Abstract
Genomic integrity is maintained by the coordinated interaction of many DNA damage response pathways, including checkpoints, DNA repair processes, and cell cycle restart. In Saccharomyces cerevisiae, the BRCA1 C-terminal domain-containing protein Rtt107/Esc4 is required for restart of DNA replication after successful repair of DNA damage and for cellular resistance to DNA-damaging agents. Rtt107 and its interaction partner Slx4 are phosphorylated during the initial phase of DNA damage response by the checkpoint kinases Mec1 and Tel1. Because the natural chromatin template plays an important role during the DNA damage response, we tested whether chromatin modifications affected the requirement for Rtt107 and Slx4 during DNA damage repair. Here, we report that the sensitivity to DNA-damaging agents of rtt107Δ and slx4Δ mutants was rescued by inactivation of the chromatin regulatory pathway leading to H3 K79 trimethylation. Further analysis revealed that lack of Dot1, the H3 K79 methyltransferase, led to activation of the translesion synthesis pathway, thereby allowing the survival in the presence of DNA damage. The DNA damage-induced phosphorylation of Rtt107 and Slx4, which was mutually dependent, was not restored in the absence of Dot1. The antagonistic relationship between Rtt107 and Dot1 was specific for DNA damage-induced phenotypes, whereas the genomic instability caused by loss of Rtt107 was not rescued. These data revealed a multifaceted functional relationship between Rtt107 and Dot1 in the DNA damage response and maintenance of genome integrity.
Keywords: Chromatin, DNA Damage, DNA Repair, DNA Replication, Genetics, Histone Modification, Protein Phosphorylation
Introduction
Multiple mechanisms cooperate to maintain genome integrity, thus ensuring proper transmission of genetic information from one generation to the next. DNA damage is detected by sensors that activate the DNA damage checkpoint, which in turn elicits various cellular responses, including cell cycle arrest, DNA repair, apoptosis, and/or DNA damage-induced transcriptional programs (1, 2).
In Saccharomyces cerevisiae, the kinase proteins Mec1 and Tel1, the yeast homologues of mammalian ATR (ATM and Rad3-related) and ATM (ataxia-telangiectasia mutated), are crucial for transducing signals in the S phase checkpoint response (3, 4). The downstream signaling cascade leads to cell cycle arrest, replication fork stabilization, and DNA damage repair (5). Following successful DNA repair, the checkpoint must be deactivated to allow resumption of the cell cycle and restart of stalled replication forks. Although one of the main steps in this process is dephosphorylation of the effector kinase Rad53, checkpoint deactivation is further coordinated by many different proteins, including phosphatases, proteases, and helicases (6–9). In the event of irreparable DNA damage, tolerance mechanisms allow bypass of DNA lesions therefore enabling cells to survive (10). One of these pathways is the translesion synthesis (TLS)6 pathway that uses special error-prone polymerases to allow replication past DNA lesions, resulting in an increased mutation frequency (11).
One of the downstream phosphorylation targets of Mec1 is Rtt107/Esc4, which is required for reinitiating replication after repair of alkylating DNA damage (12, 13). Accordingly, yeast lacking the nonessential RTT107 gene or carrying an allele encoding for a nonphosphorylatable Rtt107 protein are hypersensitive to different DNA-damaging agents (12). These include the DNA-alkylating agent methyl methanesulfonate (MMS), the nucleotide reductase inhibitor hydroxyurea, and the topoisomerase I poison camptothecin (12–14). Moreover, rtt107Δ mutants have a chromosome instability phenotype and an increased incidence of Rad52 foci, indicative of homologous recombination occurring because of stalled DNA replication forks (15, 16). Aside from these roles in genome integrity, Rtt107 functions to repress the mobility of Ty1 transposons and to establish silent chromatin (17, 18).
Rtt107 contains several BRCA1 C-terminal homology domains, which often serve as phospho-binding modules to recruit signaling complexes and repair factors to DNA damage-induced lesions (12, 19). Consistent with a role as a scaffold for protein-protein interactions during the DNA damage response, Rtt107 interacts with a number of DNA repair and recombination proteins (13, 20, 21). Of these, the interaction of Rtt107 with the structure-specific endonuclease Slx4 is best characterized and indicative of a close functional relationship between the two proteins. Slx4 is required for Mec1-dependent phosphorylation of four (S/T)Q motifs in the C-terminal half of Rtt107 and, like Rtt107, facilitates resumption of DNA replication after DNA damage (12, 13).
In addition to the complex regulation of the DNA damage response by signaling cascades, chromatin structures in the cell also play many roles in regulating access to DNA during the repair process. One example of the emerging interface between chromatin and the DNA damage response pathways is the DNA damage-induced recruitment of Rtt107 to chromatin by the H3 K56 acetyltransferase Rtt109 and the cullin Rtt101 (20). There are many other chromatin modifications involved in the DNA damage response, such as the well studied H2A phosphorylation and H3 K79 methylation pathways (22). H2A S129 is phosphorylated by Mec1 in response to DNA damage, triggering the assembly of many repair proteins and chromatin modifiers acting at subsequent steps (22–25). To allow resumption of the cell cycle and DNA replication after successful completion of DNA repair, H2A S129 needs to be dephosphorylated by either Pph3 or Glc7, depending on the exact nature of the initial damage (26, 27). Dot1-mediated H3 K79 methylation, which is regulated by Bre1-mediated H2B K123 ubiquitination, is required for G1 and S phase checkpoints (28–32). In part, this requirement is mediated through a functional linkage to the Rad9 adaptor protein (31, 33). Several lines of evidence suggest that Dot1 plays an additional role in DNA repair pathways, such as nucleotide excision repair, sister chromatid recombination, and repair of ionizing radiation damage (34–36). In contrast, Dot1 negatively regulates the error-prone TLS pathway through an unknown mechanism, thereby allowing bypass of DNA replication blocks (7). Aside from the function of Dot1 in DNA damage, it is also involved in gene silencing as well as differential H3 K79 methylation during the cell cycle (37, 38).
This study established a close connection between Rtt107 and the pathway resulting in a specific chromatin modification, H3 K79 trimethylation. Specifically, loss of H3 K79 trimethylation suppressed the DNA damage sensitivity of rtt107Δ and slx4Δ mutants. This suppression was not linked to restoration of Rtt107 or Slx4 phosphorylation but instead was dependent on the presence of a functional TLS pathway. Moreover, deletion of DOT1 partially suppressed the cell cycle delay and the defect in resuming DNA replication of rtt107Δ mutants during the recovery from MMS-induced DNA damage. In contrast, deletion of DOT1 rescued neither the chromosome instability phenotype nor the increased incidence of spontaneous Rad52 foci caused by loss of Rtt107.
