Abstract
Operant self-administration procedures are used to assess the neural basis of ethanol-seeking behavior under a wide range of experimental conditions. Rats do not spontaneously self-administer ethanol in pharmacologically meaningful amounts. This unit provides a step-by-step guide for training rats to self-administer quantities of ethanol that produce moderate to high blood-alcohol content. Different protocols are used for rats that are genetically heterogeneous versus rats that are selectively bred for high alcohol preference. Also, these protocols have different sets of advantages and disadvantages, for example, control for caloric intake or taste between two solutions that maintain operant lever-press behavior. Basic self-administration protocols can also be altered to focus on different aspects of the motivational properties of ethanol (for example, those related to dependence). This unit provides multiple protocols for producing alcohol intake in rats that can be pharmacologically probed relative to a variety of control conditions.
Keywords: Ethanol, Alcohol, Self-administration, Neuropharmacology, Operant Learning
Introduction
The procedures described in this unit are designed to evaluate the capacity of potential pharmacotherapeutic agents to selectively reduce alcohol-motivated behaviors (i.e., trained lever pressing) in rats. In addition, important features that enable the study of the neural mechanisms regulating alcohol drinking behaviors are discussed. The protocols are particularly useful for dissociating nonspecific effects of a test agent on liquid ingestive behaviors. Both procedural and interpretive details are provided to assist the researcher in implementing, as well as understanding the data obtained from each of the five Basic Protocols. The methods can be used to initiate simple ethanol drinking in outbred rats, or to obtain rapid acquisition in lines of rats selectively bred for ethanol consumption. While all the protocols in this unit have been designed to determine the capacity of a test agent to selectively decrease motivated responding for alcohol, each protocol is an independent evaluative tool and may be used to make inferences about different aspects of alcohol-drinking behaviors and particular neural substrates responsible for mediating alcohol's effects.
To systematically evaluate the selectivity of pharmacological treatments that suppress ethanol responding, a four-phase strategy is used. Basic Protocol 1 is employed to evaluate the capacity of a pharmacological agent to produce dose and time-course effects when rats are presented with an ethanol reinforcer on a fixed-ratio (FR)-4 schedule. In an FR-4 schedule, a rat will press a lever four times to receive a 0.10-ml reinforcer. This protocol determines both potency and duration of an agent to suppress ethanol responding. After being trained to respond for ethanol versus water on fixed-ratio reinforcement schedules, animals can subsequently be tested for operant ethanol-self-administration behavior under a variety of conditions, for example progressive-ratio ethanol responding (Support Protocol 2) and dependence-induced elevations in drinking (Support Protocol 3). Basic Protocol 2 is used to evaluate the capacity of a pharmacological agent to produce dose and time-course effects when rats are presented with a saccharin reinforcer. The concentration of the saccharin reinforcer is chosen such that both response profiles and rates approximate the ethanol reinforcer in Basic Protocol 1. The goal of Basic Protocol 2 is to obtain information on the potency and duration of action of an agent that suppresses responding to a highly palatable reinforcer. When the data are interpreted from Basic Protocols 1 and 2, inferences can be made as to whether antagonism is specific to ethanol. Basic Protocol 3 is used to evaluate the capacity of a pharmacological agent to produce dose and time course effects when a concurrent schedule procedure is employed (i.e., both ethanol and a saccharin reinforcer are simultaneously available). Because basal response rates will be equated (i.e., equal or near equal), the concurrent schedule addresses the issue of whether the capacity of an agent to decrease ethanol and not saccharin reinforcement in Basic Protocols 1 and 2 is due to differences in reinforcing efficacy (i.e., strength). Basic Protocol 4 is performed to evaluate the capacity of a pharmacological agent to produce dose and time-course effects in a concurrent schedule procedure when two isocaloric alternative solutions are presented. In the isocaloric concurrent procedure, the post-ingestional effects of the nondrug reinforcer are also taken into account. Basic Protocol 5 is used to produce binge-like patterns of ethanol self-administration by allowing rats to respond for sweetened ethanol vs. water, while controls respond for sweetened vehicle vs. water. This protocol produces high levels of ethanol self-administration and allows testing for the effects of pharmacological agents on self-administration of ethanol relative to its effects on a similarly flavored ethanol-free reinforcer.
While a step-by-step procedural description appears in each of the Basic Protocols, it is strongly recommended that less experienced investigators consult a seasoned researcher to assist with understanding the written procedures, data interpretation, and to provide some basic understanding of the theoretical foundation from which many of the principles inherent within the Basic Protocols were derived.
Strategic Planning
Pharmacological Manipulations/Drug Effects
Following initiation and stabilization of ethanol-maintained operant responding, pharmacological compounds are often tested for their effects on self-administration behavior. In these protocols, pharmacological agents are typically administered directly prior to self-administration sessions via systemic injections, oral delivery (i.e., gavage using a feeding needle), drug pellets, or microinfusion techniques (i.e., intracerebral or intracerebroventricular delivery). The goals of these pharmacological manipulations are similar in that they all attempt to make inferences about particular neural mechanisms regulating alcohol-seeking behaviors. This unit does not provide the reader with technical information on drug administration techniques but instead presents a set of procedures that enables the researcher to arrive at the point where pharmacological manipulations/drug evaluations may begin.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
Basic Protocol 1: Train Rats to Initiate Ethanol-Maintained Responding on an FR-4 Schedule
In this protocol, the operant software is programmed to record number of responses (i.e., response rates) and earned reinforcers (i.e., stimuli) for each solution presented (saccharin, ethanol-saccharin cocktail, and ethanol) under each schedule of reinforcement (FR-1, FR-4). While all data should be recorded and archived during the acquisition phase, the data obtained once the animals exhibit stable response rates are the primary concern of the investigator. Most operant software permits a direct transfer of these data to spreadsheets (e.g., Excel). Once the number of responses and reinforcers of the saccharin, ethanol-saccharin cocktail, and ethanol have been determined for the post-stabilization period, analysis of these data will allow evaluation of the reinforcing efficacy (i.e., strength) of the various solutions. The effects of drug treatments on these measures are also easily determined following the post-stabilization period. The amount of absolute ethanol intake (i.e., g/kg) should also be evaluated.
Materials
Ten 2- to 3-month-old naive outbred rats or rats selectively bred for alcohol consumption (∼200 to 300 g, female or male)
Standard rodent diet
0.10% (w/v) saccharin (Fisher Scientific) solution in distilled water
2% (v/v) ethanol/0.075% (w/v) saccharin solution
5% and 10% (v/v) ethanol solutions
Wire-mesh stainless-steel cages or plastic tubs
Personal computer with standard operant software packages (e.g., from Coulbourn Instruments or Med Associates) to record responses and control reinforcements
Stopwatch
Ten standard operant chambers (Coulbourn Instruments; Fig. 9.12.1A) equipped with two removable levers (Fig. 9.12.1B) and two dipper fluid delivery systems (Fig. 9.12.1C) enclosed in sound-attenuated cubicles (Fig. 9.12.1D). Dipper presentations should provide 1.5-sec access to a 0.10-ml dipper solution, followed by a 3-sec time-out period; the amount of the earned reinforcer delivered following the various response requirement in Basic Protocols 1 to 4 is 0.10 ml. Above each lever, a stimulus light (red, green, or yellow) is present and is illuminated upon presentation of the stimulus delivery/reinforcer (Fig. 9.12.1B).
Animal balance for weighing rats
Figure 9.12.1.

