Abstract
The basement membrane (BM) proteins laminins, which consist of α, β, and γ chains, support tissue structures and cellular functions. To date only α4 and α5 types of laminins have been identified in the BMs of blood vessels. Our recent study suggested the presence of novel α3B-containing laminins in vascular BMs. Here we identified and characterized the third member of vascular laminins, laminin-3B11 (Lm3B11). RT-PCR analysis showed that microvascular endothelial (MVE) cells and umbilical vein endothelial cells expressed the messages for the α3B, β1, β2, and γ1 chains. In the culture of MVE cells, α3B was associated with β1 and γ1, producing Lm3B11. Recombinant Lm3B11 was overexpressed by introducing the cDNAs of the three chains into HEK-293 cells and purified to homogeneity. Purified Lm3B11 exhibited relatively weak cell adhesion activity through both α3β1 and α6β1 integrins. Most characteristically, Lm3B11 strongly stimulated MVE cells to extend many lamellipodial protrusions. This pseudopodial branching was blocked by an inhibitor for Src or phosphatidylinositol 3-kinase. Consistently, Lm3B11 stimulated the phosphorylation of Src and Akt more strongly than other laminins, suggesting that the integrin-derived signaling is mediated by these factors. The unique activity of Lm3B11 appears to be favorable to the branching of capillaries and venules.
Keywords: Basement Membrane, Endothelium, Extracellular Matrix, Integrin, Laminin
Introduction
Laminin, a family of large heterotrimeric glycoproteins consisting of α, β, and γ chains, is a major cell-adhesive component present in the basement membranes (BMs)2 of various tissues. It plays critical roles in the maintenance of tissue architectures and the regulation of cellular functions such as cell adhesion, migration, proliferation, apoptosis, and differentiation (1). Different combinations of five α, three β, and three γ chains give rise to more than 15 laminin isoforms (2). These laminins are distributed in a tissue-specific manner and are thought to play differential roles in the individual tissues. Such specific distribution and function of laminins are mainly determined by the α chains. For example, α2 laminins (laminin-211 and -221) are localized in the BMs of muscle and neurons, whereas α4 laminins (laminin-411 and -421) are localized in vascular BMs.
There are two splicing variants of laminin α3 chain, the truncated form α3 (or α3A) and the full-sized form α3B (3, 4), gene expression of which is regulated by alternative splicing and different promoters (5). Laminin-3A32 (Lm3A32), which is previously known as laminin-5 and consists of all truncated chains (α3A, β3, and γ2), is a major laminin isoform in the skin, esophagus, lung, breast, and other epithelial tissues. This laminin has extensively been studied because it has unique structure and biological activity as compared with other laminins (6). In the skin, Lm3A32 in the BM associates with integrin α6β4 of basal keratinocytes to form the hemidesmosome structures and contributes to their stable anchorage to the underlying connective tissues (7, 8). The laminin α3A chain also associates with the full-sized β chains (β1 and β2) and the full-sized, γ1 chain, thus producing laminin-3A11 (Lm3A11) and laminin-3A21 (Lm3A21) (9–11).
Although the α3B chain is known to be more widely expressed than the α3A chain in vivo, α3B-containing laminins have been poorly investigated (12–14). We previously expressed laminin-3B32 (Lm3B32; α3B/β3/γ2) as a recombinant protein and revealed its biological activity (15). In addition, our recent immunohistochemical study showed that the α3B chain is colocalized with the β3 and γ2 chains in the epithelial BMs of the skin, esophagus, breast, and lung, whereas in the BMs of blood vessels it is colocalized with the β1/2 and γ1 chains, suggesting the presence of novel vascular laminin isoforms, laminin-3B11 (Lm3B11) and laminin-3B21 (Lm3B21) in the vascular BMs (16). However, no previous studies have identified the α3B-containing, full-sized Lm3B11/3B21.
The network of blood vessels dynamically changes under various physiological and pathological conditions such as tumor growth and inflammation. The angiogenesis in tumor tissues is essential for tumor growth and metastasis. It is expected that vascular laminins play some important roles in the formation of blood vessels or the maintenance of vascular functions, because they regulate the proliferation, adhesion, migration, and differentiation of vascular endothelial cells (17, 18). It is well known that vascular BMs contain two major laminin groups, i.e. the α4 laminin-411/421 (Lm411/421) and the α5 laminin-511/521 (Lm511/521) (18). To identify and characterize the new laminin isoform Lm3B11 (Lm3B11), we examined its expression in cultured human vascular endothelial cells and expressed it as a recombinant protein in HEK-293 cells. The present study for the first time identifies Lm3B11 protein and shows its unique activity.
EXPERIMENTAL PROCEDURES
Adhesive Proteins, Antibodies, and Other Materials
Human placental laminin (Lm511/521) was purchased from Invitrogen. Mouse EHS laminin-111 (Lm111) and fibronectin were purchased from Chemicon (Temecula, CA). Human recombinant Lm3B32 was purified as described previously (15). Mouse monoclonal antibodies against the N-terminal regions of human laminin α3A chain (LSα3c3) and laminin α3B chain (F7) have been prepared before (15). A monoclonal antibody against human laminin β1 chain (LT-3) was purchased from Chemicon, and one against human laminin γ1 chain (number 22) was from BD Bioscience. Function-blocking anti-integrin antibodies used are the anti-α2-integrin antibody P1E6, the anti-α3-integrin antibody P1B5, the anti-α5-integrin antibody P1D6, and the anti-β1-integrin antibody 6S6 from Chemicon and the anti-α6-integrin antibody GoH3 from Pharmingen. Human epidermal growth factor (EGF) and human fibroblast growth factor (FGF) were purchased from Wako (Osaka, Japan). Rabbit monoclonal antibodies against Akt (pan) (C67E7) and phospho-Akt (Ser473) (D9E) were purchased from Cell Signaling (Beverly, MA), rabbit polyclonal antibody against c-Src (SRC-2) was from Santa Cruz (Santa Cruz, CA), and rabbit polyclonal antibody against Src (pY418) was from BIOSOURCE (Camarillo, CA). Signal inhibitors including Wortmannin (phosphatidylinositol 3-kinase (PI3K)), Y-27632 (ROCK), and PP1 analog (Src) were provided from the Screening Committee of Anticancer Drugs supported by a grant-in-aid for Cancer from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Cells and Culture
The human embryonic kidney cell line HEK-293 (ATCC CRL-1573) and the human mammary gland epithelial cell line MCF-10A (ATCC CRL-10317) were obtained from the American Type Culture Collection (Manassas, VA). The Buffalo rat liver-derived epithelial cell line BRL and the human glioblastoma cell line T98G have been used in previous studies (19). HEK-293 and BRL were maintained in DME/F-12 medium (Invitrogen) supplemented with 10% fetal calf serum (FCS), penicillin, and streptomycin sulfate. MCF-10A cells were cultured in DME/F-12 supplemented with 20 ng/ml EGF, 100 ng/ml cholera toxin, 0.01 mg/ml insulin, 500 ng/ml hydrocortisone, and 5% horse serum. Human skin microvascular endothelial (MVE) cells and human umbilical vein endothelial (UVE) cells were purchased from Kurabo (Osaka, Japan). MVE cells and UVE cells were maintained on gelatin-coated plates in Humedia-EB2 (Kurabo) supplemented with 10 ng/ml EGF, 5 ng/ml FGF, 10 μg/ml heparin, 1 μg/ml hydrocortisone, 8.8 mm dibutylyl cyclic AMP, 50 μg/ml gentamicin, 50 ng/ml amphotericin, and 5% FCS.
RT-PCR
Total RNAs were extracted from cultured cells according to the manufacture's protocol by Invitrogen and used as templates for cDNA synthesis. cDNAs were synthesized from 5 μg of the total RNAs as the templates using an RT-PCR kit (Toyobo, Osaka, Japan). cDNA fragments encoding each of the three laminin α chains, three laminin β chains, and two γ chains were amplified by PCR using specific primers. The primers used are listed in Table 1. The PCR was performed under the following conditions: 30 cycles of 1 min at 95 °C, 1 min at 58 °C, and 1 min at 72 °C for all genes. Aliquots of the PCR products were electrophoresed on 1% agarose gels in Tris borate-EDTA buffer and stained with ethidium bromide.