EXPERIMENTAL PROCEDURES
Yeast Strains
All yeast strains used in this study are listed in supplemental Table 1 and were created using standard yeast genetic techniques (39). Complete gene deletions and integration of a triple FLAG tag at the 3′ end of genes (40) were achieved using one-step gene integration of PCR-amplified modules (41). Plasmid shuffling experiments were performed using 5-fluoroorotic acid as a counter-selecting agent for the URA3 plasmid (pRS316, HHT2-HHF2) and shuffling in plasmids containing histone H3 K79 mutations (pRS314, hht2-HHF2) (42). Catalytically inactive Dot1 mutants were expressed from pRS315 plasmids (43), and a nonphosphorylatable mutant of Rtt107 (four Ser-Gln motifs substituted by Ala-Gln) was expressed from a plasmid (pRS315, rtt107–4AQ) that was a generous gift from Grant Brown and Tania Roberts (University of Toronto). BrdU-Incorporating (BrdU-Inc) wild-type and mutant strains containing constitutively expressed herpes simplex virus-thymidine kinase and human equilibrative nucleoside transporter (hENT1) were generated by genetic crosses with a previously published parental strain (44).
Sensitivity Measurements
Overnight cultures grown in YPD or SC-Leu at 30 °C were diluted to 0.3 A600. The cells were 10-fold serially diluted and spotted onto solid YPD plates or SC-Leu plates with MMS (Sigma) at various concentrations. The plates were then incubated at 30 °C for 2–3 days.
Protein Extracts and Protein Blot Analysis
Overnight cultures were diluted to 0.3 A600, grown in YPD to 0.8 A600, and then treated with 0.03% MMS for 1 h. The FLAG-tagged Slx4 protein was extracted by an alkaline method using 0.2 m NaOH (45). Slx4-FLAG proteins were visualized using anti-FLAG M2 antibodies (Sigma) and SuperSignal-enhanced chemiluminescence (Pierce). The procedure for analytical scale immunoprecipitation of the FLAG-tagged Rtt107 protein was adapted from a previous report (46). Briefly, yeast cells were harvested and lysed in TAP-IP buffer (50 mm Tris, pH 7.8, 150 mm NaCl, 1.5 mm MgAc, 0.15% Nonidet P-40, 1 mm DTT, 10 mm NaPPi, 5 mm EGTA, 5 mm EDTA, 0.1 mm Na3VO4, 5 mm NaF, CompleteTM protease inhibitor mixture) using acid-washed glass beads and mechanically disrupted using a bead beater (BioSpec Products). Rtt107-FLAG fusion proteins were captured using anti-FLAG M2-agarose beads (Sigma) and subsequently washed in TAP-IP buffer. Captured material was analyzed by protein blotting with anti-FLAG M2 (Sigma) and visualized using the Odyssey Infrared Imaging System (Licor).
Flow Cytometric Analysis and BrdU Incorporation Experiments
Cells were prepared under the same conditions for flow cytometric analysis and BrdU incorporation experiment. For the latter, we used wild-type and mutant strains containing the BrdU-Inc cassette (44) to allow for BrdU uptake in yeast. Briefly, cells were arrested in G1 by addition of 2 μg/ml α-factor for 2 h at 30 °C in YPD, then washed with sterile 1× PBS, and resuspended in YPD containing 0.03% MMS for 1 h. MMS was removed by treating with 2% sodium thiosulfate and washing with sterile 1× PBS. The cells were resuspended in YPD and incubated at 30 °C during the recovery phase. For the BrdU incorporation experiment, 400 μg/ml of BrdU was added to the cultures during the recovery phase. Aliquots were removed at the indicated times and processed further for flow cytometric analysis or measurement of BrdU incorporation.
For flow cytometric analysis, cells were collected in 70% ethanol with 0.2 m Tris-HCl, pH 7.5, and prepared as described previously (47). Samples were analyzed using the BD FACSCalibur instrument and the Flow Jo software (Tree Star Inc., OR). For the BrdU incorporation experiments, cells were collected in buffer containing 100 mm EDTA, pH 8.0, 10 mm Tris-HCl, pH 8.0, and 0.1% NaH3. Total genomic DNA was extracted by bead beating and use of the DNeasy kit (Qiagen) and sonicated using Bioruptor (Diagenode). The DNA concentration was adjusted to 20 ng/μl, then heat-denatured, and snap-cooled. 1 μg of DNA was spotted onto a nitrocellulose membrane (Bio-Rad) pre-soaked with 2× SSC using the Convertible Filtration Manifold System (Invitrogen) and subjected to ultraviolet cross-linking in a Stratalinker (Stratagene). Subsequently, the membrane was blocked with 5% milk in TTBS, probed with an anti-BrdU antibody (GE Healthcare), and visualized using the Odyssey Infrared Imaging System (Licor).
Quantitative Bimater Assay
The procedure for the bimater assay was modified from a previous method to allow quantification (16). Briefly, 12 independent colonies from each homozygous diploid strain were grown in YPD overnight at 30 °C and diluted to 2.0 A600. Cells were plated on to solid YPD at appropriate dilutions to determine the total number of cells. Equal volumes of MATa mating tester cultures (10.0 A600) and the homozygous diploid strain cultures (2.0 A600) were plated onto solid media containing no amino acids and incubated at 30 °C for 3–4 days. Mating rates and 95% confidence intervals were calculated with the Ma-Sandri-Sarkar Maximum Likelihood Estimator method using the on-line Fluctuation Analysis Calculator (48).
Microscopy
Nuclear morphology was determined by treating cells as for flow cytometric analysis, except α-factor incubation was omitted, and SC-complete medium was used to minimize autofluorescence. Aliquots were removed at the indicated times and treated with 4% formaldehyde solution (Sigma) for 10 min. Cells were immobilized on a glass slide with a solution of 1 mg/ml polylysine (Sigma) and then stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma). Cells with medium to large buds were counted as being in G2/M phase. Ambiguous cases where cells with separate nuclei were insufficiently spread were considered to be in G1 phase.
To visualize Rad52-GFP foci, cells were grown at 30 °C in SC-complete medium to logarithmic phase and then immobilized on a glass slide with a solution of 1.0% agarose in double distilled H2O. Multiple images were obtained at 0.3-μm intervals along the z axis, and Rad52-GFP foci were counted by inspection of all focal planes. At least 300 cells were counted for each time point. All imaging was done with the Zeiss Axioplan 2 fluorescence microscope using the Metamorph software. Statistical significance was assessed using Student's t test.
Measurement of Mutation Rates
Forward mutation rates were measured by mutations at the CAN1 locus, which when mutated renders cells sensitive to canavanine (49). Cells from 12 independent colonies for each strain were grown in YPD to logarithmic phase; 0.005% MMS was added to half of each culture, and cells were further incubated at 30 °C for 20 h. Cells plated on SC-Arg were diluted 1:200,000, and cells plated on SC-Arg containing 50 μg/ml canavanine (Sigma) were diluted by a factor of 2. Plates were incubated at 30 °C for 2 days, and colonies were counted. The mutation rates and 95% confidence intervals were calculated with the Ma-Sandri-Sarkar Maximum Likelihood Estimator method using the on-line Fluctuation Analysis Calculator (48).