(A) Modular operant chamber (Coulbourn Instruments) used in training rats to initiate self-administration of ethanol, saccharin, sucrose, or an ethanol cocktail solution. (B) Manipulandum (i.e., response lever) for the operant chamber. Depending on the protocol, 1 or 2 levers may be inserted into the operant chamber. The lever extends 0.75 in. past the front panel. A triple cue light module (green, yellow, and red) may be used in conjunction with the response lever for generating a visual cue to initiate the lever press response. While the visual cue can be an intricate component of any of the four Basic Protocols, this was not included in the present unit. (C) Liquid dipper dispenser module with trough (located on bottom) designed to deliver any type of liquid reinforcer. Interchangeable dipper-cup sizes are available from Coulbourn Instruments (0.02 to 0.10 ml). Whenever a lever is inserted in the operant chamber, it must be accompanied by a liquid dipper dispenser module and trough. (D) Modular operant chamber enclosed in sound-attenuated cubicle. The cubicle is designed to isolate the subject from distracting ambient light, sound, and other laboratory activity. A peep-hole is built in the front panel so the investigator can monitor the subject's activity. Also, enclosed along the rear wall of the chamber is an exhaust fan (not visible from picture), which regulates the air flow within the cubicle. The fan is powered by 28 V from the control interface.
Begin initiation phase
-
1
One week prior to experiment, handle 10 rats daily and allow them to become acclimated to the vivarium and light cycle (either a normal or reversed 12-hr light/dark cycle is appropriate). House animals individually in wire-mesh stainless steel cages or plastic tubs. Maintain the vivarium at 21°C and provide ad libitum access to food and water, except during the first days of the training phase. Weigh animals once weekly.
Training and experimental sessions should take place between 10 a.m. and 3 p.m.
If animals are being trained after a stereotaxic surgical procedure, delay the beginning of the initiation phase for at least 1 week.
-
2
Provide food ad libitum to the rats; the only exception being that no food should be available during the 60-min operant session. To begin the initiation phase, place only the right lever (Fig. 9.12.1B), dipper assembly, and fluid delivery system (i.e., trough; Fig. 9.12.1C) in the operant chamber. Fill the liquid reservoir with 15 ml of 0.10% saccharin solution.
-
3
Select and define the testing parameters for the operant session: for example, determine if the data will be gathered for 30 or 60 min. Then, determine if these data will be gathered in 2-, 5-, or 10-min blocks. Program this information in the computer using the commercial Coulbourn or Med Associates software.
The block data will be important in illustrating the within-session response profiles. The cumulative session data is one type of within-session response profile that is often desired by the researcher. With outbred rats, a 30-min session is generally adequate, but when working with animals with very high response rates, such as genetically selected rat lines, a 1-hr session can generate substantially more data, increasing the power of the analysis (for comparison see June et al., 1998a). When investigating drugs that have a very short onset or half-life, it is advisable to record the data in 2-, 5-, or 10-min blocks so a more accurate determination of the time course of effects of a particular drug treatment can be obtained.
-
4
Prior to any experimentation, use a stopwatch to determine an appropriate duration of stimulus delivery. During the first phase of initiation, use a fixed ratio (FR)-1 schedule that provides the rat with one 0.1-ml dose of liquid reinforcer for a specified duration of stimulus delivery (e.g., 1.5 to 3.0 sec) each time the animal makes a single lever press. Use the stopwatch data to define the duration of stimulus delivery and response requirements and program this information in the operant software.
Typically, the duration of stimulus delivery has been set by the software manufacturer and will range from 1.5 to 3.0 sec. Any time within this range is sufficient to provide either a nondeprived or fluid-deprived rat an opportunity to consume its reinforcing solution. Longer periods are not recommended and would be considered nonstandard.
The FR-1 response requirement is synonymous with the term “continuous reinforcement” and the terms are used interchangeably throughout this unit. The computer software will provide a recording of the number of earned reinforcers and the number of responses made during the session. The total number of responses at the end of the 30- or 60-min session will also be calculated. The block data discussed in step 3 can easily be obtained by specifying these parameters on the computer.
-
5
Deprive each animal of fluid for 22 hr prior to experimentation.
-
6
Once the testing parameters, duration of stimulus delivery, and response requirements have been specified, remove the fluid-deprived rat from its home cage and place it in the operant chamber for the 1-hr session. Close the door to the operant chamber and then close the door to the sound-attenuated chamber (the animal will be visible through the peep-hole in the sound-attenuated chamber; Fig. 9.12.1D). During the 1-hr session, simply observe the rat.
The deprived rat will begin to press the lever by engaging in what is termed appetitive behaviors (i.e., restless seeking behaviors). It is advisable that daily observations be completed during the beginning of this initial training phase to learn about the different response profiles that occur during the acquisition phase of the different types of response-contingent behaviors. Most deprived rats will lever press for a highly palatable saccharin or sucrose reinforcer during the very first day of the training phase. The greater the fluid-deprivation period and palatability (i.e., concentration) of the reinforcer, the more likely the rat will initiate lever pressing the very first day in the operant chamber. A 0.1% saccharin reinforcer (June et al., 1998b) or 10% sucrose reinforcer (Samson, 1987) is recommended as these are highly palatable reinforcers to a deprived rat.
-
7
To facilitate consistent and robust lever pressing, deprive rats of fluid for 22 hr prior to the 1-hr operant session for at least 2 to 3 days; however, once the rats are shaped and responding rapidly on the continuous reinforcement (CRF) schedule (i.e., for two or three sessions), rats should receive no additional fluid restriction throughout the experiment.
-
8
After the last day of fluid deprivation, train the rats for 5 additional days under the 60-min daily access paradigm to respond for the 0.1% saccharin solution on an FR-1 schedule.
-
9
On day 6, discontinue the FR-1 schedule and increase the response requirement to an FR-4 schedule. Continue until stable responding is obtained (about day 14).
The FR-4 schedule provides the rat with a 0.1-ml dose of liquid reinforcer each time the animal makes four lever presses. While the response requirement changes, the amount of the earned reinforcer (0.1 ml) remains the same as the FR-1 schedule.
Stable responding on the FR-4 schedule for the 0.10% saccharin reinforcer should be obtained around day 14. Stability is defined as at least five consecutive sessions that show neither a systematic increase nor decrease in responding. The authors use a criterion of +/-20% of the average responses for 5 consecutive days in evaluating systematic increases or decreases in responding. It is always assumed (based on the authors' experience) that over a 5-day period, whether responding for a single solution using a single lever or responding for two different solutions with two levers, stability in responding will be obtained by this time. On the rare occasions when this does not occur, the authors suggest that rats showing such response profiles be given 2 or 3 additional sessions to stabilize. If after these additional sessions stabilization still does not occur, these animals should be eliminated from the study and training of additional rats should begin. When the term “stability” is used throughout the unit, the above criteria should be applied.
-
10
Train rats to respond for a 2% ethanol/0.075% saccharin reinforcer on an FR-4 schedule (0.1 ml/four responses).
When ethanol is combined with a sweetened solution this mixture is referred to as a “cocktail.”
-
11
After development of stable FR-4 responding (i.e., typically five sessions) for the ethanol-saccharin cocktail, expose rats to six successive FR-4 sessions in which the drinking solution is alternated daily from the ethanol-saccharin cocktail to 5% ethanol only (0.1 ml/four responses).
-
12
After the 6-day alternation procedure, raise the ethanol concentration in the ethanol-saccharin cocktail solution each day from 2% to 5%, 7%, 9%, and 10% and concurrently decrease the concentration of saccharin from 0.075%, to 0.05%, 0.025%, and 0.0125% until saccharin is eventually eliminated at the 10% ethanol concentration level.
