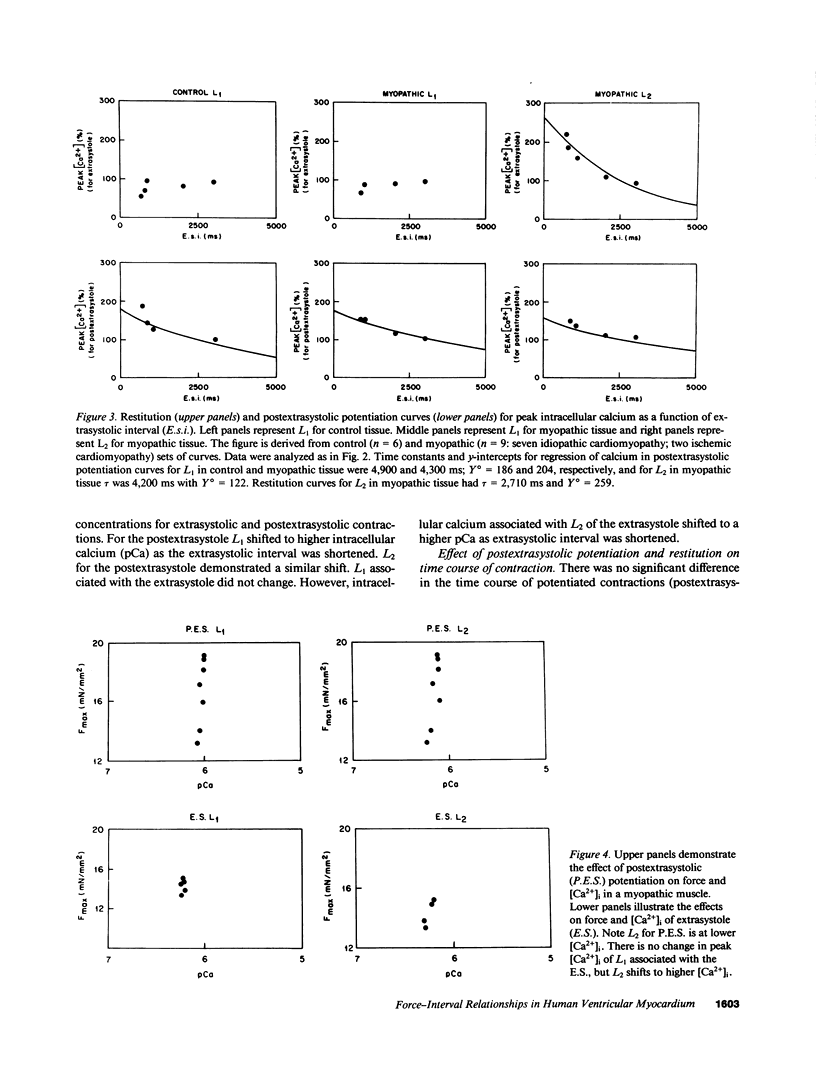

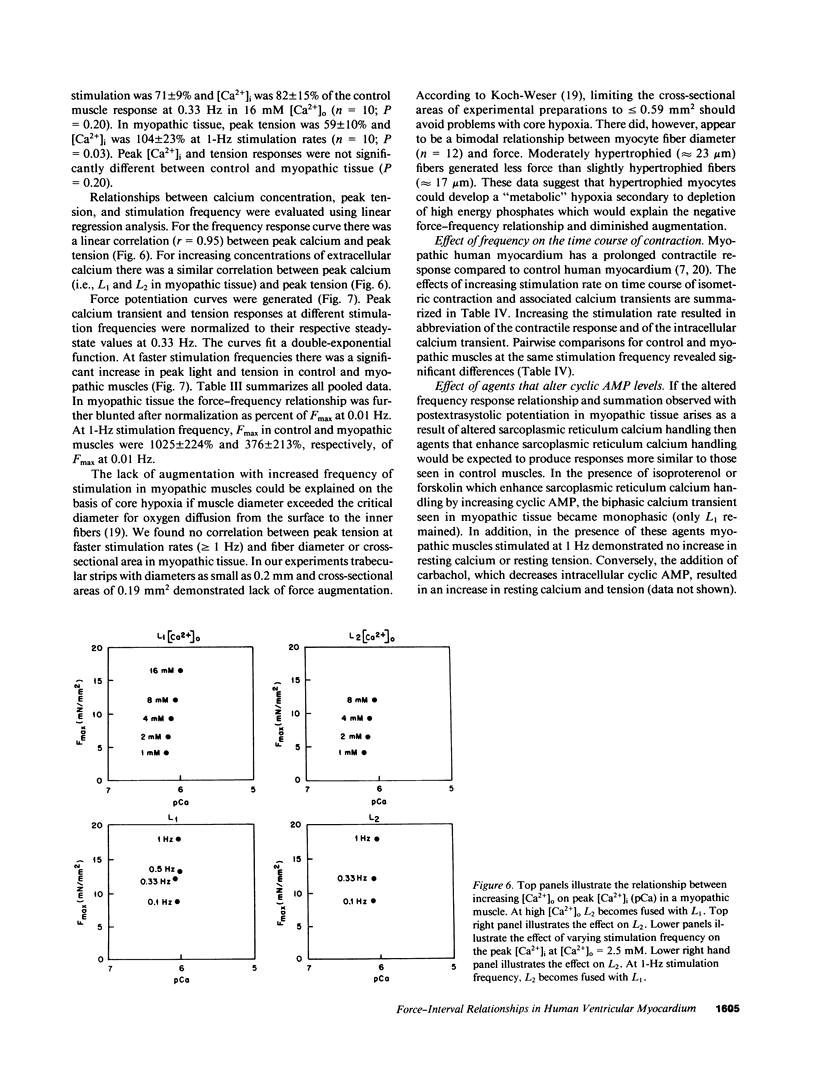



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R., KOCH-WESER J. Analysis of the effects of changes in rate and rhythm upon myocardial contractility. J Pharmacol Exp Ther. 1961 Dec;134:373–389. [PubMed] [Google Scholar]
- Blinks J. R. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966 Feb;151(2):221–235. [PubMed] [Google Scholar]
- Buckley N. M., Penefsky Z. J., Litwak R. S. Comparative force-frequency relationships in human and other mammalian ventricular myocardium. Pflugers Arch. 1972;332(4):259–270. doi: 10.1007/BF00588574. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. D., Copelas L., Gwathmey J. K., Phillips P., Warren S. E., Schoen F. J., Grossman W., Morgan J. P. Deficient production of cyclic AMP: pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987 Feb;75(2):331–339. doi: 10.1161/01.cir.75.2.331. [DOI] [PubMed] [Google Scholar]
- Garvey J. L., Kranias E. G., Solaro R. J. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem J. 1988 Feb 1;249(3):709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman W., McLaurin L. P., Rolett E. L. Alterations in left ventricular relaxation and diastolic compliance in congestive cardiomyopathy. Cardiovasc Res. 1979 Sep;13(9):514–522. doi: 10.1093/cvr/13.9.514. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Morgan J. P. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res. 1985 Dec;57(6):836–843. doi: 10.1161/01.res.57.6.836. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Slawsky M. T., Briggs G. M., Morgan J. P. Role of intracellular sodium in the regulation of intracellular calcium and contractility. Effects of DPI 201-106 on excitation-contraction coupling in human ventricular myocardium. J Clin Invest. 1988 Nov;82(5):1592–1605. doi: 10.1172/JCI113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN B. F., BINDLER E., SUCKLING E. E. Postextrasystolic potentiation of contraction in cardiac muscle. Am J Physiol. 1956 Apr;185(1):95–102. doi: 10.1152/ajplegacy.1956.185.1.95. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Hausworth O., Noble D., Tsien R. W. The dependence of plateau currents in cardiac Purkinje fibres on the interval between action potentials. J Physiol. 1972 Apr;222(1):27–49. doi: 10.1113/jphysiol.1972.sp009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig J. W., Peterson J. W., Rüegg J. C., Solaro R. J. Vanadate and phosphate ions reduce tension and increase cross-bridge kinetics in chemically skinned heart muscle. Biochim Biophys Acta. 1981 Jan 21;672(2):191–196. doi: 10.1016/0304-4165(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., Kramer M. F., Fifer M. A., Lorell B. H., Shemin R., Grossman W., Allen P. D. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985 Oct 24;313(17):1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- KOCH-WESER J. Effect of rate changes on strength and time course of contraction of papillary muscle. Am J Physiol. 1963 Mar;204:451–457. doi: 10.1152/ajplegacy.1963.204.3.451. [DOI] [PubMed] [Google Scholar]