RESULTS
Elimination of H3 K79 Methylation Suppresses the Sensitivity of rtt107Δ and slx4Δ Mutants to the DNA-damaging Agent MMS
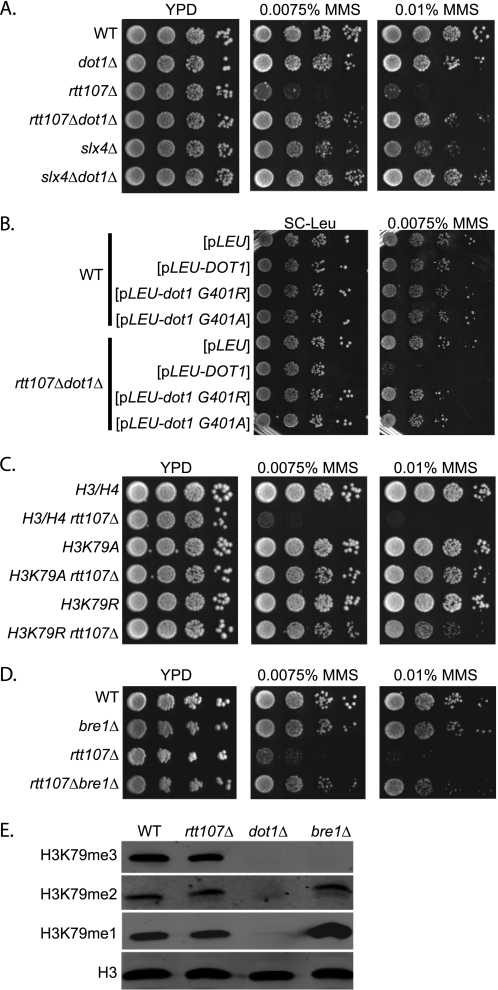
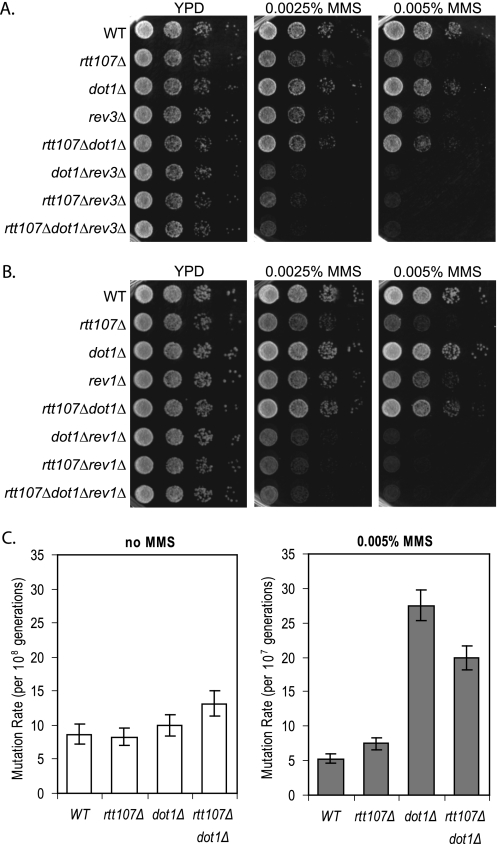
Rtt107 and its interaction partner Slx4 are required for yeast cells to survive exposure to DNA damage conditions, such as those caused by the alkylating agent MMS (13, 14). Given the importance of the natural chromatin template during the DNA damage response, and the existing link between Rtt107 and the histone acetyltransferase Rtt109 (20, 30, 50), we hypothesized that chromatin modifications might affect the requirement for Rtt107 and Slx4 during DNA damage repair. For this purpose, we created strains that, in addition to deletion of either RTT107 or SLX4, lacked genes encoding several chromatin modifiers with roles in the DNA damage response to test whether their absence enhanced or suppressed the sensitivity of rtt107Δ or slx4Δ mutants to MMS. Although the majority of double mutants grew equally well as rtt107Δ or slx4Δ mutants, we found that deletion of DOT1, a nonessential gene encoding a histone methyltransferase catalyzing mono-, di-, and trimethylation of histone H3 K79, almost completely rescued the MMS sensitivity of rtt107Δ and slx4Δ mutants (Fig. 1A).
FIGURE 1.
Abrogation of H3 K79 trimethylation suppressed the MMS sensitivity of strains lacking Rtt107 or Slx4. 10-Fold serial dilutions of the indicated strains were plated onto media containing 0.0075 or 0.01% MMS. A, loss of Dot1 suppressed MMS sensitivity of rtt107Δ and slx4Δ mutants. B, loss of Dot1 catalytic activity; C, H3 K79A, K79R; or D, loss of Bre1 suppressed MMS sensitivity of rtt107Δ mutants. E, Bre1 affected mainly H3 K79 trimethylation and not di- or monomethylation. Whole cell extracts of indicated strains were analyzed by protein blotting with anti-H3 K79 tri-, di-, or monomethyl antibodies. Antibodies against H3 were used as a loading control.
To determine whether this effect was dependent on the catalytic activity of Dot1, alleles encoding catalytically inactive Dot1 proteins were compared with the complete loss of Dot1 and with the presence of wild-type Dot1. The strains carrying dot1G401R and dot1G401A alleles, encoding for catalytically inactive forms of Dot1, suppressed the MMS sensitivity phenotype similar to the complete deletion (Fig. 1B). As expected, re-introducing wild-type DOT1 in rtt107Δdot1Δ double mutants restored MMS sensitivity to levels similar to that of rtt107Δ single mutants. These data suggested that eliminating the catalytic activity of Dot1 enabled cells lacking Rtt107 to survive otherwise detrimental conditions during exposure to MMS. The same results were obtained for slx4Δ mutants, except that slx4Δ mutants were less sensitive to MMS than rtt107Δ mutants (supplemental Fig. S1A).
The only known target of Dot1 methyltransferase activity to date is the Lys-79 residue located in the core of histone H3, but formally it is possible that Dot1, similar to other chromatin modifiers, has other enzymatic targets not yet identified. To examine whether the suppression of the MMS sensitivity of rtt107Δ mutants by loss of Dot1 was due to lack of H3 K79 methylation, strains with mutated forms of H3 K79, which cannot be methylated, were tested for their ability to survive chronic MMS exposure in the absence of Rtt107. Changing lysine 79 to either alanine or arginine rescued the DNA damage sensitivity of the rtt107Δ mutants, analogous to the DOT1 deletion (Fig. 1C). Therefore, we concluded that the reversal of the MMS sensitivity of rtt107Δ mutants was due to the loss of Dot1-mediated H3 K79 methylation. Similarly, H3 K79A and H3 K79R mutants also suppressed the MMS sensitivity of the slx4Δ strain (supplemental Fig. S1B).
Methylation of H3 K79 by Dot1 is regulated through cross-talk with another histone modification, mono-ubiquitination of H2B K123, which is catalyzed by the Bre1/Rad6 enzyme complex (28, 29, 32, 51–53). Thus, we wanted to test whether upstream regulators of H3 K79 methylation would have a similar effect on the MMS sensitivity of rtt107Δ and slx4Δ mutants. Indeed, deletion of BRE1 also rescued the MMS sensitivity of the strains lacking Rtt107 or Slx4 (Fig. 1D and supplemental Fig. S1C).
To learn more about the biochemical nature underlying the observed effects, we assessed the total levels of mono-, di-, or trimethylated H3 K79 in whole cell extracts. Interestingly, although Dot1 broadly catalyzes mono-, di-, and trimethylation of H3 K79 (43, 54, 55), Bre1 primarily affected Lys-79 trimethylation (Fig. 1E). These results suggested that specifically a lack of H3 K79 trimethylation caused the suppression of the MMS sensitivity of rtt107Δ and slx4Δ mutants.