TABLE 1.
PCR primers used for detection of laminin chain messages by RT-PCR
| Gene | Sequences (5′–3′) | Product |
|---|---|---|
| bp | ||
| α3A chain | 543 | |
| 5′ | AGGGTGCCATTTCTTCAGCCTC | |
| 3′ | GTCGCAATCATCACATTCTTCTGC | |
| α3B chain | 785 | |
| 5′ | CCACAATTGGTGATAAACTCGAGTG | |
| 3′ | GTTGATGTGAATGTGAAGAGCTCC | |
| α5 chain | 656 | |
| 5′ | ATGAGCATCACATTCCTGGA | |
| 3′ | AGTGGGTTCCCAAAGAATCC | |
| β1 chain | 679 | |
| 5′ | TTGGACCAAGATGTCCTGAG | |
| 3′ | CAATATATTCTGCCTCCCCG | |
| β2 chain | 484 | |
| 5′ | TGGTGATTACAGAAGCCCAGAAGG | |
| 3′ | GTCAGTTGACCTGAGCATACCCAT | |
| β3 chain | 398 | |
| 5′ | CCAATATCATGCCCTGGTGAGCTA | |
| 3′ | TGCAGAACAGTAGCTGAGTCTGTG | |
| γ1 chain | 687 | |
| 5′ | GCAAGACTGAACAGCAGACC | |
| 3′ | TCCTATCAAGATCGCTGACC | |
| γ2 chain | 484 | |
| 5′ | AGTGACAAGACCCAGCAAGCA | |
| 3′ | ATTGGTACAGGAAGACATCCAC | |
| GAPDH | 450 | |
| 5′ | TCCACCACCCTGTTGCTGTA | |
| 3′ | ACCACAGTCCATGCCATCAC | |
Construction and Transfection of Lm3B11 Expression Vectors
The expression vector of human laminin α3B chain has been prepared previously (15). Human laminin β1 (5361 bp)- and γ1 (4830 bp)-chain cDNAs were constructed as follows. To obtain overlapping cDNA clones, a human lung 5′ stretch Plus cDNA library (Clontech, CA) was screened by the ECL direct nucleic acid labeling/detection system and amplified by PCR with Ex Taq polymerase (Takara, Tokyo, Japan). All primers used for PCR are listed in supplemental Tables S1 and S2. Amplified cDNA fragments were cloned into the pGEM T-Easy vector (Promega, Madison, WI). Cloned cDNA sequences were compared with reported sequences (β1, GenBankTM accession number M61916; γ1, GenBankTM accession number J03202), and their sequences were verified. Multiple cDNA fragments were combined at appropriate restriction enzyme sites to construct the complete laminin β1 and γ1 cDNAs. These β1 and γ1 cDNAs were inserted into pcDNA3.1/Zeo (+) and pcDNA3/Neo (+) (Invitrogen), respectively. HEK-293 cells were transfected with the expression vectors of the γ1 and β1 cDNAs in this order using the Lipofectamine PLUS reagents (Invitrogen). After each cDNA transfection and subsequent selection with 500 μg/ml G418 (neomycin, Calbiochem) for γ1 or 300 μg/ml zeocin (Invitrogen) for β1, a cell clone secreting the γ1 or β1 chain at a high level was selected. Finally, the HEK cell clone expressing both γ1 and β1 chains was transfected with the laminin α3B pcDNA3.1 Hygro (+), and stable transfectants were selected with 100 μg/ml hygromycin (Wako, Osaka). A HEK-293 cell clone most highly expressing the α3B chain was selected and used as Lm3B11-HEK.
Purification of Lm3B11
For purification of Lm3B11 protein, Lm3B11-HEK cells were grown to confluence in the growth medium in 150-mm dishes that had been coated with poly-l-lysine. The cultures were washed 3 times with PBS and then incubated in the serum-free DME/F-12 medium. The resultant conditioned medium was collected every 2 days and pooled. The pooled conditioned medium was directly applied to a Q-Sepharose column (Amersham Biosciences) that had previously been equilibrated with 20 mm Tris-HCl (pH 7.5). The column was washed with the buffer, and proteins bound to the column were eluted with 20 mm Tris-HCl (pH 7.5) buffer containing 0.5 m NaCl, 0.01% (w/v) Brij 35, and 0.1% CHAPS. The eluted fractions containing Lm3B11 were pooled and applied to a gelatin-Sepharose 4B column to remove fibronectin and matrix metalloproteinases 2 and 9. Lm3B11 in the unbound fractions from the gelatin column was finally purified by immunoaffinity chromatography with the anti-laminin α3B antibody F7. Bound proteins were eluted from the affinity column with 0.05% (v/v) trifluoroacetic acid and immediately neutralized to pH 7.0 to 7.5. The Lm3B11 protein thus purified was stored in the presence of 0.01% Brij 35 and 0.1% CHAPS. Protein concentrations were determined using a Bio-Rad protein assay kit with bovine serum albumin (BSA) as a standard. Purity was confirmed by SDS-PAGE. Approximately 100 μg of pure Lm3B11 was obtained from 1 liter of the Lm3B11-HEK cell-conditioned medium.
SDS-PAGE and Immunoblotting
SDS-PAGE was performed on 5% gels or 3.0–7.5% gradient gels under reducing or nonreducing conditions. Separated proteins were stained with Coomassie Brilliant Blue. For immunoblotting analysis, proteins resolved by SDS-PAGE were transferred to PVDF membranes and detected by the ECL detection method (Amersham Biosciences).
Cell Adhesion Assay
Cell adhesion assay was performed as described previously (15). Briefly, each well of the 96-well enzyme-linked immunosorbent assay plates (Costar, Cambridge, MA) was coated with various concentrations of laminins at 4 °C overnight and then blocked with 1% (w/v) BSA. We confirmed that under these conditions Lm3B11, Lm3B32 and Lm511 were coated on the plastic plates at an efficiency of nearly 80% or more without significant difference. Cells (3 × 104) were inoculated per each well containing serum-free Humedia EB-2 or DME/F-12 medium and incubated at 37 °C for 1 h. After nonadherent cells were removed, adherent cells were fixed and stained with Hoechst 33432. The fluorescent intensity of each well of the plates was measured using the fluorescence plate reader Plate Chameleon (Hidex, Turku, Finland). To identify integrins responsible for cell adhesion to laminins, the function-blocking, anti-integrin antibodies shown above were used at a concentration of 20 μg/ml. Cells were incubated with each function-blocking antibody for 20 min at room temperature and then placed onto the laminin-coated plates. The cell attachment to the plates was determined as described above.
Assays of Cell Scattering and Migration
To assay the scattering activity of laminins, BRL cells were suspended in DME/F-12 plus 1% FCS and inoculated into each well of 24-well plates at a density of 7 × 103 cells/well. Each test sample was directly added to the cultures and incubated at 37 °C for ∼40 h. Cell scattering was determined by counting scattered cells and total cells in three randomly selected microscopic fields. To measure cell migration on laminin substrates, MVE cells (2 × 104 cells) were suspended in Humedia EB-2 supplemented with 10 ng/ml EGF, 5 ng/ml FGF, 10 μg/ml heparin, 1 μg/ml hydrocortisone, 8.8 mm dibutylyl cyclic AMP, 50 μg/ml gentamycin, and 50 ng/ml amphotericin, the cell suspension (500 μl) containing 2 × 104 cells was inoculated per well of 24-well plates precoated with each substrate protein. After preincubation for 1.5 h at 37 °C, cell movement was monitored under a phase-contrast microscope using a time-lapse video equipment for 8 h.