-
13
Obtain stable responding for the 10% ethanol solution for at least 2 weeks before beginning experimental drug treatments.
To ensure stability of responding prior to the drug treatment, the response rates for the last 5 days before the drug treatment should be no more than +/-20% of the average daily response rate for the sample of 10 rats.
Analyze data
-
14
Divide the number of lever presses obtained under the FR-4 schedule by 4 to determine the number of earned reinforcers.
-
15
Calculate the volume (ml) of solution consumed.
For example, a rat presses the lever 350 times for a 10% ethanol solution in a 60-min session. 350/4 = 87 earned reinforcers, and 87 × 0.10 ml = 8.7 ml total solution.
-
16
Calculate the grams of ethanol consumed: one earned reinforcer using a 0.10-ml dipper cup volume = 0.10 ml volume of 10% ethanol
Continuing with the example from step 15: 8.7 ml total solution × 10% ethanol × 0.793 g/ml = 0.69 g absolute ethanol consumed.
-
17
Divide grams of ethanol consumed by the weight of the rat to obtain a result in g/kg.
0.69 g ethanol consumed/0.370 kg rat = 1.86 g/kg dose of absolute ethanol
-
18
To determine the pattern of ethanol intake prior to drug treatment (baseline phase), analyze data by a repeated measures analysis of variance (ANOVA) for the factors day (day 1, 2, and 3), and response interval (0 to 10, 11 to 20, 21 to 30, 31 to 40, 41 to 50, 51 to 60 min, and total: 10 to 60 min). Analyze both factors as repeating variables. Conduct separate analyses on each dependent measure (i.e., number of responses, reinforcers, and intake (g/kg) of the ethanol and saccharin/sucrose solutions).
-
19
Analyze the data from the drug treatment phase by repeated measures ANOVA with drug treatment, response interval, and consumption day (day 1, 2, and 3) as the independent factors. Conduct separate analyses on each dependent measure (i.e., number of responses, reinforcers, and intake (g/kg) of the ethanol and saccharin/sucrose solutions). Determine the drug treatment × response interval interaction to obtain a detailed analysis of the profile of antagonism by a test agent across the full length of the 60-min session. Make post-hoc comparisons using the Newman-Keuls test (or similar measures) during the baseline and drug treatment phases.
Illustrative example. Using 4 doses of “drug x” and one placebo treatment (e.g., saline), the 5 experimental manipulations would become the factor “drug treatment condition”, comprising 5 levels. The pooled no-injection baseline (e.g., average of 5 days before the drug treatment) would be the 6th drug treatment level (i.e., no-injection control). Hence, the analysis for response rate would be a 6 × 7 repeated measures ANOVA. The latter factor with 7 levels would be response interval (0 to 10, 11 to 20, 21 to 30, 31 to 40, 41 to 50, 51 to 60 min, total: 0 to 60 min). If the experimenter is not interested in evaluating the time-course data, then the analysis would be reduced to a one-way repeated measures ANOVA for only the 60-min data.
Support Protocol 1: Blood Alcohol Content (BAC)
It is extremely important to validate operant ethanol self-administration procedures by assessing post-session BACs. This procedure is easy, fast, and inexpensive, and confirms that rats are consuming pharmacologically relevant amounts of ethanol following lever presses. Tail blood samples are typically collected from animals under baseline conditions (i.e., when animals are not receiving a drug treatment). The Analox Analyzer (Analox Instruments) can be used to measure BAC and is highly recommended, although other methods may also be used. However, compared with many previously described HPLC methods (June et al., 1999), this new methodology is more efficient in measuring BAC content and real-time data can be obtained.
Materials
Rats (see Basic Protocols 1 to 4)
Heparin-coated microcentrifuge tubes
Clinical analyzer (GL-5 MicroStat, Analox Instruments)
Clark-type amperometric oxygen electrode
NOTE: Use alcohol reagent buffer solutions (pH 7.4) and alcohol oxidase enzymes provided by the manufacturer (Analox Instruments) in all samples tested.
After the first 30 min of an operant session, collect ∼100 ul of whole blood from the tip of the rat's tail into a heparin-coated microcentrifuge tubes.
Immediately microcentrifuge the blood for 5 min at 1100 rpm, room temperature.
-
Collect duplicate 5-ul plasma samples with a pipet and inject them directly into an analyzer.
Microanalysis will consist of measuring the oxygen consumption in the reaction between the sample of alcohol and alcohol oxidase (AOD) using a Clark-type amperometric oxygen electrode.
Results are calculated in units of mg/dl and printed within 20 sec of each trial.
Use the mean of the duplicate samples as an index of the level of BAC content for each rat.
Correlate data obtained from the BAC measurements with ethanol response rates and absolute ethanol intake (i.e., g/kg; see Fig. 9.12.2).
Figure 9.12.2.
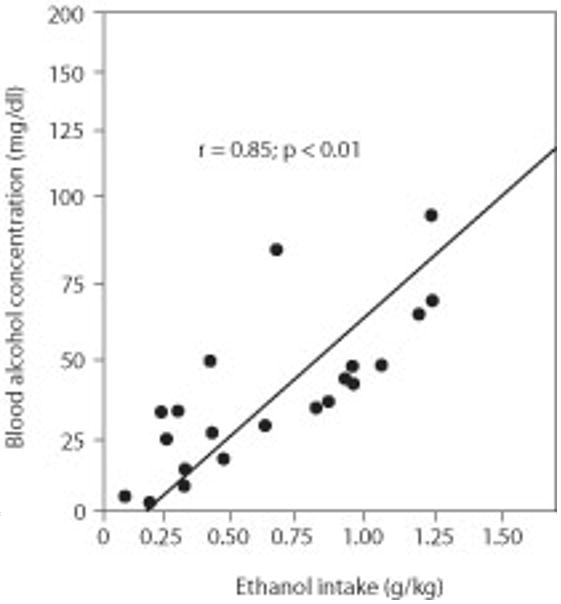
Relationship between ethanol intake and blood alcohol levels. Tail blood samples were taken from alcohol preferring (P) rats (n = 22) after having lever pressed for a concurrent presentation of 10% ethanol and 0.025% saccharin following the first 30 min of a 1-hr operant session.
Support Protocol 2: Training Rats to Respond For Ethanol on A Progressive-Ratio (PR) Schedule
The progressive-ratio schedule of reinforcement allows closer examination of the effects of pharmacological manipulations on the motivation to work for ethanol. In the PR reinforcement schedule, response requirements are gradually increased (i.e., animals have to work harder for each successive reinforcement). The dependent variables measured in PR operant testing are the break point (the point at which animals no longer lever-press for ethanol) and cumulative responses across the session. These data can then be analyzed as described in Basic Protocol 1.
Additional training
Rats can be trained to respond for ethanol on a PR schedule following any phase of testing using the FR reinforcement schedule described in Basic Protocol 1 (i.e., following training or pharmacological testing, etc.).
If animals have been trained previously on a continuous reinforcement schedule (FR-1), they should be allowed several days of responding for ethanol on partial reinforcement schedule (i.e., FR-4). If rats were initially trained on a partial reinforcement schedule, this is not necessary.
Animals are placed in operant boxes and allowed to respond for ethanol on a PR schedule of reinforcement in a single-lever situation during a 180-minute operant session. The PR schedule should be programmed using the software to follow this gradually escalating sequence of response requirements (i.e., the number of responses required to receive a single reinforcer): 1, 1, 2, 2, 3, 3, 4, 4, 5, 5, 7, 7, 9, 9, 11, 11, 13, 13, 15, 15, 18, 18, 21, 21, 24, 24, etc.