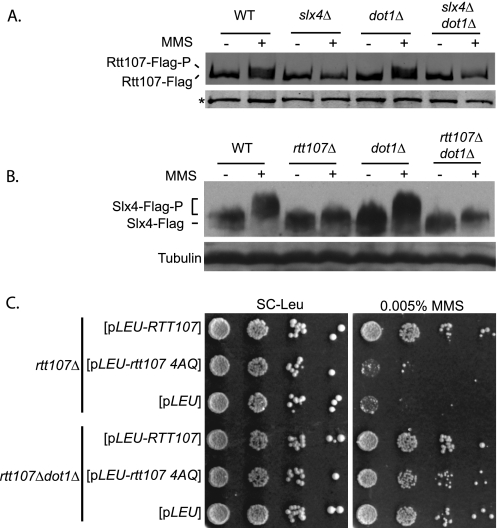
Deletion of DOT1 Suppresses DNA Damage Sensitivity in the Absence of MMS-induced Phosphorylation of Rtt107 or Slx4
In response to DNA damage induced by various agents, Rtt107 and Slx4 are phosphorylated on several Ser/Thr residues by the checkpoint kinase Mec1 (12, 13). Phosphorylation of Rtt107 is essential for its function in the DNA damage response and depends on Slx4 (12, 13). It was in principle possible that, in slx4Δdot1Δ double mutants, an alternative pathway directed Rtt107 phosphorylation in the absence of Slx4, thereby enabling cells to survive the otherwise detrimental MMS-induced DNA damage. To test this possibility, Rtt107 phosphorylation was measured in strains lacking Slx4, Dot1, or both simultaneously. As expected, exposure to MMS induced phosphorylation of Rtt107 in wild-type strains but not in strains lacking Slx4 (Fig. 2A) (13). Although deletion of DOT1 suppressed the MMS sensitivity of slx4Δ mutants, it did not overcome the requirement of Slx4 for Rtt107 phosphorylation (Fig. 2A). Loss of Dot1 had no effect on Rtt107 phosphorylation in response to MMS when Slx4 was present (Fig. 2A). Therefore, the suppression by dot1Δ did not involve a restoration of Rtt107 phosphorylation in the absence of Slx4, arguing against an alternative pathway for Rtt107 phosphorylation. Consistent with the physical interaction and close functional relationship between Rtt107 and Slx4, we found that MMS-induced phosphorylation of Slx4 was dependent on Rtt107 but not on Dot1 (Fig. 2B). Analogous to the results obtained for Rtt107, eliminating DOT1 did not restore Slx4 phosphorylation in the absence of Rtt107 (Fig. 2B).
FIGURE 2.
Suppression of rtt107Δ MMS sensitivity by deletion of DOT1 was not dependent on the phosphorylation of Slx4 and vice versa. A, cells expressing Rtt107-FLAG were untreated or treated with 0.03% MMS for 1 h. Analytical scale immunoprecipitations of Rtt107-FLAG were performed and analyzed by protein blotting with anti-FLAG antibodies. The reduced mobility of Rtt107-FLAG indicated phosphorylation of the protein. Background bands (*) were used as a loading control. B, cells expressing Slx4-FLAG were treated as described in A. Whole cell extracts were analyzed by protein blotting with anti-FLAG antibodies, with reduced mobility of Slx4-FLAG being indicative of phosphorylation. Antibodies against tubulin were used as a loading control. C, deletion of DOT1 suppressed the MMS sensitivity of the mutants expressing the nonphosphorylatable Rtt107–4AQ. 10-fold serial dilutions were plated onto SC-Leu containing 0.005% MMS.
To test whether the suppression by deletion of DOT1 was linked to the phosphorylation of Rtt107 at specific Ser-Gln sites, we utilized mutants expressing the nonphosphorylatable form of Rtt107. Using a plasmid expressing rtt107–4AQ in cells lacking Dot1, we tested the MMS sensitivity of the double mutants. Consistent with the importance of Rtt107 phosphorylation, deletion of DOT1 suppressed the MMS sensitivity of the rtt107 4AQ mutants (Fig. 2C). Taken together, these results indicated that MMS sensitivity of mutants lacking Rtt107 phosphorylation was suppressed by deletion of DOT1.
Requirement of Rtt107 for Resumption of Cell Cycle after S Phase Damage Is Partially Suppressed by Lack of Dot1
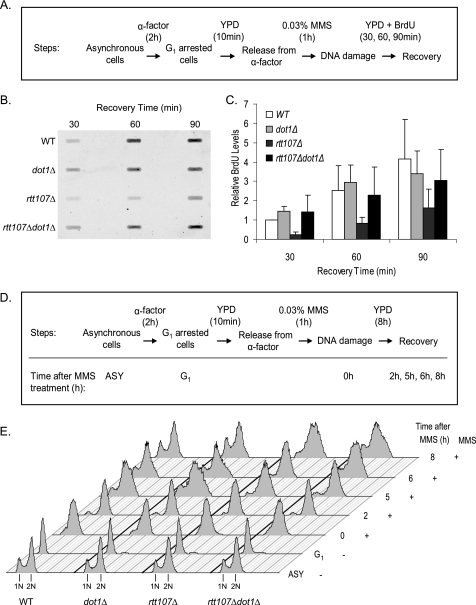
To further understand the molecular mechanism leading to the suppression of rtt107Δ MMS sensitivity, we tested whether loss of Dot1 could compensate for the requirement of Rtt107 during the restart of DNA replication (12, 13). Cells arrested in G1 were released into S phase in the presence of MMS for 1 h, and restart of DNA replication was directly measured by BrdU incorporation into nascent genomic DNA (Fig. 3A). Because BrdU was added after MMS treatment, it serves as a quantitative indicator of newly replicated DNA during the recovery process. As expected, BrdU levels increased in wild-type cells during the course of the experiment, indicating successful resumption of DNA replication (Fig. 3B). In contrast, BrdU levels in rtt107Δ mutants were consistently lower than the wild-type at each time point. Although the levels of BrdU incorporation in dot1Δ mutants increased similar to the wild-type cells, rtt107Δdot1Δ mutants incorporated BrdU at intermediate levels between wild-type and rtt107Δ mutants (Fig. 3C). This result suggested that loss of Dot1 could partly suppress the defect of rtt107Δ mutants in resuming DNA replication.
FIGURE 3.
Requirement of Rtt107 for resumption of DNA replication and cell cycle after DNA damage was partially suppressed by deletion of DOT1. A, diagram of experimental strategy used for BrdU incorporation experiment. B, BrdU incorporation into nascent DNA indicated rtt107Δdot1Δ mutants more efficiently resumed DNA replication after DNA damage than rtt107Δ mutants. C, quantification of newly replicated DNA as measured by BrdU signals. All values are relative to wild type at 30 min. Error bars represent standard deviations of values from three independent experiments. D, diagram of experimental strategy used for FACS analysis. E, FACS analysis showed rtt107Δdot1Δ mutants recovered from DNA damage earlier than rtt107Δ mutants. ASY, asynchronous cells.