Immunofluorescence Microscopy
Two hundred microliters of MVE cell suspension (2 × 105 cells/ml) in serum-free Humedia EB-2 was inoculated per well of Lab-Tek 8-well chamber slides (Nunc, Naperville, IL) precoated with a test substrate protein. After incubation for 1 h at 37 °C, the cells were washed with PBS and then fixed in 3.7% (w/v) formaldehyde in PBS for 15 min. For permeabilization, the cells were treated with 0.2% (v/v) Triton X-100 in PBS for 15 min. The permeabilized cells were blocked with 1.2% BSA in PBS for 1 h, and then F-actin was stained with rhodamine phalloidin (Molecular Probes) and examined under a fluorescence microscope (Biozero; Keyence, Osaka, Japan) equipped with 100×/1.35U Plan-Apochromat oil immersion objectives.
Analysis of Lm3B11-induced Signal Transduction
To identify Lm3B11-induced signal mediators, 500 μl of MVE cell suspension (2 × 105 cells/ml) in serum-free Humedia EB-2 was inoculated per well of 24-well plate (Sumilon, Tokyo, Japan) precoated with 5 μg/ml Lm3B11 and added with signal inhibitors (Wortmannin, Y-27632, and PP1analog). After incubation for 1 h at 37 °C, cells with and without multiple pseudopodial protrusions were counted, and the ratio was determined. To analyze the phosphorylation levels of Akt and Src, 500 μl of MVE cell suspension (5 × 105 cells/ml) in serum-free Humedia EB-2 was inoculated per well of 24-well plate (Sumilon) precoated with a test substrate protein. After incubation for 1 h at 37 °C, the cells were washed with PBS and dissolved in a lysis buffer consisting of 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 2.5 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 10 mm NaF, 1 mm phenylmethylsulfonyl fluoride, and 1% Triton X-100. The resulting cell lysates were subjected to SDS-PAGE followed by immunoblotting with specific antibodies against phosphorylated and unphosphorylated signal mediators.
Statistical Analysis
Statistical significance was evaluated with an unpaired Student's t test. A p value <0.05 was considered significant.
RESULTS
Expression of Lm3B11 by Vascular Endothelial Cells
Our previous study has suggested that a laminin isoform consisting of the laminin α3B, β1, and γ1 chains, Lm3B11, is present in BMs of blood vessels. However, this laminin isoform has not been identified yet in any source. To confirm the presence of Lm3B11, we analyzed by RT-PCR the expression of selected laminin chains in two types of human vascular endothelial cells, UVE and MVE cells, as well as the human mammary gland epithelial cell line MCF-10A (Fig. 1A). The messages of the α3B and α5 chains, but not the α3A chain, were detected in the two types of endothelial cells. MCF-10A expressed the α3A and α5 chains but did not express the α3B chain. The messages of the β1, β2, β3, and γ1 chains were detected in all of the three cell lines, whereas that of the γ2 chain was found only in MCF-10A. These results suggest the expression of at least four laminin heterotrimers (Lm3B11, Lm3B21, Lm511, and Lm521) in MVE and UVE cells and that of Lm3A32 and other α3A/α5-type laminins in MCF-10A cells.
FIGURE 1.
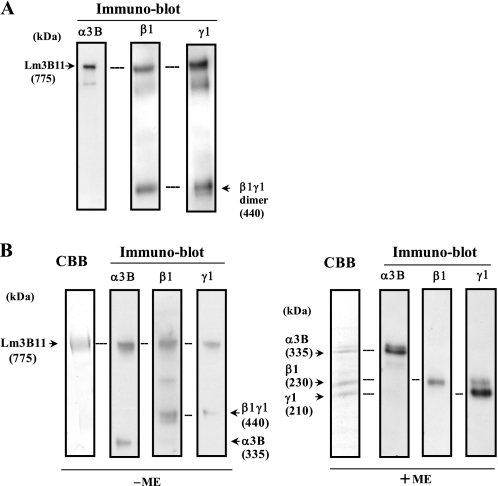
Expression of laminin chains and Lm3B11 by vascular endothelial cells. A, transcripts for laminin α3A, α3B, α5, β1, β2, β3, γ1, and γ2 chains were amplified by RT-PCR from human MVE cells and UVE cells using the primers listed in Table 1 under the conditions described under “Experimental Procedures.” The transcripts from MCF-10A cells (mammary gland epithelial cells) were also analyzed as control. The message of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as an internal loading control. B, to show whether Lm3B11 protein is secreted by UVE cells, their conditioned medium was applied to a Sepharose column conjugated with the α3B-specific antibody F7. Bound α3B-containing laminins were eluted and analyzed for the Lm3B11 chains by immunoblotting with the specific antibodies (LSα3c3 for α3, LT-3 for β1, and number 22 for γ1). The laminin chains detected are shown by arrows with apparent molecular sizes in kDa. ME, 2-mercaptoethanol.
Next, we attempted to identify the Lm3B11 heterotrimer in the conditioned medium of UVE cells. Because the α3B protein was undetectable in the concentrated conditioned medium, this protein was concentrated using a Sepharose column conjugated with the α3B-specific antibody (F7). Proteins bound to the column were eluted and analyzed for the Lm3B11 chains by immunoblotting. The eluted fraction contained not only the α3B protein of ∼335 kDa, which corresponded to the α3B chain without the LG4–5 domain (15), but also the β1 and γ1 chains, indicating that the Lm3B11 heterotrimer was indeed produced by vascular endothelial cells but at a very low level (Fig. 1B). Although the laminin α5 chain was clearly detected in the concentrated conditioned medium, it was never detected in the bound fraction of the affinity column (data not shown). This excluded the possibility that the β1 and γ1 chains in the bound fraction might derive from contaminating Lm511.
Although we stimulated vascular endothelial cells with various growth factors such as VEGF, bovine FGF, EGF, PDGF, and TGF-β1 and phorbol 12-myristate 13-acetate, the expression of the α3B chain was not affected (data not shown). Unknown special conditions seem to be required for its significant expression by vascular endothelial cells.
Expression and Purification of Recombinant Lm3B11
To show the biological activity of Lm3B11 on vascular endothelial cells, we established Lm3B11-producing cells (Lm3B11-HEK) by sequentially introducing the laminin γ1, β1, and α3B cDNAs into HEK-293 cells. When the serum-free conditioned medium of Lm3B11-HEK cells was analyzed by immunoblotting with the anti-α3B, -β1, and -γ1 monoclonal antibodies under nonreducing conditions, the Lm3B11 heterotrimer of ∼775 kDa as well as the β1/γ1 heterodimer of ∼440 kDa was detected at a high level (Fig. 2A). In addition, the immunoblot showed a minor band containing the α3B, β1, and γ1 chains at ∼600 kDa that was thought to be a Lm3B11 variant containing the 145-kDa cleaved α3B chain (15). From the conditioned medium of Lm3B11-HEK cells, Lm3B11 was purified by anion-exchange chromatography followed by immunoaffinity chromatography on an anti-α3B antibody-conjugated column. The purified protein was separated to a nearly single band of Lm3B11 at ∼775 kDa by SDS-PAGE under nonreducing conditions (Fig. 2B, left panel), whereas under reducing conditions it was separated into three major bands corresponding to the α3B, β1, and γ1 chains, respectively (right panel). These results confirmed that the laminin α3B chain is able to bind not only the β3 and γ2 chains to form Lm3B32 (15) but also the β1 and γ1 chains to form Lm3B11. It has been reported that the α3B chain is proteolytically cleaved to an N-terminal 190-kDa fragment and a C-terminal 145-kDa fragment in Lm3B32-expressing HEK-293 cell line (15). Although the Lm3B11 containing the 145-kDa cleaved α3B chain was detected in the conditioned medium of Lm3B11-HEK cells (Fig. 2A), it was not found in the purified Lm3B11 preparation because of the use of the antibody F7, which recognizes the N-terminal 190-kDa α3B fragment, in the affinity purification. However, the purified protein showed the α3B monomer and the β1/γ1 dimer when separated by SDS-PAGE under non-reducing conditions. This suggested that a part of the α3B molecules were associated with the β1/γ1 dimer without disulfide bond linkage (Fig. 2B, left panel).