Determine the point at which an individual animal stops responding (defined as no responses within a 30-minute time period). This is defined as the breakpoint and reflects the number of reinforcers earned during the session. This measure is used to determine when animals have once again achieved stabilization of responding on this new reinforcement schedule.
Train rats to respond for ethanol on a PR schedule of reinforcement until break point has stabilized, defined as ± 20% change in break point across 3 consecutive days. Once rats exhibit stable responding and break points, rats can be tested for the effects of pharmacological compounds on the motivation to consume ethanol. Changes in cumulative responding on a PR schedule reflect changes in the willingness to work for ethanol in a directly proportionate manner (see Fig. 9.12.3).
Figure 9.12.3.
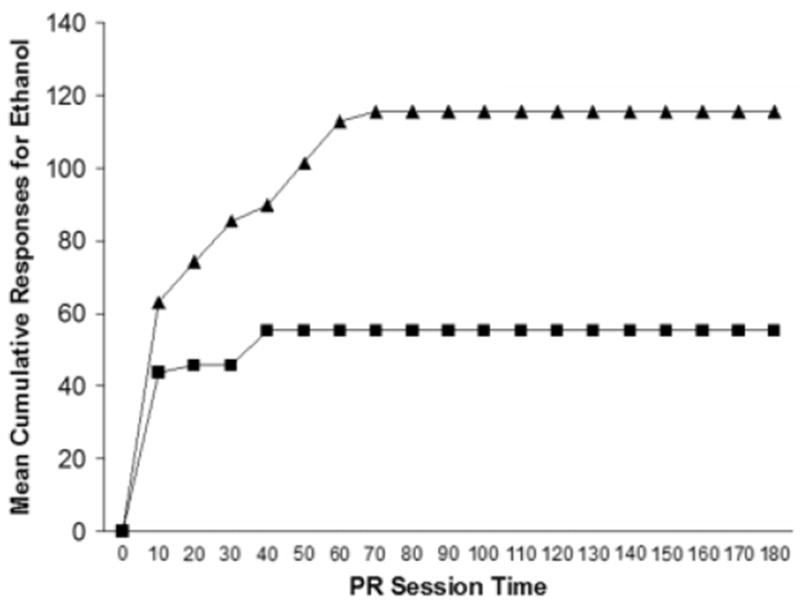
Mean cumulative responses for ethanol during a 180-minute progressive-ratio session in two groups of rats. In this case, the group represented by darkened triangles worked longer and harder to obtain ethanol, thereby exhibiting a higher motivation to consume ethanol than the group represented by darkened circles.
Support Protocol 3: Testing Ethanol Self-Administration Behavior In Alcohol-Dependent Rats
Chronic exposure to high doses of ethanol produces somatic and motivational symptoms of alcohol dependence in rats that parallel those observed in human alcoholics. Rats are typically made dependent on alcohol via one of two forced administration procedures: [1] chronic intermittent ethanol (CIE) vapor exposure (UNIT 9.29) or ethanol liquid-diet. Detailed descriptions of those procedures are beyond the scope of this Unit, but experiments are typically designed to accommodate operant testing during either acute withdrawal or protracted abstinence from chronic high-dose ethanol exposure, depending on the goals of the study.
Additional Training
Dependence induction and establishment of post-dependent baseline responding typically occur immediately following the ethanol self-administration training procedure described in Basic Protocol 1.
At the end of the baseline responding period, rats are divided into two groups, counterbalanced for daily operant ethanol intake. One group of rats is chronically exposed to high doses of ethanol (via vapor or liquid diet) and the other group is not.
Following approximately 4 weeks of exposure to high doses of ethanol, dependent and non-dependent rats can be tested for ethanol self-administration at various withdrawal and abstinence time points, as described below and as illustrated in Fig. 9.12.4.
Before any pharmacological manipulations are considered, rats should be allowed several operant sessions to re-establish baseline ethanol responding in the post-dependent state. Rats are tested intermittently (e.g., twice per week) during post-dependent re-establishment of baseline. Dependent rats reliably exhibit increases in operant ethanol responding following chronic exposure to high doses of ethanol (Gilpin et al., 2008; O'Dell et al., 2004).
Figure 9.12.4.
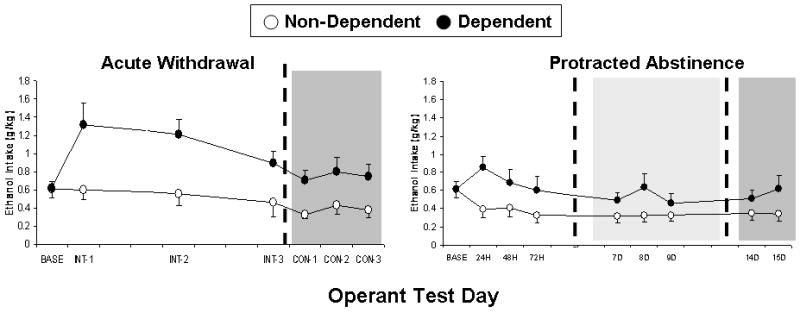
(A) Mean ± SEM ethanol intake (g/kg) by dependent (closed circles) and non-dependent (open circles) Wistar rats during pre-vapor baseline period (BASE) versus three intermittent (INT; white background) and three consecutive-days (CON; gray background) operant test sessions that occurred 6 hours into acute withdrawal; and also (B) during pre-vapor baseline period (BASE) versus three (24 hrs, 48 hrs, 72 hrs) early-abstinence (white background), three (7 days, 8 days, 9 days) middle-abstinence (light gray background), and two (14 days, 15 days) protracted-abstinence (dark gray) operant test sessions. Dependent rats exhibit significant and reliable increase in ethanol intake relative to non-dependent rats.
Within-subjects testing of a pharmacological compound lends itself better to acute withdrawal testing because rats typically experience daily withdrawal episodes (inclusion of withdrawal periods accelerates and intensifies alcohol dependence). Therefore, testing can occur during withdrawal periods without disturbing the high-dose ethanol exposure procedure. Baseline sessions and pharmacological tests are similarly conducted with the single exception of whether or not drug is administered to animals prior to the operant session.
Post-dependent operant ethanol self-administration tests can occur either on consecutive days or intermittently, either with or without vapor exposure between tests, and at various time points following termination of vapor exposure (i.e., acute withdrawal versus protracted abstinence). Tests of withdrawal- and abstinence-induced ethanol responding should be conducted at a time of day that corresponds to pre-vapor baseline testing.
Basic Protocol 2: Train Rats to Initiate Saccharin-Maintained Responding on A FR-4 Schedule
The goal of this protocol is to match the behavioral patterns and response rates obtained with the 10% (v/v) alcohol group in Basic Protocol 1.
For materials, see Basic Protocol 1.
Follow Basic Protocol 1, steps 1 to 9 using 10 naive rats.
-
On day 14, after obtaining stable responding on the FR-4 schedule, maintain rats on the 0.10% saccharin reinforcer continuing to use only the right lever in the chamber.
Rats typically stabilize rather easily on palatable solutions when given alone under FR schedules, however, 1 to 2 additional weeks (day 21 to 28) will ensure optimal stabilization. The drug-treatment phase can begin after the optimal stabilization period. Based on the author's experience with outbred and alcohol selectively-bred rats, this saccharin concentration should be in the range of 0.025% to 0.10% (see June et al., 1998a). It is recommended to start with 0.05% saccharin (i.e., a moderate concentration) and then adjust the concentration accordingly.