Next, we used FACS analysis to test whether loss of Dot1 would have a similar effect on the resumption of cell cycle progression after DNA damage (Fig. 3D). At the end of the MMS treatment (0 h), wild-type and rtt107Δ mutants were initially arrested in S phase due to activation of the DNA damage checkpoint (12, 13), although dot1Δ mutants had proceeded through S phase as judged by the shift of the signal to 2N, consistent with a requirement for Dot1 in the DNA damage checkpoint as described previously (Fig. 3E) (30, 31). 2 h after removal of MMS, rtt107Δ mutants were still in S phase, and all other strains had progressed to G2/M. Further differences between the mutants were observed as strains continued to recover from DNA damage. For example, at 5 h a substantial fraction of cells in the wild-type and dot1Δ mutant had undergone cell division as judged by the appearance of a G1 peak and S phase fraction, whereas rtt107Δ mutants had predominantly a 2N peak, suggesting that they were still residing in G2/M. These differences persisted until 8 h after recovery when the G1 peak and S phase fraction first appeared in the rtt107Δ mutants. The rtt107Δ dot1Δ double mutants had an intermediate phenotype, as the G1 peak and S phase fraction became visible at 6 h, which was earlier than rtt107Δ mutants but later than wild-type or dot1Δ mutants (Fig. 3E). Consistent with this, the delayed appearance of intact chromosomes during recovery in the rtt107Δ mutants was partially rescued by concurrent loss of Dot1 as visualized by pulsed-field gel electrophoresis (supplemental Fig. S2).
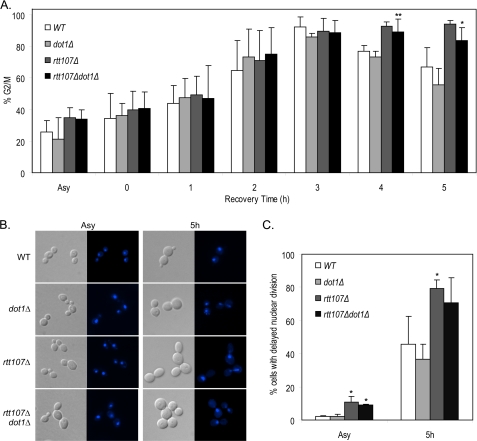
The defect of rtt107Δ mutants in completing the G2/M phase of the cell cycle during recovery from transient DNA damage can also be observed by examining nuclear morphology (13). In wild-type and dot1Δ mutants, the percentage of cells in G2/M increased after exposure to MMS, reached a peak at 3 h of recovery, and started to decrease as the cells completed mitosis (Fig. 4, A and B). As expected, the percentage of rtt107Δ mutants in G2/M also reached a peak at 3 h, but the increased level lasted up to 5 h after MMS treatment. As judged by the percentage of G2/M cells, the kinetics of recovery from DNA damage in rtt107Δdot1Δ double mutants was slower than wild-type and dot1Δ mutants but faster than rtt107Δ mutants, although this did not reach statistical significance (Fig. 4A). Another known phenotype of rtt107Δ mutants is the delay of nuclear division, as judged by the higher percentage of large budded cells with elongated nuclei spanning the bud neck. Consistent with previous reports, nuclear division was delayed in rtt107Δ mutants when compared with wild type 5 h after exposure to MMS (Fig. 4C). In contrast, dot1Δ mutants did not show any delay of nuclear division. A similar phenotype was also observed in asynchronous cultures not exposed to MMS. In both conditions, deletion of DOT1 did not rescue the defect caused by loss of Rtt107.
FIGURE 4.
Nuclear division delay of rtt107Δ mutants was not suppressed by deletion of DOT1 in the absence and presence of MMS. Cells were treated with 0.03% MMS for 1 h, washed, and then resuspended in complete media for DNA damage recovery and stained with DAPI at the indicated time points. A, increased percentage of cells in G2/M of rtt107Δ mutants after DNA damage was not suppressed by deletion of DOT1. The percentage of cells with medium to large buds (% G2/M) was calculated by dividing the number of cells with medium to large buds by the total number of cells. B, representative differential interference contrast images are shown on the left and the corresponding DAPI images on the right. C, increased percentage of cells exhibiting nuclear division delay in rtt107Δ mutants was not suppressed by loss of Dot1. The percentage of cells with delayed nuclear division was calculated by dividing the number of large budded cells with unsegregated nuclei by the total number of cells. In both A and C, at least 200 cells were counted in three independent experiments. Error bars represent standard deviations of the values. *, p < 0.05; **, p < 0.005 when compared with the wild-type strain in the same time point. ASY, asynchronous cells.
TLS Pathway Is Required for Suppression of the MMS Sensitivity of rtt107Δ Mutants by Deletion of DOT1
Next, we sought to determine the pathway by which deletion of DOT1 suppressed the requirement for RTT107 during MMS exposure. In addition to the suppression of the DNA damage sensitivity of rtt107Δ and slx4Δ mutants reported here, lack of Dot1 suppresses the MMS sensitivity of strains lacking a variety of repair proteins, and this effect is dependent on the TLS polymerases ζ and Rev1 (7). To address whether the suppression of rtt107Δ DNA damage sensitivity by loss of Dot1 was similarly dependent on the TLS pathway, we constructed triple mutants lacking two main components of the TLS pathway as follows: Rev3 (catalytic subunit of polymerase ζ) or Rev1 (dC-transferase) (56). Lack of Dot1 did not suppress the MMS sensitivity of rtt107Δ mutants in the absence of Rev3 (Fig. 5A). Similarly, Rev1 was necessary for the reversal of rtt107Δ sensitivity by deletion of DOT1, suggesting that the dot1Δ suppression was dependent on the TLS pathway in general and not specifically on Rev3 (Fig. 5B). Very low concentrations of MMS were used in this assay due to the extreme MMS sensitivity of the triple mutants. It is interesting to note that both dot1Δrev3Δ and rtt107Δrev3Δ double mutants were very sensitive to MMS, and a similar phenotype was also observed for dot1Δrev1Δ and rtt107Δrev1Δ mutants. This indicated that a functional TLS pathway became more important for DNA damage resistance in the absence of Dot1 or Rtt107.
FIGURE 5.
Suppression of the rtt107Δ MMS sensitivity by deletion of DOT1 was dependent on the TLS pathway. Deletion of REV3 or REV1 in rtt107Δdot1Δ mutants resulted in loss of the suppression. rev3Δ (A) or rev1Δ mutants (B) in combination with the indicated deletions of RTT107 and/or DOT1 were plated in 10-fold serial dilutions onto YPD containing 0.0025 or 0.005% MMS. C, loss of Dot1 resulted in increased mutation rates in presence of MMS. Mutation rates of indicated strains with and without 0.005% MMS were determined using 12 independent colonies. Mutation rates and 95% confidence intervals were calculated using the Ma-Sandri-Sarkar Maximum Likelihood Estimator method.
The TLS pathway is error-prone, and its activation would therefore be expected to cause an increased mutation rate. Using the CAN1 forward mutagenesis assay, we observed a 4–5-fold increased mutation rate in the presence of 0.005% MMS in both dot1Δ and dot1Δrtt107Δ mutants (Fig. 5C). Together, these results suggested that deletion of DOT1 led to activation of the TLS pathway, thereby allowing the survival of rtt107Δ mutants.