FIGURE 2.
Expression of recombinant Lm3B11 by HEK-293 cells and its purification. A, conditioned medium of Lm3B11-HEK cells was separated by SDS-PAGE on 3–7.5% gradient gels under nonreducing conditions and then analyzed by immunoblotting for the laminin α3B, β1, and γ1 chains. Arrows indicate the immunoreactive bands corresponding to the Lm3B11 heterotrimer or the β1γ1 heterodimer. B, recombinant Lm3B11 purified from the Lm3B11-HEK conditioned medium was analyzed by SDS-PAGE under nonreducing conditions (left panel, −ME) or reducing conditions (right panel, +ME). Separated proteins were directly stained with Coomassie Brilliant Blue dye (CBB) or identified by immunoblotting for the laminin chains. Arrows indicate identified laminin chains with apparent molecular sizes in kDa. ME, 2-mercaptoethanol.
Cell Adhesion Activity of Lm3B11 and Receptor Identification
Laminins exhibit a variety of biological activity such as cell adhesion, cell migration, scattering, and proliferation through interaction with specific cell surface receptors. These activities vary from one laminin isoform to another. Lm3B32 shares the common α3B chain with Lm3B11, but the former contains the N-terminal-truncated forms of β and γ chains, i.e. β3 and γ2 chains. Lm3B32 as well as Lm3A32 having the three truncated subunits (α3A/β3/γ2) is known to show high cell adhesion and motility activities compared with other laminins (15). Like Lm3B11, Lm511 is a vascular-type laminin and has three long subunits (α5/β1/γ1). Lm511 also has a high cell adhesion activity (20). To characterize the purified Lm3B11, we compared some biological activities between Lm3B11 and other laminin isoforms using MVE cells and two epithelial cell lines. First, cell adhesion activity toward MVE cells was determined using plastic plates precoated with varied concentrations of each laminin. Lm3B11 showed a lower cell attachment activity than Lm3B32 and Lm511, but its activity was comparable with or slightly higher than that of Lm111 (Fig. 3A). When the Buffalo rat liver-derived epithelial cell line BRL was used, Lm3B11 supported the cell attachment at higher concentrations than Lm3B32 but at lower concentrations than Lm111 (Fig. 3B).
FIGURE 3.
Comparison of cell adhesion activity of Lm3B11 and other laminins. MVE cells (A) or BRL cells (B) were suspended in serum-free MCDB131 or DME/F-12 medium, respectively, and inoculated into each well of 96-well plates precoated with the indicated concentrations of purified Lm111 (open triangles), Lm3B32 (open circles), Lm3B11 (closed circles), and Lm511 (closed triangles). After incubation for 1 h, adherent cells were stained with Hoechst 33432 fluorescent dye and measured for the fluorescent intensity. Each point represents the mean of triplicate assays. Other experimental conditions are described under “Experimental Procedures.” When the cell adhesion activities of the four laminins toward MVE cells (A) were compared at 1.25 μg/ml, Lm3B11 was significantly lower than Lm 3B32 (p = 0.0044) and Lm511 (p = 0.012) but comparable with Lm111. When compared with BRL cells (B) at 0.25 μg/ml, Lm3B11 was significantly lower than Lm3B32 (p = 0.001) but higher than Lm111 (p = 0.016).
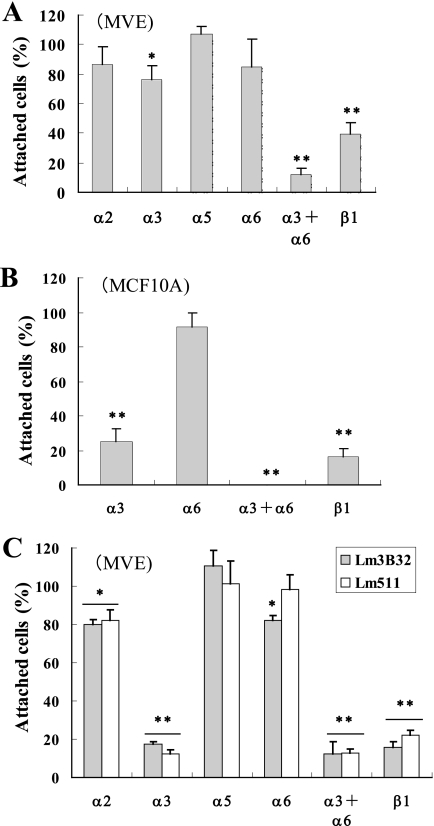
Cell adhesion to laminins is mainly mediated by integrins. Next, we examined the integrin requirement for the adhesion of MVE cells to Lm3B11 using function-blocking anti-integrin antibodies. It has previously been found that adhesion of human carcinoma cells to Lm3B32 is efficiently blocked by antibodies against integrins α3 and β1 (15). However, the adhesion of MVE cells to Lm3B11 was never or negligibly inhibited by the antibody to integrin α2, α3, α5, or α6, although it was significantly inhibited by the anti-integrin-β1 antibody (Fig. 4A). When MVE cells were treated with a combination of the anti-integrin-α3 and -α6 antibodies, their adhesion to Lm3B11 was almost completely blocked. This indicated that MVE cells equivalently use α3 and α6 integrins as the receptors for Lm3B11. On the other hand, when MCF-10A epithelial cells were used, the cell adhesion to Lm3B11 was efficiently blocked by the anti-integrin-α3 antibody alone (Fig. 4B). This suggests that Lm3B11 preferentially binds to integrin α3β1 in epithelial cells, whereas it also recognizes α6 integrins, α6β1 and possibly α6β4, at a similar affinity to integrin α3β1 in vascular endothelial cells. When a similar analysis was done with Lm3B32 and Lm511, the adhesion of MVE cells to both laminins was efficiently blocked by the anti-integrin-α3 antibody alone, indicating that these laminins preferentially recognize integrin α3β1 even in MVE cells (Fig. 4C). It has been reported that the adhesion of human microvascular endothelial cells to laminin-411 is efficiently blocked by the anti-integrin-α3 antibody alone (21). These results indicate distinct integrin recognition of Lm3B11 from other laminins in vascular endothelial cells.
FIGURE 4.
Inhibitory effects of anti-integrin antibodies on cell adhesion to Lm3B11. MVE (A and C) or MCF10-A (B) cells were incubated with the indicated function-blocking, anti-integrin antibodies at a concentration of 20 μg/ml for 20 min at room temperature and then placed onto 96-well plates precoated with 5 μg/ml Lm3B11 (A and B), 1 μg/ml Lm3B32 (C, dark gray bar), or 2.5 μg/ml Lm5111 (C, clear bar) followed by incubation for 1 h at 37 °C. The relative number of adherent cells was determined as described in Fig. 3. The fluorescent intensity of the culture treated with mouse IgG was taken 100%. Each point represents the mean ± S.D. for triplicate assays. Other experimental conditions are described under “Experimental Procedures.” Statistical differences from the control: *, p < 0.05; **, p < 0.01.