Analyze the saccharin-maintained responding data identical to the ethanol-maintained responding data as discussed in steps 18 to 19 of Basic Protocol 1.
The number of responses (i.e., response rates) and earned reinforcers for the saccharin solution (0.025% to 0.10%) will be recorded under the FR-4 schedule of reinforcement by the operant software. Once the number of responses and reinforcers for the saccharin have been determined for the post-stabilization period, it will allow evaluation of the reinforcing efficacy of the saccharin. The effects of drug treatments on these measures are also easily determined.
Basic Protocol 3: Train Rats to Lever Press Concurrently for Ethanol and Saccharin Under an FR-4 Schedule
In a concurrent procedure, a 10% ethanol reinforcer is presented in the operant chamber simultaneously with a saccharin reinforcer (e.g., 0.025% to 0.1%), which provides response rates and patterns similar to those obtained with ethanol (Samson and Grant, 1985; Petry and Heyman, 1995; June et al., 1998a).
In general, high- to very high-responding rats that lever-press for ethanol or saccharin tend to perform substantially better in a concurrent protocol situation (see June et al., 1998a, 1999, 2001). Thus, beginning this protocol with a sample size two times that of Basic Protocols 1 and 2 (i.e., n = 20) will provide the researcher with a greater opportunity to obtain the desired sample of rats capable of responding similarly for two concurrently presented reinforcers. It is important to note that while the experimental demands inherent within the concurrent protocol are much greater than Basic Protocols 1 and 2, the capacity to show that a pharmacological agent can selectively alter ethanol consumption in the absence of modifying another highly palatable oral reinforcer is regarded as an optimal test for preclinical screening of agents that might hold promise in reducing alcohol intake in humans (Carroll et al., 1991; Petry, 1997; June et al., 1999; Rodefer et al., 1999).
For materials, see Basic Protocol 1.
-
1
Perform Basic Protocol 1, steps 1 to 13 using 20 naive rats.
-
2
After obtaining stable responding on the FR-4 schedule for 10% ethanol (around day 35 to 40), maintain rats on the ethanol reinforcer for an additional 1 week, continuing to use only the right lever in the chamber.
-
3
After the final week of ethanol stabilization with the single right lever, insert the left lever and dipper assembly system into the operant chamber. Fill the left reservoir with ∼15 ml of saccharin solution. Use the saccharin concentration determined in Basic Protocol 2 that matched the patterns and response rates of the 10% ethanol.
-
4
With 10% ethanol in one dipper assembly and saccharin in the other, train rats for 10 days in 60-min daily sessions under the FR-4 schedule. Alternate the position of the levers and associated dippers for each reinforcer for each session to avoid the establishment of lever preference.
For example, if the ethanol reinforcer appeared in the right dipper assembly and the saccharin reinforcer appeared under the left on Monday, then on Tuesday, the ethanol reinforcer should appear in the left dipper assembly and the saccharin reinforcer in the right.
An alternative and equally effective strategy to avoid establishment of a lever preference is to use the mixed-alternation procedure. In this procedure, the ethanol reinforcer is presented for 2 consecutive days under the left lever, while the saccharin reinforcer is presented for 2 consecutive days under the right lever. Following the 2-day right, 2-day left procedure, the experimenter changes the presentation sequence to a 1-day right, 1-day left procedure. This mixed presentation procedure can begin with the 1-day or 2-day alternation strategy. Following a 10-day evaluation, the experimenter should determine if responding for the two reinforcers is similar or equal. It may be necessary to adjust the saccharin concentration up or down to match the ethanol response rates. The authors generally accepts rates of saccharin responding that are at least 70% that of ethanol, and no greater than 25% higher than ethanol (that is, between 70-125% of the ethanol response rate). Even these criteria might prove very difficult to attain with certain rat lines and strains. Nevertheless, every attempt should be made to equate the basal response rates of the two qualitatively different reinforcers. Only animals with “compatible” (i.e., similar) ethanol and saccharin response profiles should be permitted in the study. Hence, it may be necessary to train additional animals.
-
5
Obtain stable responding for the concurrently presented 10% ethanol and saccharin reinforcers for at least 2 weeks before beginning experimental drug treatments.
-
6
Analyze data as described in Basic Protocol 1, steps 18 to 19.
-
7
Collect BAC data (see Support Protocol 1) as necessary throughout the drug-treatment phase.
Basic Protocol 4: Train Rats to Lever Press Concurrently for Alcohol And an Isocaloric Alternative Solution Under An FR-4 Schedule
This protocol not only addresses the issue of post-ingestional properties of reinforcers, but also the issue of specificity of a pharmacological agent to suppress responding. This is accomplished by employing a modification of the Heyman and Oldfather (1992) procedures for presenting alcohol concurrently with isocaloric alternative solutions to outbred rats. The isocaloric ratio is determined by the following formulas: ethanol: (1 ml ethanol/10 ml solution) × (7.1 cal/g ethanol) × (0.79 g ethanol/ml ethanol) = 0.56 cal/ml; and sucrose: (1.42 g sucrose/10 ml solution) × (3.95 cal/g sucrose) = 0.56 cal/ml. The procedure entails the use of both right and left levers and their dipper systems in the operant chamber.
Additional Materials (also see Basic Protocol 1)
10% (w/v) sucrose solution
-
1
Follow Basic Protocol 1, steps 1 to 8, using 10 naive rats, but in step 8, use a 10% sucrose solution instead of the 0.10% saccharin solution.
-
2
Continue the FR-1 schedule on the 10% sucrose solution for 7 consecutive days. Around day 8, after obtaining stable responding on the FR-1 schedule for the sucrose (i.e., +/-20% of the average responses for 5 consecutive days), gradually add increasing concentrations of ethanol (2.5%, 5%, 7.5%, 10%, 15%, 20%, and 25%) to the sucrose solution. Maintain animals on each of the sucrose plus ethanol cocktails for 2 days.
Hence, the sucrose plus ethanol phase will last for 14 days.
Begin concurrent schedule presentation
-
3
After completing the induction phase, insert the right lever and dipper assembly into the operant chamber (Figures 9.12.1B, C). Present 10% ethanol + 10% sucrose on one lever and water on the second lever to begin the concurrent schedule presentation.
It does not matter whether the cocktail or water appears on the left or right position on the first day of the concurrent schedule presentation.
-
4
On the second day of the concurrent schedule presentation, begin gradually increasing the concentration of sucrose in the water reservoir over 5 days (i.e., 2%, 4%, 6%, 8%, and 10%) while continuing to present the 10% ethanol/10% sucrose cocktail on the second lever. Place both solutions on alternating sides (i.e., maintaining the left/right orientation) of the operant chamber to ensure sampling of both alternatives and prevention of establishing a lever preference.
Use the following schedule to proceed with concurrent schedule presentation over the next 5 days after the initial concurrent 10% ethanol + 10% sucrose (lever 1) and water (lever 2) choice:
Day 2: 10% ethanol + 10% sucrose (right lever); 2% sucrose (left lever)
Day 3: 10% ethanol + 10% sucrose (left lever); 4% sucrose (right lever)
Day 4: 10% ethanol + 10% sucrose (right lever); 6% sucrose (left lever)
Day 5: 10% ethanol + 10% sucrose (left lever); 8% sucrose (right lever)
Day 6: 10% ethanol + 10% sucrose (right lever); 10% sucrose (left lever)
-
5
On day 7, begin training on the concurrent schedule with the two isocaloric alternatives. Over the next 5 days, the rats should receive 10% ethanol + 10% sucrose + 0.25% saccharin (w/v) (lever 1), and 10% (w/v) sucrose (lever 2).