Rtt107 Has Functions in Maintaining Genomic Integrity That Are Independent of Dot1 Activity
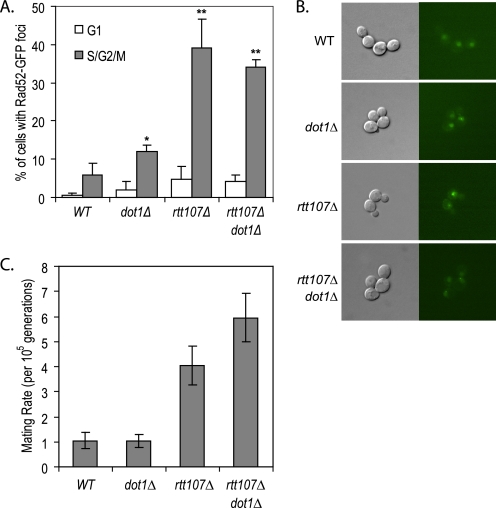
The role of Rtt107 in the maintenance of genome stability is not restricted to its specific function of restarting the cell cycle during S phase after DNA damage. During normal cell cycle progression, cells lacking Rtt107 have increased numbers of Rad52 and Ddc2 foci, indicative of spontaneous DNA damage and/or replication fork stalling (13, 15). Consistent with this, we observed that the percentage of cells with Rad52-GFP foci in S/G2/M phase was ∼7-fold higher in rtt107Δ mutants than in wild type (Fig. 6, A and B). Deletion of DOT1 in the rtt107Δ background did not significantly alter the number of cells containing Rad52-GFP foci. However, consistent with published data, we observed a 2-fold increase in the number of cells with Rad52-GFP foci in dot1Δ mutants, suggesting that RTT107 was epistatic to DOT1 in suppressing spontaneous DNA damage and/or replication fork stalling (7).
FIGURE 6.
Genomic instability of the rtt107Δ mutants was not suppressed by deletion of DOT1. A, increased number of Rad52-GFP foci in rtt107Δ mutants was not suppressed in the absence of Dot1. The percentage of cells in G1 or S/G2/M phase containing Rad52-GFP foci was calculated by dividing the number of cells in G1 or S/G2/M phase containing Rad52-GFP foci by the total number of cells in G1 or S/G2/M, respectively. At least 200 cells were counted in three independent experiments. Error bars represent standard deviations of the values. *, p < 0.05; **, p < 0.005 when compared with the wild-type strain for the same cell cycle phase. B, representative differential interference contrast images are shown on the left and the corresponding GFP images on the right. C, deletion of DOT1 did not suppress the increased loss of heterozygosity in rtt107Δ mutants. Mating rates of homozygous diploids of indicated strains were determined by using 12 independent colonies. Mating rates and 95% confidence intervals were calculated using the Ma-Sandri-Sarkar Maximum Likelihood Estimator method.
Further indicative of a broader role of Rtt107 in genome stability is the chromosome instability phenotype of rtt107Δ mutants (16). Compared with wild-type cells, rtt107Δ homozygous diploid mutants have a higher loss of heterozygosity at the MATa and MATα loci, which is due to either enhanced mitotic recombination between homologous chromosomes, chromosome loss, rearrangement, or gene conversion (16). Using a quantitative version of the original Bimater screen used to define RTT107 as a chromosome instability gene (57), we tested whether this phenotype was suppressed by loss of Dot1. Consistent with increased loss of heterozygosity, strains lacking Rtt107 had a 4-fold increase in mating rate when compared with wild-type or dot1Δ mutants (Fig. 6C). Deletion of DOT1 did not rescue the chromosome instability phenotype of rtt107Δ mutants, but rather it resulted in a further increase in the mating rate to 6-fold as compared with wild type. Hence, it appeared that rather than rescuing the requirement for Rtt107 in preventing loss of heterozygosity, Dot1 cooperated with Rtt107 in this process.
DISCUSSION
In this study, we uncover a close functional relationship between chromatin and the cellular processes regulated by the BRCA1 C-terminal domain-containing protein Rtt107 and its interaction partner Slx4. Loss of Dot1, likely mediated by loss of histone H3 K79 trimethylation, suppressed the DNA damage sensitivity of rtt107Δ mutants through a mechanism that was dependent on the presence of a functional TLS pathway. The DNA damage-induced phosphorylation of Rtt107 and Slx4, which was mutually dependent, was not restored in the absence of Dot1. Furthermore, deletion of DOT1 partially reversed the cell cycle progression and replication fork restart defect caused by the lack of Rtt107. In contrast, other genomic instability defects of rtt107Δ mutants were worsened or unaffected by loss of DOT1. Together, these data point to a complex functional relationship between Rtt107 and Dot1 in both the DNA damage response and preservation of genome integrity.
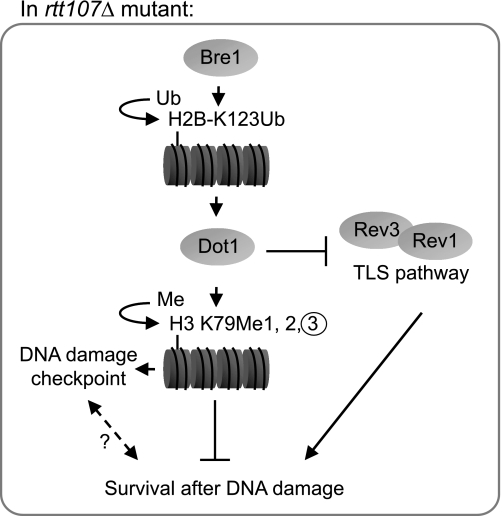
We propose a model to explain the inhibitory effect of H3 K79 trimethylation on growth during DNA damage conditions in yeast cells lacking Rtt107 or Slx4 (Fig. 7). Bre1-mediated H2B K123 ubiquitination is required for Dot1 to catalyze H3 K79 trimethylation, which in turn prevents rtt107Δ and slx4Δ mutants from surviving DNA damage conditions. This effect likely is mediated through inhibition of the TLS pathway by H3 K79 trimethylation, either directly or indirectly through a nexus to the Dot1-mediated DNA damage checkpoint. We favor a direct mechanism that could involve binding to the H3 K79 trimethylation mark by a protein that inhibits TLS. Alternatively, H3 K79 trimethylation might directly create a chromatin conformation that in some way is refractory to TLS. An indirect enhancement of TLS might be caused by the compromised DNA damage checkpoint due to loss of Dot1, allowing rtt107Δ mutants to survive DNA damage conditions. However, currently there is no evidence linking the roles of Dot1 in the TLS and DNA damage checkpoint. Moreover, UV exposure of DNA damage checkpoint-deficient mutants does not result in an increased mutation rate, thereby disfavoring a link between the DNA damage checkpoint and TLS (58). Finally, it is formally possible that the suppression is an indirect effect of an altered transcriptional response caused by lack of Dot1, Bre1, or H3 K79 methylation, which could involve reduced expression of an unknown inhibitor of the TLS. In any case, given our finding that the suppression of rtt107Δ phenotypes was linked to loss of trimethylation of H3 K79, it is tempting to speculate that specific genomic regions might be more prone to mediate this effect than others. This is supported by a genome-wide analysis showing that regions containing H3 K79 trimethylation are distinct from those containing H3 K79 dimethylation (38). Further studies are required to elucidate the precise mechanism whereby Dot1-mediated H3 K79 trimethylation inhibits the TLS pathway.