Cell Migration and Proliferation
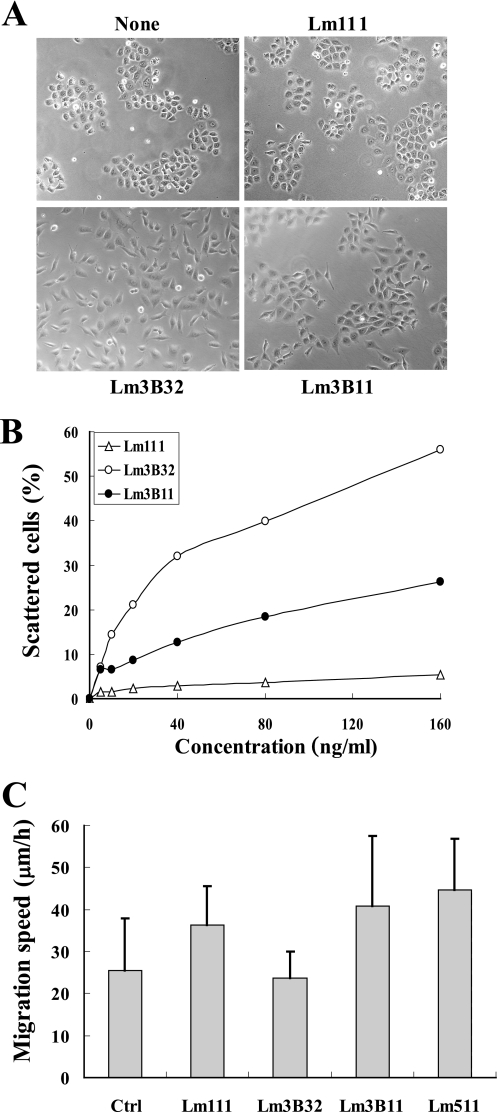
Lm3B32 and Lm3A32 promote scattering of BRL cells when coated onto culture plates or added into culture medium, whereas Lm511 shows this activity only in a coated form (15). When assayed by directly adding to the culture medium containing 1% FCS, Lm3B11 promoted scattering of BRL cells dose-dependently but at a far lower activity than Lm3B32 (Fig. 5, A and B). In contrast, Lm111 showed little cell scattering activity.
FIGURE 5.
Effects of Lm3B11 and other laminins on cell migration and scattering. A, cell-scattering activity of Lm3B11, Lm111, and Lm3B32 toward BRL cells is shown. Five hundred microliters of BRL cell suspension (1.4 × 104 cells/ml in DME/F-12 plus 1% FCS) was inoculated per well of 24-well plates. Each laminin isoform was directly added to the culture of BRL cells to make a final concentration of 0.02 μg/ml. Cell morphology was examined under a phase-contrast microscope after incubation for 40 h. None, cells incubated without test sample. Original magnification, ×100. B, to compare the cell-scattering activity of the three laminins quantitatively, BRL cells were incubated with varied concentrations of Lm3B11 (closed circles), Lm111 (open triangles), or Lm3B32 (open circles), and scattered cells were counted in three randomly selected microscopic fields. Each point represents the mean of the percentages of scattered cells in triplicate cultures. When statistical differences of scattering activity were determined at 160 ng/ml, Lm3B11 was significantly higher than Lm111 (p = 0.0008) but lower than Lm3B32 (p = 0.0002). C, shown are the effects of Lm3B11 and four other matrix proteins on migration of MVE cells. Five hundred microliters of MVE cell suspension (3 × 104 cells/ml in MCDB131 plus 1% FCS) was inoculated per well of 24-well plates precoated with 40 μg/ml gelatin as control (Ctr) or the following minimal effective concentrations of laminins determined for the adhesion of MVE cells (see Fig. 3A): 10 μg/ml Lm111, 1 μg/ml Lm3B32, 5 μg/ml Lm3B11, and 2.5 μg/ml Lm511. After incubation for 1.5 h to allow cell attachment, the migration on each substrate was monitored by video microscopy for 8 h. Each bar represents the mean ± S.D. of the migration speeds of 20 cells. The activity of Lm3B11 was significantly higher than that of Lm3B32 (p = 0.0044) but comparable with those of Lm111 and Lm511.
Next, cell migration activity toward vascular endothelial cells was compared between Lm3B11 and three other laminins (Fig. 5C). On the plastic plates coated with these laminins, MVE cells showed relatively poor cell migration although they actively extended pseudopodial protrusions. Lm3B11, Lm511, and Lm111 appeared to promote the migration of MVE cells at similar levels. Unexpectedly, Lm3B32 as well as gelatin as a control substrate scarcely promoted the MVE cell migration.
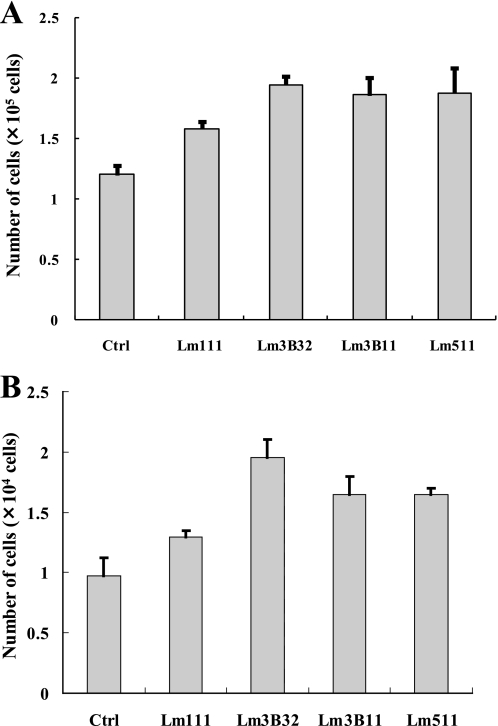
Growth effects of laminins were also examined using MVE cells and the human mammary epithelial cell line MCF-10A. Lm3B11 and Lm511 showed a tendency to support the growth of MVE cells more efficiently than Lm111 but less efficiently than Lm3B32, although the differences were insignificant (Fig. 6A). Similar growth effects were observed with MCF-10A cells (Fig. 6B).
FIGURE 6.
Comparison of cell growth activity of Lm3B11 and other laminins. A, MVE cells (5 × 103 cells in MCDB131 plus 1% FCS) was inoculated per 35-mm dish precoated with 40 μg/ml gelatin (Ctrl) or, 3 μg/ml each of Lm111, Lm3B32, Lm3B11, or Lm511 and incubated for 9 days with medium changes every 3 days. Grown cells were harvested by trypsinization and counted with a hemocytometer. Each point represents the mean ± S.D. of the numbers of cells in triplicate cultures. In the cell growth activity, Lm3B11 appeared slightly higher than Lm111 (p = 0.10) and slightly lower than Lm3B32 (p = 0.44), but the differences were not significant. B, MCF-10A (5 × 103 cells in DMEM/F-12 plus 1% FCS) was inoculated per 35-mm dish precoated with Lm111, Lm3B32, Lm3B11, or Lm511 and incubated for 3 days. Each point represents the mean ± S.D. of the numbers of cells in triplicate cultures. Statistical difference compared with Lm3B11 was Lm111 (p = 0.0014) and Lm3B32 (p = 0.056).
Stimulation of Pseudopodia Formation of MVE Cells by Lm3B11
Vascular endothelial cells are known to form capillary-like structures on the reconstituted BM Matrigel (22). Interaction with BM laminins is thought to play important roles in vascular functions. Therefore, we examined morphological change of MVE cells induced by Lm3B11 and other cell adhesion proteins including gelatin and fibronectin.
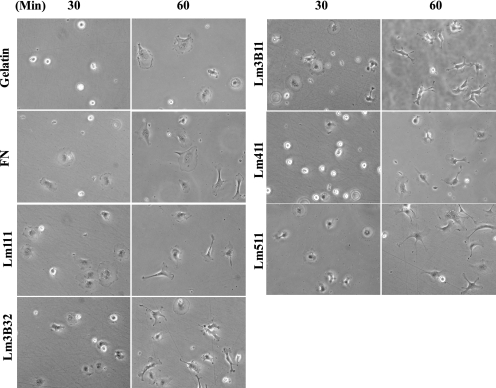
When MVE cells were placed on plastic plates precoated with each cell adhesion protein, they became more quickly spread on Lm3B11, Lm111, Lm3B32, Lm511, and fibronectin than gelatin and Lm411 (Fig. 7). After longer incubation, MVE cells on laminin-coated plates formed pseudopodia-like protrusions with lamellipodia at the leading edges. Most notably, MVE cells extended many thin and long pseudopodial protrusions on Lm3B11. Similar morphological changes were less prominently observed with cells on Lm3B32, Lm511, and Lm411.