The addition of the saccharin to the cocktail will increase the palatability of the ethanol and, in turn, increase the efficacy (i.e., reward strength) of the ethanol cocktail.
-
6
Gradually fade the sucrose by daily reducing the concentration (i.e., 7.5%, 5%, 0%) in the 10% ethanol + 10% sucrose + 0.25% saccharin cocktail (lever 1) while continuing to present the 10% sucrose (lever 2). Place both solutions on alternating sides of the operant chamber to ensure sampling of both alternatives and prevention of establishing a lever preference. The concurrent schedule presentation would proceed as follows over the next 6 days:
Day 1: 10% ethanol + 10% sucrose + 0.25% saccharin (right lever); 10% sucrose (left lever)
Day 2: 10% ethanol + 7.5% sucrose + 0.25% saccharin (left lever); 10% sucrose (right lever)
Day 3: 10% ethanol + 5% sucrose + 0.25% saccharin (right lever); 10% sucrose (left lever)
Day 4: 10% ethanol + 2.5% sucrose + 0.25% saccharin (left lever); 10% sucrose (right lever)
Day 5: 10% ethanol + 0% sucrose + 0.25% saccharin (right lever); 10% sucrose (left lever)
Day 6: 10% ethanol (left lever); 10% sucrose (right lever)
-
7
Continue the 10% ethanol (lever 1) and 10% sucrose (lever 2) regimen for 2 additional days.
-
8
On day 9, increase the reinforcement schedule to an FR-4 schedule and increase the 10% sucrose solution to a 14.2% solution. (The 10% ethanol and the 14.2% sucrose solutions are isocaloric.) Maintain the animals on the FR-4 schedule for 7 days with the two isocaloric fluids. Place solutions on alternating sides of the operant chamber to ensure sampling of both alternatives and prevention of establishing a lever preference.
This period will stabilize the animals under the FR-4 schedule for the two isocaloric fluids. The stability criteria outlined in Basic Protocol 1 (i.e., neither systematic increase or decrease in responding over at least five consecutive sessions using a reference point of +/-20%) should also be applied to the two-lever situation. However, each solution must be viewed individually for stabilization purposes; for example, stabilization should occur across time in a “single” solution, not “between” the two solutions. After attaining the stabilization phase, experimental manipulations may begin.
Analyze data
-
9
The number of responses and reinforcers of the concurrently presented ethanol and sucrose solutions should be recorded under the FR-1 and FR-4 schedules of reinforcement by the operant software. The number of responses and reinforcers of the sucrose and ethanol solutions during the stabilization and post-stabilization periods are the principal data of interest. For investigators that are interested in conducting an interval by time analyses, the operant software can be programmed to collect data in 1-, 2-, 5-, or 10-min blocks/bins for within-session analyses. Even if the investigator is not sure that she/he will analyze the within-session data, the author suggests these data be recorded. The amount of absolute ethanol intake (i.e., g/kg) should also be evaluated and recorded as noted in Basic Protocols 1 and 3.
-
10
Collect BAC data (see Support Protocol 1) as necessary throughout the drug-treatment phase.
Basic Protocol 5: Train Rats To Initiate Binge-Like Ethanol Responding in an Operant Situation
The major utility of this protocol is that it produces binge-like patterns of ethanol self-administration by allowing rats to respond for sweetened ethanol vs. water, while controls respond for sweetened vehicle vs. water. This allows between-subjects testing for the effects of pharmacological agents on self-administration of ethanol relative to its effects on a similarly flavored ethanol-free reinforcer, and simultaneous within-subjects testing of pharmacological effects on consumption of experimental solutions vs. water. Following are guidelines the authors have used (Ji et al., 2008), although this procedure could be adjusted to better serve the goals of a particular study (e.g., changing the sweetener concentrations to maximize alcohol binge responding or to match response rates in alcohol binge groups vs. controls). Binge models that utilize rats selectively bred for high alcohol preference will likely use a different protocol than the one described below (Bell et al., 2006).
Materials
Rats
Glucose-saccharin (“Supersac”) solution: 3% glucose + 0.0125% saccharin in water
Sweetened Alcohol: 10%(w/v) Ethanol + 3% glucose + 0.0125% saccharin in water
Begin initiation phase
-
1
One week prior to experiment, handle 20 rats daily and allow them to become acclimated to the vivarium and light cycle (a reversed 12-hr light/dark cycle is ideal). House animals as described in Basic Protocol 1.
Training and experimental sessions should take place at the start of the dark cycle.
-
2
Provide food ad libitum to the rats; the only exception is that no food should be available during the 30-min operant session. To begin the initiation phase, place only the right lever (Fig. 9.12.1B), dipper assembly, and fluid delivery system (i.e., trough; Fig. 9.12.1C) in the operant chamber. Fill the liquid reservoir with Supersac solution.
-
3
Steps 3 and 4 are the same as described in Basic Protocol 1.
-
5
Once the testing parameters have been specified, remove the rat from its home cage and place it in the operant chamber for the 30-min session. Close the door to the operant chamber and then close the door to the sound-attenuated chamber (the animal will be visible through the peep-hole in the sound-attenuated chamber; Fig. 9.12.1D). Using the operant software, set the maximum number of rewarded responses at 200 (i.e., 20 ml), after which the lever retracts. During the 30-min session, simply observe the rat. All rats should be given 3 operant sessions responding for supersac on an FR-1 schedule in a single-lever situation.
-
6
Following 3 days of single-lever training, give rats 3 more sessions of responding for supersac vs. water on FR-1 schedule in a 2-lever situation (i.e., introduce the left lever into the chamber for these and all subsequent operant sessions). Again, allow rats a maximum of 200 responses on either of the two levers, after which levers are retracted and are no longer available.
-
7
Divide rats into two groups that are counterbalanced for supersac responding.
For example, match the two highest responders and randomly assign one of them to group A, then match the next two highest responders and randomly assign one of them to Group A, etc. Then randomly assign one of the two binge conditions to Group A and the other to Group B. One group will be the alcohol binge group and the other will be the control binge group.
-
8
Place rats into operant chambers daily for 30-min sessions. Rats in the alcohol binge group will lever press for Supersac + ethanol cocktail vs. water, and rats in the control binge group will lever press for Supersac vs. water in a 2-lever situation until rats exhibit reliable responding (i.e., intakes are ±20% across 3 consecutive days). This is likely to require anywhere from 10-15 operant sessions.
-
9
Once rats have exhibited stable responding, collect tail blood samples 15 min after the end of the operant session to determine BACs (see Support Protocol 1). Tail blood samples should also be collected from control binge rats (although BACs must be zero) to account for the stress of this procedure in both groups. This step is critical for this procedure because NIAAA defines “binge” drinking patterns by BACs achieved in short drinking periods. The NIAAA cut-off for binge drinking is a BAC of 80 mg/dl.
Commentary
Background Information
Several procedures have been successfully used to initiate oral ethanol intake in rats. The most widely used and accepted method in the alcohol field is the sucrose-fading technique developed by Samson (1986, 1987). Samson (1987), as well as others (Petry and Heyman, 1995), has validated this procedure for limited access (i.e., 30 min) and more prolonged exposure periods. Subsequent modifications to the procedure wherein non-food and water deprived rats trained to initiate ethanol responding are allowed to obtain water or ethanol by responding at one of two levers, has produced an operant choice paradigm that can be used to investigate the neuropharmacological bases of alcoholism (Weiss and Koob, 1991). Some of the important measures incorporated in this model include: (1) maintenance of responding for ethanol as a measure of ethanol reinforcement; (2) an index of preference for ethanol over water, independent of the absolute amounts of ethanol consumed; (3) a control for nonspecific drug effects on ingestive behaviors; and (4) dissociation of the pharmacological motivation from consummatory processes (see Weiss and Koob, 1991).