FIGURE 7.
Model for repressive effect of chromatin modifications on DNA damage survival in rtt107Δ mutants. During DNA damage response, Bre1-mediated H2B K123Ub and by extension, Dot1-mediated H3 K79Me3, are required for checkpoint function. In rtt107Δ mutants, the presence of H3 K79Me3 is inhibitory to the yeast survival in DNA damage conditions. Loss of Dot1 increases the activity of the TLS pathway that bypasses the requirement of Rtt107 for cell survival. Ub, ubiquitin.
Our data showed that Mec1-mediated phosphorylation of Rtt107 was dependent on Slx4, consistent with earlier reports (13). We also showed that MMS-induced phosphorylation of Slx4 was dependent on Rtt107, suggesting a mutual requirement of these two proteins for their respective phosphorylation. Interestingly, neither Rtt107 nor Slx4 was phosphorylated in slx4Δ or rtt107Δ mutants, respectively, when DOT1 was also deleted. Furthermore, the MMS sensitivity of a strain containing a nonphosphorylatable form of Rtt107 was rescued by deletion of DOT1. Together, this biochemical and genetic evidence suggested that DNA damage-dependent phosphorylation of Rtt107 is essential for resistance to MMS only when Dot1 is present to methylate H3 K79. Although it is not clear what mechanistic change is triggered by Rtt107 phosphorylation, it is likely to involve a DNA damage-induced protein-protein interaction. Whatever the mechanism might be, it is clear that Rtt107 phosphorylation in the DNA damage response becomes dispensable when H3 K79 trimethylation is inhibited.
The suppression of rtt107Δ by deletion of DOT1 was restricted to situations of induced DNA damage, suggesting that the functional interaction between Rtt107 and Dot1 was context-dependent. Confirming published data from a high throughput screen for regulators of Rad52 foci formation, we found that loss of Rtt107 caused a significant increase in the number of Rad52 foci-positive cells (15). In contrast to the suppression of MMS sensitivity of rtt107Δ mutants, this phenotype was not rescued by loss of Dot1. This suggested that Rtt107 had a role in preventing spontaneous DNA damage, likely caused by stalled DNA replication forks, which was not negatively regulated by H3 K79 methylation. Furthermore, our work uncovered additional evidence for a complex relationship between Dot1 and Rtt107 in the maintenance of genomic integrity. Rtt107 was required for chromosome stability, as determined by a genetic assay (16). Although loss of Dot1 alone did not affect chromosome stability, it enhanced the defect caused by loss of Rtt107. This suggested that in the absence of Rtt107, Dot1 plays a minor role in maintenance of chromosome stability. Taken together, these data point to multiple activities of Rtt107, where only those induced by external DNA-damaging agents were suppressed by deletion of DOT1. Presumably, both the increased number of Rad52 foci and the chromosome instability in rtt107Δ mutants were not suppressed by deletion of DOT1 because the TLS pathway is unlikely to be activated in these conditions.
The suppression of DNA damage sensitivity by loss of Dot1 reported here is interesting in light of other findings suggesting certain chromatin modifications act as negative regulators of DNA replication, recombination, and repair. For example, the H3 K36 histone methyltransferase Set2 and the ATP-dependent chromatin remodeler Chd1 exert an inhibitory effect on DNA replication, as deletions of these genes suppress the hydroxyurea sensitivity of mutations in several genes involved in DNA replication (59). In addition, deletion of CHD1 can suppress the lethality normally caused by disruption of the gene encoding either Mec1 or Rad53 DNA damage checkpoint kinases (59). The UV sensitivity and G2/M checkpoint defects of rad9Δ and mec1-21 mutants can be suppressed by loss of genes encoding components of the Rpd3/Sin3 histone deacetylase (60). Rpd3 and the aforementioned Set2 also repress meiotic recombination at the HIS4 meiotic recombination hot spot (61). However, not all chromatin modifications involved in DNA metabolism exert a negative effect, as mutations in the genes encoding members of the histone acetyltransferase complex NuA4, the ATP-dependent chromatin remodelers RSC, Swi/Snf, SWR1-C, and INO80 cause sensitivity to MMS, as does loss of the histone variant H2A.Z or the Mec1-dependent phosphorylation targets in H2A (62, 63). Together, all these data suggest a complex and differentiated role for the chromatin template in DNA repair, recombination, and replication. Our work revealed that the role of Dot1 in the DNA damage response is multifaceted and extends to regulation of the TLS pathway and maintenance of chromosome stability, although the mechanisms are still unclear. The challenge of future research will be to uncover the intricate network between chromatin modifiers and DNA damage response effectors.
Supplementary Material
Acknowledgments
We thank O. Aparicio, D. Gottschling, A. Kirchmaier, G. Brown, and T. Roberts for yeast strains and plasmids; S. Taubert, A. Wang, J. Schulze, M. Aristizabal, and L. Lee for critical reading of the manuscript; and A. Shilatifard for helpful discussion.
This work was supported in part by a scholar award from the Michael Smith Foundation for Health Research.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Table 1, Figs. S1 and S2, and additional references.
- TLS
- translesion synthesis
- MMS
- methyl methanesulfonate.