FIGURE 7.
Morphological change of MVE cells on Lm3B11 and other cell adhesion substrates. MVE cells (1 × 104 cells in MCDB131 plus 1% FCS) were plated onto 24-well culture plates precoated with 40 μg/ml gelatin, 10 μg/ml fibronectin (FN), 10 μg/ml Lm111, 1 μg/ml Lm3B32, 5 μg/ml Lm3B11, 5 μg/ml Lm411, or 2.5 μg/ml Lm511 and incubated. Phase-contrast micrographs were taken 30 and 60 min after plating. Original magnification, ×200.
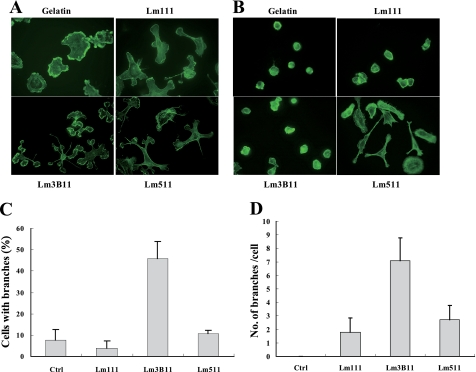
Fluorescent staining of filamentous actin more clearly showed the prominent morphological change of MVE cells on the Lm3B11 substrate. They extended many lamellipodial protrusions, which often had multiple branches, from single cells (Fig. 8A). The number of MVE cells with more than two protrusions and that of protrusions per cell were much greater on Lm3B11 than Lm511, Lm111, or gelatin (Fig. 8, C and D). When MCF-10A epithelial cells were plated on these proteins, the cells on Lm3B11, Lm111, and gelatin were poorly spread and showed few protrusions, whereas they well spread and extended lamellipodia on Lm511 (Fig. 8B). These results demonstrate that Lm3B11 strongly and specifically stimulates vascular endothelial cells to extend multiple lamellipodial protrusions.
FIGURE 8.
Actin cytoskeleton of MVE cells on Lm3B11 and other cell adhesion substrates. MVE cells (A) and MCF-10A cells (B) were plated onto glass-bottom culture plates precoated with 40 μg/ml gelatin, 10 μg/ml Lm111, 5 μg/ml Lm3B11, or 2.5 μg/ml Lm511 and incubated for 1 h. After the incubation, the cells were stained by FITC-phalloidin, and their actin cytoskeleton was examined under a fluorescent microscope. Original magnification, ×300. C, the percentage of cells with more than two pseudopodial protrusions (branches) was determined. Statistical differences compared with Lm3B11: control (p = 0.0059), Lm111 (p = 0.0035), and Lm511 (p = 0.017). D, the number of protrusions (branches) per cell was determined. Each point represents the mean ± S.D. in triplicate cultures. Statistical differences compared with Lm3B11: control (Ctrl, p = 0.025), Lm111 (p = 0.046), and Lm511 (p = 0.066).
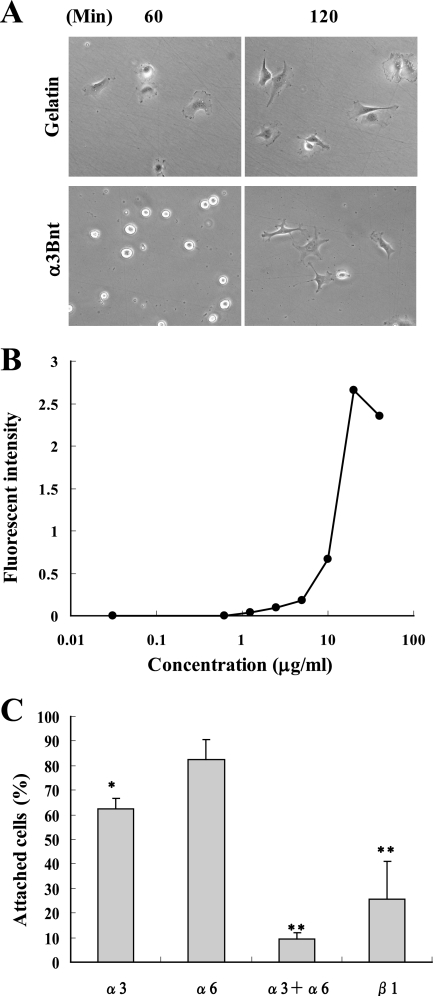
The α3B chain of Lm3B11 has an extended N-terminal region that contains a secondary integrin binding region compared with the α3A chain (15). It is also known that an N-terminal 190-kDa sequence is cleaved from the α3B chain by endogenous proteinases. The N-terminal 190-kDa fragment (α3Bnt) supported the adhesion of MVE cells more weakly than gelatin (Fig. 9, A and B). However, α3Bnt stimulated MVE cells to extend multiple protrusions. Like Lm3B11, the cell adhesion activity of α3Bnt was mediated by both α3 and α6 integrins (Fig. 9C). This suggests that not only the LG domain of the α3B chain but also α3Bnt is involved in the unique activity of Lm3B11.
FIGURE 9.
Activity of 190-kDa N-terminal fragment of α3B chain (α3Bnt). A, shown is the morphology of MVE cells adhered to α3Bnt. The α3Bnt fragment was purified as reported previously (15). MVE cells in serum-free medium was placed on plates precoated with 40 μg/ml gelatin or 20 μg/ml α3Bnt. After incubation for 60 and 120 min, the cell morphology was examined under a phase-contrast microscope. Original magnification, ×200. B, shown is the effect of varied concentrations of α3Bnt on attachment of MVE cells. MVE cells were incubated on plates precoated with the indicated concentrations of α3Bnt for 2 h. Relative number of adherent cells were determined as described in Fig. 3C. C, identification of integrins responsible for attachment of MVE cells to α3Bnt is shown. Inhibitory effects of the indicated, function-blocking anti-integrin antibodies on the cell attachment to the α3Bnt-coated plates were assayed as described in Fig. 4. The relative number of adherent cells in the presence of mouse IgG was taken 100%. Each point represents the mean ± S.D. for triplicate assays. Statistical differences compared with the control: *. p < 0.05; **, p < 0.01.
Lm3B1-induced Signal Transduction in MVE Cells
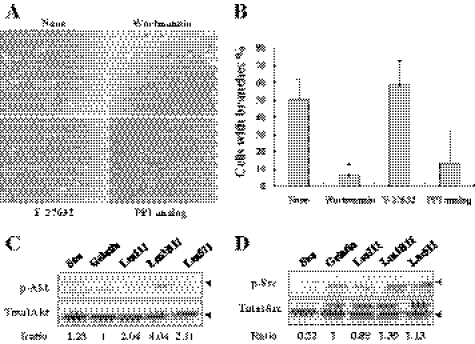
During angiogenic sprouting, endothelial tip cells directionally branch from existing vessels. Such endothelial cell branching is negatively regulated by ROCK-mediated myosin II activity in three-dimensional culture conditions (23). As shown above, Lm3B11 promoted similar endothelial cell branching in two-dimensional culture conditions. It is expected that Lm3B11 induces some intracellular signal transduction through both integrins α3β1 and α6 β1, resulting in the remodeling of actin cytoskeleton. To identify intracellular signal mediators responsible for the pseudopodia formation, we briefly examined the effects of some signal inhibitors on the Lm3B11-induced morphological change of MVE cells. The PI3K inhibitor (Wortmannin) significantly blocked the pseudopodia formation induced by Lm3B11 (Fig. 10, A and B). A Src inhibitor (PP1 analog) showed a similar inhibitory tendency, but a ROCK inhibitor (Y27632) did not.
FIGURE 10.