- Kort A. A., Lakatta E. G. Bimodal effect of stimulation on light fluctuation transients monitoring spontaneous sarcoplasmic reticulum calcium release in rat cardiac muscle. Circ Res. 1988 Nov;63(5):960–968. doi: 10.1161/01.res.63.5.960. [DOI] [PubMed] [Google Scholar]
- Kort A. A., Lakatta E. G. Spontaneous sarcoplasmic reticulum calcium release in rat and rabbit cardiac muscle: relation to transient and rested-state twitch tension. Circ Res. 1988 Nov;63(5):969–979. doi: 10.1161/01.res.63.5.969. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Lakatta E. G. Intracellular calcium transients and developed tension in rat heart muscle. A mechanism for the negative interval-strength relationship. J Gen Physiol. 1985 Nov;86(5):637–651. doi: 10.1085/jgp.86.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky Z. J., Buckley N. M., Litwak R. S. Effect of temperature and calcium on force-frequency relationships in mammalian ventricular myocardium. Pflugers Arch. 1972;332(4):271–282. doi: 10.1007/BF00588575. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Bailey J. C., Surawicz B. Alternans of action potential duration after abrupt shortening of cycle length: differences between dog Purkinje and ventricular muscle fibers. Circ Res. 1988 May;62(5):1027–1040. doi: 10.1161/01.res.62.5.1027. [DOI] [PubMed] [Google Scholar]
- Urthaler F., Walker A. A., Reeves D. N., Hefner L. L. Maximal twitch tension in intact length-clamped ferret papillary muscles evoked by modified postextrasystolic potentiation. Circ Res. 1988 Jan;62(1):65–74. doi: 10.1161/01.res.62.1.65. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Yue D. T. Intracellular calcium transients underlying the short-term force-interval relationship in ferret ventricular myocardium. J Physiol. 1986 Jul;376:507–530. doi: 10.1113/jphysiol.1986.sp016167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Burkhoff D., Franz M. R., Hunter W. C., Sagawa K. Postextrasystolic potentiation of the isolated canine left ventricle. Relationship to mechanical restitution. Circ Res. 1985 Mar;56(3):340–350. doi: 10.1161/01.res.56.3.340. [DOI] [PubMed] [Google Scholar]
- Yue D. T. Intracellular [Ca2+] related to rate of force development in twitch contraction of heart. Am J Physiol. 1987 Apr;252(4 Pt 2):H760–H770. doi: 10.1152/ajpheart.1987.252.4.H760. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