REFERENCES
- 1.Putnam C. D., Jaehnig E. J., Kolodner R. D. (2009) DNA Repair 8, 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper J. W., Elledge S. J. (2007) Mol. Cell 28, 739–745 [DOI] [PubMed] [Google Scholar]
- 3.Abraham R. T. (2001) Genes Dev. 15, 2177–2196 [DOI] [PubMed] [Google Scholar]
- 4.Shiloh Y. (2003) Nat. Rev. Cancer 3, 155–168 [DOI] [PubMed] [Google Scholar]
- 5.Segurado M., Tercero J. A. (2009) Biol. Cell. 101, 617–627 [DOI] [PubMed] [Google Scholar]
- 6.Harrison J. C., Haber J. E. (2006) Annu. Rev. Genet. 40, 209–235 [DOI] [PubMed] [Google Scholar]
- 7.Conde F., San-Segundo P. A. (2008) Genetics 179, 1197–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szyjka S. J., Aparicio J. G., Viggiani C. J., Knott S., Xu W., Tavaré S., Aparicio O. M. (2008) Genes Dev. 22, 1906–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neill B. M., Szyjka S. J., Lis E. T., Bailey A. O., Yates J. R., 3rd., Aparicio O. M., Romesberg F. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9290–9295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K. Y., Myung K. (2008) Mol. Cells 26, 5–11 [PMC free article] [PubMed] [Google Scholar]
- 11.Waters L. S., Minesinger B. K., Wiltrout M. E., D'Souza S., Woodruff R. V., Walker G. C. (2009) Microbiol. Mol. Biol. Rev. 73, 134–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouse J. (2004) EMBO J. 23, 1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts T. M., Kobor M. S., Bastin-Shanower S. A., Ii M., Horte S. A., Gin J. W., Emili A., Rine J., Brill S. J., Brown G. W. (2006) Mol. Biol. Cell 17, 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang M., Bellaoui M., Boone C., Brown G. W. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16934–16939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvaro D., Lisby M., Rothstein R. (2007) PLoS Genet. 3, e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuen K. W., Warren C. D., Chen O., Kwok T., Hieter P., Spencer F. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 3925–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholes D. T., Banerjee M., Bowen B., Curcio M. J. (2001) Genetics 159, 1449–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrulis E. D., Zappulla D. C., Alexieva-Botcheva K., Evangelista C., Sternglanz R. (2004) Genetics 166, 631–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammad D. H., Yaffe M. B. (2009) DNA Repair 8, 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts T. M., Zaidi I. W., Vaisica J. A., Peter M., Brown G. W. (2008) Mol. Biol. Cell 19, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin J. K., Bashkirov V. I., Heyer W. D., Romesberg F. E. (2006) DNA Repair 5, 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Attikum H., Gasser S. M. (2009) Trends Cell Biol. 19, 207–217 [DOI] [PubMed] [Google Scholar]
- 23.Downs J. A., Lowndes N. F., Jackson S. P. (2000) Nature 408, 1001–1004 [DOI] [PubMed] [Google Scholar]
- 24.Redon C., Pilch D. R., Rogakou E. P., Orr A. H., Lowndes N. F., Bonner W. M. (2003) EMBO Rep. 4, 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers A. L., Downs J. A. (2007) Biochem. Soc. Trans. 35, 1519–1524 [DOI] [PubMed] [Google Scholar]
- 26.Keogh M. C., Kim J. A., Downey M., Fillingham J., Chowdhury D., Harrison J. C., Onishi M., Datta N., Galicia S., Emili A., Lieberman J., Shen X., Buratowski S., Haber J. E., Durocher D., Greenblatt J. F., Krogan N. J. (2006) Nature 439, 497–501 [DOI] [PubMed] [Google Scholar]
- 27.Bazzi M., Mantiero D., Trovesi C., Lucchini G., Longhese M. P. (2010) Mol. Cell. Biol. 30, 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dover J., Schneider J., Tawiah-Boateng M. A., Wood A., Dean K., Johnston M., Shilatifard A. (2002) J. Biol. Chem. 277, 28368–28371 [DOI] [PubMed] [Google Scholar]
- 29.Shilatifard A. (2006) Annu. Rev. Biochem. 75, 243–269 [DOI] [PubMed] [Google Scholar]
- 30.Giannattasio M., Lazzaro F., Plevani P., Muzi-Falconi M. (2005) J. Biol. Chem. 280, 9879–9886 [DOI] [PubMed] [Google Scholar]
- 31.Wysocki R., Javaheri A., Allard S., Sha F., Côté J., Kron S. J. (2005) Mol. Cell. Biol. 25, 8430–8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood A., Krogan N. J., Dover J., Schneider J., Heidt J., Boateng M. A., Dean K., Golshani A., Zhang Y., Greenblatt J. F., Johnston M., Shilatifard A. (2003) Mol. Cell 11, 267–274 [DOI] [PubMed] [Google Scholar]
- 33.Grenon M., Costelloe T., Jimeno S., O'Shaughnessy A., Fitzgerald J., Zgheib O., Degerth L., Lowndes N. F. (2007) Yeast 24, 105–119 [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri S., Wyrick J. J., Smerdon M. J. (2009) Nucleic Acids Res. 37, 1690–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conde F., Refolio E., Cordón-Preciado V., Cortés-Ledesma F., Aragón L., Aguilera A., San-Segundo P. A. (2009) Genetics 182, 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Game J. C., Williamson M. S., Baccari C. (2005) Genetics 169, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer M. S., Kahana A., Wolf A. J., Meisinger L. L., Peterson S. E., Goggin C., Mahowald M., Gottschling D. E. (1998) Genetics 150, 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze J. M., Jackson J., Nakanishi S., Gardner J. M., Hentrich T., Haug J., Johnston M., Jaspersen S. L., Kobor M. S., Shilatifard A. (2009) Mol. Cell 35, 626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amberg D. C., Burke D. J., Strathern J. N. (2005) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, pp. 155–160, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 40.Gelbart M. E., Rechsteiner T., Richmond T. J., Tsukiyama T. (2001) Mol. Cell. Biol. 21, 2098–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longtine M. S., McKenzie A., 3rd., Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 42.Yang B., Britton J., Kirchmaier A. L. (2008) J. Mol. Biol. 381, 826–844 [DOI] [PubMed] [Google Scholar]
- 43.van Leeuwen F., Gafken P. R., Gottschling D. E. (2002) Cell 109, 745–756 [DOI] [PubMed] [Google Scholar]
- 44.Viggiani C. J., Aparicio O. M. (2006) Yeast 23, 1045–1051 [DOI] [PubMed] [Google Scholar]
- 45.Kushnirov V. V. (2000) Yeast 16, 857–860 [DOI] [PubMed] [Google Scholar]
- 46.Kobor M. S., Venkatasubrahmanyam S., Meneghini M. D., Gin J. W., Jennings J. L., Link A. J., Madhani H. D., Rine J. (2004) PLoS Biol. 2, E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haase S. B., Lew D. J. (1997) Methods Enzymol. 283, 322–332 [DOI] [PubMed] [Google Scholar]
- 48.Hall B. M., Ma C. X., Liang P., Singh K. K. (2009) Bioinformatics 25, 1564–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grenson M., Mousset M., Wiame J. M., Bechet J. (1966) Biochim. Biophys. Acta 127, 325–338 [DOI] [PubMed] [Google Scholar]
- 50.Bassal S., El-Osta A. (2005) Hum. Mutat. 25, 101–109 [DOI] [PubMed] [Google Scholar]
- 51.Foster E. R., Downs J. A. (2009) J. Cell. Biol. 184, 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahbazian M. D., Zhang K., Grunstein M. (2005) Mol. Cell 19, 271–277 [DOI] [PubMed] [Google Scholar]
- 53.Ng H. H., Xu R. M., Zhang Y., Struhl K. (2002) J. Biol. Chem. 277, 34655–34657 [DOI] [PubMed] [Google Scholar]
- 54.Ng H. H., Feng Q., Wang H., Erdjument-Bromage H., Tempst P., Zhang Y., Struhl K. (2002) Genes Dev. 16, 1518–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacoste N., Utley R. T., Hunter J. M., Poirier G. G., Côte J. (2002) J. Biol. Chem. 277, 30421–30424 [DOI] [PubMed] [Google Scholar]
- 56.Kunz B. A., Straffon A. F., Vonarx E. J. (2000) Mutat. Res. 451, 169–185 [DOI] [PubMed] [Google Scholar]
- 57.Gerring S. L., Spencer F., Hieter P. (1990) EMBO J. 9, 4347–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagès V., Santa Maria S. R., Prakash L., Prakash S. (2009) Genes Dev. 23, 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas D., Takahata S., Xin H., Dutta-Biswas R., Yu Y., Formosa T., Stillman D. J. (2008) Genetics 178, 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott K. L., Plon S. E. (2003) Mol. Cell. Biol. 23, 4522–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merker J. D., Dominska M., Greenwell P. W., Rinella E., Bouck D. C., Shibata Y., Strahl B. D., Mieczkowski P., Petes T. D. (2008) DNA Repair 7, 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karagiannis T. C., El-Osta A. (2007) Leukemia 21, 195–200 [DOI] [PubMed] [Google Scholar]
- 63.Morrison A. J., Shen X. (2009) Nat. Rev. Mol. Cell Biol. 10, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.