A number of laboratories have reported pharmacologically relevant ethanol concentrations in rats using other alcohol initiation procedures (see Linseman, 1987; Hubbell and Reid, 1990; June et al., 1994). For example, the ascending two-bottle water choice paradigm (often referred to as a “home cage” procedure) is capable of producing ethanol intake levels in outbred rats that resemble operant conditioning (Linseman, 1987; June et al., 1994). Unfortunately, the investigator has little control over the contingent relations between responses and ingestion in this procedure. As in studies of other abused drugs, the appropriate instrument is the operant conditioning chamber where the contingency between responding and drinking can be specified and the volume of liquid ingested per completed schedule unit can be controlled. The operant methodology is especially important in analyzing complex dose-response relationships of a test agent (June et al., 1998a,b) and when examining the effects of a test agent on ethanol and another concurrently available reinforcer (Samson and Grant, 1985; Petry, 1997; June et al., 1998a, 1999, 2001). Hence, the operant paradigm has a number of advantages over simple home cage drinking procedures.
While a number of laboratories are currently evaluating the efficacy of pharmacological treatments on scheduled-controlled responding for ethanol using the Samson (1987) fading procedure, studies in which controls are used for rate-dependency and the post-ingestional properties of the test solutions are generally lacking in the alcohol literature. Many of these published reports are very difficult to interpret in relation to the selectivity of a test agent reducing alcohol drinking on the one hand, and the degree to which inferences are made from these studies to neural mechanisms of action on the other. The issue of selectivity on alcohol drinking should be a very important concern among researchers given that most studies in the literature report that test compounds “all” reduce motivated responding for alcohol. Thus, to eliminate confounding of results/interpretation, it is important to use rigorous control procedures in alcohol-self-administration studies.
Neuroanatomical controls
Although the four Basic Protocols in this unit incorporate rigorous behavioral control procedures, it is important that neuroanatomical controls are also used when making inferences about CNS mechanisms that regulate ethanol-seeking behaviors. This is rarely performed in many of the published reports in the alcohol field. CNS substrates are primarily studied in alcohol research using microinjection procedures (i.e., intracerebral drug delivery; Samson and Hodge, 1996; McBride and Li, 1998). The authors recommend two types of neuroanatomical control groups that will assist in data interpretation.
A researcher may use a “region-specific” control wherein a second infusion is performed 1 to 2 mm away from the designated brain site. The second infusion may be administered rostral, caudal, anterior, or dorsal to the designated brain site. Studies that attempt to compare the anterior and posterior ventral tegmental area (VTA), or the nucleus accumbens (NACC) shell and core, or the NACC and ventral striatum are also examples of “region-specific” comparisons. See Nowak et al. (1998) or June et al. (1998b) for illustrative examples of such control procedures.
A researcher may also use an entirely different locus as the control site. This type of control is referred to as an “alternate region” control. The alternate region control is particularly important when different neuronal populations, or subunit configurations are of interest. See June et al. (2001) for illustrative examples of such control procedures.
Critical Parameters and Troubleshooting
Training of outbred versus rats selectively bred to prefer alcohol
Alcohol-preferring rats are animals that have been bred over successive generations to self-administer 10% alcohol (and higher concentrations) in preference to water using a two-bottle choice situation (Lumeng et al., 1995; McBride and Li, 1998). Despite selective breeding, it is still necessary to train these rats to lever press for alcohol in the operant chamber. The question arises as to whether the sucrose-fading procedure is necessary to initiate operant responding in selected rat lines. While the fading procedure is not necessary with certain lines (e.g., P rats), it appears essential with others (e.g., HAD-1 and HAD-2). Nevertheless, to initiate rapid lever-press responding in the operant chamber, the authors use the fading procedure with all selected rat lines. Most alcohol-selectively bred rat lines will initiate ethanol-maintained responding quicker than outbred rat lines. For example, ethanol-maintained responding can be initiated in P rats in 5 to 10 days using an ultra-short fading procedure. The P rats can reach complete stabilization around days 15 to 20. In contrast, outbred rats may require 35 to 40 days to reach complete stabilization on an FR-4 schedule. If required by time constraints, ethanol self-administration behavior can be more quickly stabilized in outbred rats by reducing the response requirement (e.g., FR-1 schedule in lieu of FR-4 schedule). A quick formula to use in some selected lines is to reduce all the steps required to initiate alcohol drinking by ∼50%. For example, during the beginning of Basic Protocol 1, a researcher would deprive outbred rats of fluid for 2 to 4 days while initiating saccharin drinking, however, with the P rat, this could be reduced to 1 to 2 days. Also, in contrast to P rats, HAD-1 or HAD-2 lines (Lumeng et al., 1995) do not initiate ethanol-maintained responding in <30 days. A 25- to 30-day period (which includes partial stabilization) is also typical for ethanol initiation of responding in outbred rats (Samson, 1987; Weiss and Koob, 1991; June et al., 1998a). Furthermore, the absolute amount of ethanol consumed is also greater in alcohol-selectively bred rat lines (Weiss and Koob, 1991; June et al., 1998a). Even Wistar and Sprague-Dawley rats differ in the rate in which they acquire operant responding for alcohol, with the former tending to lever-press sooner than the latter (June, unpub. observ.). In outbred rats, a 30-min session is typically sufficient to obtain an adequate amount of lever responding to reliably assess a test agent. In selected rat lines, a 60-min operant session is often employed to obtain a greater number of responses and to increase the overall power of the statistical analyses. The length of the operant session might also vary according to the pharmacokinetic profile (e.g., half-life) of the pharmacological agent to be tested, as well as the anticipated effects (e.g., time course) of that agent on operant ethanol drinking.
Once trained, is there a need for retraining?
A frequently asked question from investigators learning operant self-administration procedures is whether once animals are trained, is there a need for retraining? Or, do the animals simply forget the operant if not placed in the operant chamber for some period of time? The answer to both questions is an unequivocal no. It is strongly recommended, however, that animals be re-stabilized following a period of absence from the operant chambers. Furthermore, it is necessary to establish a new baseline for operant behavior following manipulations of the history of the animal (e.g., dependence induction in Support Protocol 3) or of the operant response requirement (e.g., change to progressive ratio response requirement in Support Protocol 2). Nevertheless, one of the advantages of the rather extended procedures in each of the protocols in this unit is the response reliability once the animals have fully learned the operant task. This makes operant analysis of behavior a robust and powerful behavioral tool for studying classical motivated behaviors (e.g., eating, drinking) and drug-seeking behaviors.
Frequent procedural errors made in training rats
Inconsistency or missed training days within a given protocol prevents the animals from acquiring robust and reliable responding for a given reinforcer. Nonetheless, once animals have acquired the basic operant response behavior, short scheduled periods without operant access (e.g., weekends) can elevate baseline operant ethanol responding by animals upon return to operant chambers. Attempting to shorten the training protocols also prevents the animals from acquiring robust and reliable response profiles. Although rats will still show some degree of lever pressing for the reinforcer of interest, the extended training protocols are required to obtain optimal responding (i.e., reliable, high rates of responding).