Analysis of signal mediators responsible for Lm3B11-induced protrusion formation in MVE cells. A, shown are the effects of three signal inhibitors on Lm3B11-induced morphological change of MVE cells. MVE cells (5 × 104 cells) in 0.1 ml of serum-free Humedia EB-2 was inoculated per well of 96-well plate precoated with 5 μg/ml Lm3B11 and incubated without (None) or with 10 nm Wortmannin (PI3K inhibitor), 10 μm Y-27632 (ROCK inhibitor), or PP1 analog (Src inhibitor) at 37 °C for 1 h. After the incubation cell morphology was examined under a phase-contrast microscopy. B, quantitative analysis of inhibitory effects is shown. In the culture shown in A, the percentage of cells with more than two pseudopodial protrusions (branches) was determined. Each point represents the mean ± S.D. for triplicate assays. Statistical differences compared with the control: Wortmannin (*, p = 0.025) and PP1 analog (p = 0.085). C and D, effects of cell adhesion substrates on phosphorylation of Akt and Src. Five hundred microliters of MVE cell suspension (5 × 105 cells/ml) in serum-free Humedia EB-2 was inoculated per well of 24-well plate precoated with gelatin, Lm111, Lm3B11, or Lm511 and incubated at 37 °C for 1 h as shown in Fig. 8. As a negative control, the cells in suspension (Sus) were incubated in a plastic test tube. After the incubation, the cells were lysed and subjected to immunoblotting with specific antibodies against phosphorylated and unphosphorylated Akt (C) or Src (D). Relative intensity of the immunopositive bands was measured by the computer software ImageJ. Values indicate the ratio of the relative intensity of the phosphorylated Akt band in each culture to that in the culture on the gelatin substrate. Other experimental conditions are described under “Experimental Procedures.” In C, the position of the p-Akt band (upper arrow, 60 kDa) corresponded to that of the total Akt band (lower arrow). An additional band (50 kDa) of p-Akt found in suspension (Sus) and gelatin seemed to be a nonspecific band because it was sometimes undetectable. In D, the major p-Src band corresponded to the major total Src band as shown by arrows. We could not identify the upper minor band (about 65 kDa) of total Src. It may be a different member of the Src family because the polyclonal antibody recognizes other Src family members.
To confirm the results obtained with signal inhibitors, the phosphorylation levels of Akt and Src were analyzed after MVE cells were incubated on Lm3B11. Both phosphorylation levels of Akt and Src were significantly enhanced by Lm3B11 as compared with gelatin, Lm111 and Lm511 (Fig. 10, C and D).
DISCUSSION
In the present study we identified a novel laminin isoform consisting of the laminin α3B, β1, and γ1 chains, i.e. Lm3B11, and revealed its biological activity as a recombinant protein. Previous studies have shown that the α3B chain is expressed widely in various human organs and tissues, but unlike α3A, it is scarcely detected in cultured cells (12). The present RT-PCR analysis showed that the two types of vascular endothelial cells, MVE and UVE cells, expressed the messages for the laminin α3B, β1, β2, β3, and γ1 genes but neither α3A nor γ2. This suggested that the Lm3B11/Lm3B21 heterotrimers, but not Lm3B32, could be produced by the endothelial cells. Indeed, the α3B chain was associated with the β1 and γ1 chains in the culture of MVE cells. These results are consistent with our recent finding that the α3B chain is colocalized with the β1 and γ1 chains but neither the β3 nor γ2 chain in vascular BMs of various tissues (16). However, the expression level of Lm3B11 in the cultured endothelial cells appeared to be very low.
The laminin α3A and α3B chains preferentially bind with the β3 and γ2 chains, both of which have truncated short arms, producing the laminin heterotrimers Lm3A32 and Lm3B32 (15, 24). These laminins have very high activities of cell adhesion and migration as compared with other laminins (6). The α3A chain also binds with the β1/2 and γ1 chains, producing Lm3A11/3A21 (11). In this study we for the first time expressed Lm3B11 as a recombinant protein by introducing the cDNAs of the α3B, β1, and γ1 chains into HEK-293 cells. Lm3A11 has a truncated α chain, whereas Lm3B11, like Lm111 and Lm511, has a typical laminin structure with the three full-length chains. As compared with Lm3B32, Lm3B11 had relatively low cell adhesion activity. This is similar to the case of Lm3A11 (11). It seems likely that the long short arms of the β1 and γ1 chains suppress the cell adhesion activity of Lm3A11 and Lm3B11. However, Lm3B11 exhibited relatively strong cell motility activity toward vascular endothelial cells as compared with epithelial cells. This may be related to a special integrin recognition of Lm3B11 by endothelial cells. Our experiments with neutral integrin antibodies indicated that an α3 integrin, most likely integrin α3β1, was primarily responsible for the attachment of epithelial cells to Lm3B11, whereas both α3 and α6 integrins, such as integrins α3β1, α6β1, and α6β4, equally contributed to the attachment of endothelial cells. In contrast, Lm511 and Lm3B32 preferentially recognized the α3 integrin for the attachment of both epithelial and endothelial cells.
Most prominent activity of Lm3B11 was the induction of a unique morphological change of MVE cells. When MVE cells were seeded on the Lm3B11 substrate, they extended multiple and branching lamellipodial protrusions. Such endothelial cell branching was less evidently seen on Lm3B32, Lm511, and Lm111 but not on fibronectin or gelatin at all. It is well known that laminins regulate cytoskeletal change through interaction with integrins (21). The Rho family of small GTPases plays critical roles in the formation of actin cytoskeleton and in the capillary assembly of endothelial cells (25–27). Our data suggest that Lm3B11 activates intracellular signal transduction mediated by Src, PI3K, and Akt. The PI3K signaling is known to activate small G-proteins through the downstream signaling kinase Akt (28). The signaling pathway of Src induces capillary assembly through activation of Rac (27). Taken together, it is expected that the interaction of Lm3B11 with α3 and α6 integrins induces the Src and then PI3K signaling pathways, which may activate Rac rather than Rho, leading to the unique cytoskeletal change that allows the cells to produce many thin and long lamellipodial protrusions. The α3Β chain contains the second integrin-binding site in its N-terminal region in addition to the LG1–3 domain (15, 29). In this study the N-terminal fragment of the α3Bnt weakly stimulated the protrusion formation in MVE cells, suggesting that the N-terminal integrin binding site contributes to the unique activity of Lm3B11 toward MVE cells.
It has long been believed that there are only two groups of laminin isoforms, Lm411/421 and Lm511/521, in vascular BMs. Their distinctive roles are still unclear. Lm411 is expressed in the BMs of all types of blood vessels including developing embryonic endothelium (E8.5) (30), whereas Lm511 occurs in mature vessels at later stages, predominantly in capillaries (31). A study with laminin α4 null mice indicates that this laminin is not essential for blood vessel formation, but it plays roles in maturation of vessels (32). Because the laminin α5 chain is expressed in not only the endothelium but also various kinds of epithelial tissues, laminin α5 null mice die during embryogenesis before it appears in vascular BMs. An in vitro study with mouse embryoid bodies also showed that although both α4 and α5 laminins are deposited on the surface of capillary-like tubes, the laminin deposition is dispensable for vasculogenesis (33). This study also showed that laminin-deficient embryoid bodies produced vascular structures with large lumens, but the addition of purified laminins increased capillary branches and produced more slender vessels. Taken together, it is considered that vascular laminins play roles in organizing and stabilizing blood vessels. In addition, these laminins may influence the barrier function of blood vessels and the transmigration of leukocytes and tumor cells. T-cell transmigration through the endothelial cell BM occurs exclusively at sites having the α4 but not α5 laminins (34).
Lm3B11 found in this study is the third member of vascular laminins. The laminin α3B chain has the highest homology to the α5 chain (14). Therefore, Lm3B11 is most close to Lm511 in structure. Like the laminin α5 chain, the α3B chain is expressed in both endothelial and epithelial cells. However, the α3B chain seems to exist as Lm3B32 in epithelial BMs because of its higher affinity to the β3 and γ2 chains than the β1 and γ1 chains. The present study demonstrates that Lm3B11 is distinguished from Lm511 and Lm3B32 with respect to the biological activity and integrin requirement. At present it is unknown what vascular function is attributed to the prominent activity of Lm3B11 in promoting the formation of multiple pseudopodial protrusions of MVE cells. Endothelial cell branching is required for the angiogenic process (23). The unique activity of Lm3B11 appears to be favorable to the branching of capillaries during angiogenesis. However, we previously found that the α3B laminin chain was less frequently detected in human skin cancer tissues as compared with normal skin tissues (16). It seems possible that Lm3B11 contributes to the formation or maintenance of complex vascular network in normal tissues. To clarify the biological function of Lm3B11, it is important to investigate its precise distribution in comparison with Lm411 and Lm511 in blood vessels of various tissues including cancers.
Supplementary Material
Acknowledgment
We thank Yuta Yoshimura for excellent technical assistance.
This work was supported by a Grant-in-aid for Scientific Research on Priority Areas 17014077 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- BM
- basement membrane
- Lm
- laminin
- MVE cell
- microvascular endothelial cell
- UVE
- umbilical vein endothelial cell
- DME
- Dulbecco's modified Eagle's medium.
REFERENCES
- 1.Colognato H., Yurchenco P. D. (2000) Dev. Dyn. 218, 213–234 [DOI] [PubMed] [Google Scholar]
- 2.Aumailley M., Bruckner-Tuderman L., Carter W. G., Deutzmann R., Edgar D., Ekblom P., Engel J., Engvall E., Hohenester E., Jones J. C., Kleinman H. K., Marinkovich M. P., Martin G. R., Mayer U., Meneguzzi G., Miner J. H., Miyazaki K., Patarroyo M., Paulsson M., Quaranta V., Sanes J. R., Sasaki T., Sekiguchi K., Sorokin L. M., Talts J. F., Tryggvason K., Uitto J., Virtanen I., von der Mark K., Wewer U. M., Yamada Y., Yurchenco P. D. (2005) Matrix Biol. 24, 326–332 [DOI] [PubMed] [Google Scholar]
- 3.Ryan M. C., Tizard R., VanDevanter D. R., Carter W. G. (1994) J. Biol. Chem. 269, 22779–22787 [PubMed] [Google Scholar]
- 4.Galliano M. F., Aberdam D., Aguzzi A., Ortonne J. P., Meneguzzi G. (1995) J. Biol. Chem. 270, 21820–21826 [DOI] [PubMed] [Google Scholar]
- 5.Ferrigno O., Virolle T., Galliano M. F., Chauvin N., Ortonne J. P., Meneguzzi G., Aberdam D. (1997) J. Biol. Chem. 272, 20502–20507 [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki K. (2006) Cancer Sci. 97, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivirikko S., McGrath J. A., Baudoin C., Aberdam D., Ciatti S., Dunnill M. G., McMillan J. R., Eady R. A., Ortonne J. P., Meneguzzi G. (1995) Hum. Mol. Genet. 4, 959–962 [DOI] [PubMed] [Google Scholar]
- 8.Baker S. E., DiPasquale A. P., Stock E. L., Quaranta V., Fitchmun M., Jones J. C. (1996) Exp. Cell Res. 228, 262–270 [DOI] [PubMed] [Google Scholar]
- 9.Marinkovich M. P., Lunstrum G. P., Burgeson R. E. (1992) J. Biol. Chem. 267, 17900–17906 [PubMed] [Google Scholar]
- 10.Champliaud M. F., Lunstrum G. P., Rousselle P., Nishiyama T., Keene D. R., Burgeson R. E. (1996) J. Cell Biol. 132, 1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirosaki T., Tsubota Y., Kariya Y., Moriyama K., Mizushima H., Miyazaki K. (2002) J. Biol. Chem. 277, 49287–49295 [DOI] [PubMed] [Google Scholar]
- 12.Mizushima H., Miyagi Y., Kikkawa Y., Yamanaka N., Yasumitsu H., Misugi K., Miyazaki K. (1996) J. Biochem. 120, 1196–1202 [DOI] [PubMed] [Google Scholar]
- 13.Garbe J. H., Göhring W., Mann K., Timpl R., Sasaki T. (2002) Biochem. J. 362, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doliana R., Bellina I., Bucciotti F., Mongiat M., Perris R., Colombatti A. (1997) FEBS Lett. 417, 65–70 [DOI] [PubMed] [Google Scholar]
- 15.Kariya Y., Yasuda C., Nakashima Y., Ishida K., Tsubota Y., Miyazaki K. (2004) J. Biol. Chem. 279, 24774–24784 [DOI] [PubMed] [Google Scholar]
- 16.Kariya Y., Mori T., Yasuda C., Watanabe N., Kaneko Y., Nakashima Y., Ogawa T., Miyazaki K. (2008) J. Mol. Histol. 39, 435–446 [DOI] [PubMed] [Google Scholar]
- 17.Doi M., Thyboll J., Kortesmaa J., Jansson K., Iivanainen A., Parvardeh M., Timpl R., Hedin U., Swedenborg J., Tryggvason K. (2002) J. Biol. Chem. 277, 12741–12748 [DOI] [PubMed] [Google Scholar]
- 18.Hallmann R., Horn N., Selg M., Wendler O., Pausch F., Sorokin L. M. (2005) Physiol. Rev. 85, 979–1000 [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki K., Kikkawa Y., Nakamura A., Yasumitsu H., Umeda M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 11767–11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikkawa Y., Sanzen N., Sekiguchi K. (1998) J. Biol. Chem. 273, 15854–15859 [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara H., Gu J., Sekiguchi K. (2004) Exp. Cell Res. 292, 67–77 [DOI] [PubMed] [Google Scholar]
- 22.Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. (1989) Cell 58, 933–943 [DOI] [PubMed] [Google Scholar]
- 23.Fischer R. S., Gardel M., Ma X., Adelstein R. S., Waterman C. M. (2009) Curr. Biol. 19, 260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kariya Y., Ishida K., Tsubota Y., Nakashima Y., Hirosaki T., Ogawa T., Miyazaki K. (2002) J. Biochem. 132, 607–612 [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki D., Suetsugu S., Miki H., Kataoka Y., Nishikawa S., Fujiwara T., Yoshida N., Takenawa T. (2003) Nature 424, 452–456 [DOI] [PubMed] [Google Scholar]
- 26.Connolly J. O., Simpson N., Hewlett L., Hall A. (2002) Mol. Biol. Cell. 13, 2474–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y., Senger D. R. (2004) FASEB J. 18, 457–468 [DOI] [PubMed] [Google Scholar]
- 28.Krasilnikov M. A. (2000) Biochemistry 65, 59–67 [PubMed] [Google Scholar]
- 29.Hirosaki T., Mizushima H., Tsubota Y., Moriyama K., Miyazaki K. (2000) J. Biol. Chem. 275, 22495–22502 [DOI] [PubMed] [Google Scholar]
- 30.Frieser M., Nöckel H., Pausch F., Röder C., Hahn A., Deutzmann R., Sorokin L. M. (1997) Eur. J. Biochem. 246, 727–735 [DOI] [PubMed] [Google Scholar]
- 31.Sorokin L. M., Pausch F., Frieser M., Kröger S., Ohage E., Deutzmann R. (1997) Dev. Biol. 189, 285–300 [DOI] [PubMed] [Google Scholar]
- 32.Thyboll J., Kortesmaa J., Cao R., Soininen R., Wang L., Iivanainen A., Sorokin L., Risling M., Cao Y., Tryggvason K. (2002) Mol. Cell. Biol. 22, 1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakobsson L., Domogatskaya A., Tryggvason K., Edgar D., Claesson-Welsh L. (2008) FASEB J. 22, 1530–1539 [DOI] [PubMed] [Google Scholar]
- 34.Spessotto P., Yin Z., Magro G., Deutzmann R., Chiu A., Colombatti A., Perris R. (2001) Cancer Res. 61, 339–347 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.