Anticipated Results
If a test compound causes a dose-dependent reduction of ethanol-maintained responding (see Basic Protocol 1), but not saccharin-maintained responding (see Basic Protocol 2), or produces a concomitant increase in saccharin responding (see Basic Protocol 3), this will be taken as evidence of the potential selectivity of a test agent on ethanol reward processes. A flat dose-response profile of a test agent would also be indicative of effects on ethanol-maintained responding that are not specific for ethanol. The concurrent procedure proposed in the present unit permits response rates of ethanol and an alternative reinforcer to be equated/matched prior to any pharmacological manipulation. When response rates are matched prior to drug administration, the issue of rate dependency can be ruled out as a possible explanation of the drug treatment (Samson et al., 1989). A concurrent schedule where ethanol and the alternative reinforcer are matched also permits determination of whether ethanol-reinforced responding or responding for the alternative reinforcer (e.g., saccharin) is more sensitive to the effects of a pharmacological treatment, given that the two reinforcers appear to be equally efficacious (see Basic Protocol 3). An evaluation of the post-ingestional properties of reinforcing solutions are also important since the caloric value of the reinforcer itself could take on secondary reinforcing properties that might sustain or maintain scheduled reinforced responding (see Basic Protocol 4). While it is predicted that rates of responding will be greater for the sweetened alternative reinforcer compared with the sweetened ethanol cocktail in Basic Protocols 4 and 5 (see Heyman and Oldfather, 1992; Petry and Heyman, 1995), the investigator will be able to make inferences about a given pharmacological treatment when the post-ingestional properties of a drug and non-drug reinforcer are matched for either calories or flavorants. On the other hand, while Basic Protocol 3 has the advantage of having similar basal response rates, a disadvantage is that the two reinforcers have different post-ingestional properties (i.e., caloric content versus no caloric content). Sometimes difficulties arise in trying to match behaviors with qualitatively different reinforcers. For example, a researcher may find it difficult to match response rates for two qualitatively different reinforcers with certain rat lines and strains. Some pharmacological agents may have similar effects on operant responding for ethanol and an alternative reinforcer that are not related to caloric content of solutions. In these situations, effects may be attributable to non-specific effects on locomotor activity, a hypothesis that can be tested easily with one of several behavioral assays that measure locomotor activity of rats (UNIT 8.1) or motor coordination (UNIT 8.12). A final consideration is changes in body weight that occur over what is very often an extended time course in protocols for ethanol self-administration. Making comparisons between groups of rats that have different histories (e.g., extensive experience responding for caloric versus non-caloric solutions in Basic Protocols 1 and 2, or being made dependent on alcohol in Support Protocol 3) can be complicated by different rates of weight gain in those groups. The simplest way to account for this discrepancy is to convert operant response and raw consumption data to account for body weight in the denominator (for example, grams ethanol consumed per kilogram body weight).
BAC analyses
BAC data may not always correlate with response rates or actual absolute alcohol intake, obtained by multiplying response rate × 0.10 ml dipper volume (see Fig. 9.12.2). Some explanations for this are as follows: operant responding accounts for ∼50% to 60% of the variance in BACs. The remainder of the variance could be accounted for by several variables, such as pattern of responding and consumption of the alcohol solution, circadian time point at which testing occurs, variations in food intake prior to the operant session, and/or the body weight to fat ratio differences among rats (June et al., 1998b, 2001).
Pharmacological Manipulation (Example 1)
Analysis of responding for the total number of lever presses during a 60-min operant session
Nucleus accumbens (NACC) as an experimental target region
Figure 9.12.5 illustrates the effects of nalmefene (10 to 60 ug), a nonselective opioid antagonist, following unilateral injection in the nucleus accumbens (NACC) core and shell on responding maintained by 10% ethanol and 0.025% saccharin during a 1-hr operant session. The topography of the shell and core are well documented (Stratford and Kelley, 1997). Note that basal ethanol and saccharin-maintained responding are similar in the no-injection control condition (i.e., BL1 = baseline 1). The BL1 represents the average of 5 days prior to the randomized drug treatment administrations. Similar response rates for ethanol and saccharin responding are also apparent following the artificial cerebral spinal fluid (aCSF) control condition. A single factor ANOVA examining the effect of drug treatment (i.e., aCSF, 10, 40, 60 ug) was conducted on both the ethanol and saccharin data. The results revealed that, compared with the aCSF and no-injection control condition (e.g., BL1, BL2), ethanol-maintained responding was dose-dependently reduced with the 10- to 60-ug treatments by 29% to 74% of control levels [F(5,40) = 3.5, p < 0.0101]. Newman Keuls post-hoc analyses confirmed the suppression with the two higher doses (p < 0.01). The lower portion of Figure 9.12.5 shows that, unlike the effects seen on ethanol-maintained responding compared with the aCSF and no-injection control conditions (e.g., BL 1, BL2), saccharin-maintained responding was not altered [F(5,40) = 0.526, p > 0.755]. Following the drug treatment phase, a second no-injection control condition (i.e., BL2 = baseline 2) should also be determined in a manner similar to BL1. Typically, a 3- to 4-day washout period is sufficient before restabilizing the rats on a 5-day baseline phase. The BL2 condition not only permits an investigator to determine if a drug treatment has residual effects, it also provides an indication of the reliability of responding. The BL1 and BL2 measures are often similar and can be pooled prior to any primary statistical analyses (see Fig. 9.12.3).
Figure 9.12.5.
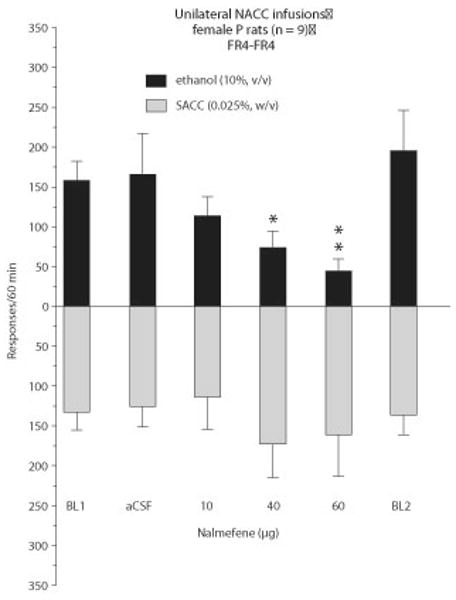
Effects of nalmefene (10 to 60 ug) following unilateral injection in the nucleus accumbens (NACC) core and shell on responding maintained by 10% ethanol and 0.025% saccharin during a 1-hr operant session. Five min after the microinfusions, rats were placed in the operant chamber for a 60-min session. Data are shown as mean (+/−S.E.M.) (n = 9). *P <0.05, **P <0.01, nalmefene versus artificial cerebrospinal fluid (aCSF) and the no-injection control conditions (e.g., BL = baseline 1; BL2 = baseline 2) by ANOVA and post-hoc Newman-Keuls test.
Ventral tegmental area (VTA) as a control target region
While some researchers have reported that certain opioid receptors in the VTA play a role in ethanol self-administration (Herz, 1997; McCullough et al., 1998), others have questioned the significance of opioid receptors in the VTA in regulating ethanol responding (Hyytia and Kiianmaa, 2000) and intake of palatable solutions such as sucrose and saccharin (Ragnauth et al., 1997). Hence, the VTA represents an ideal control locus that may be used for comparison with the NACC. In the study described in the previous section, a separate group of rats was unilaterally infused with nalmefene into the VTA (i.e., an alternate neuroanatomical control locus). Data analysis indicated that intra-VTA infusions of nalmefene produced a non-specific suppression of operant responding maintained by ethanol and saccharin, contrary to the ethanol-specific suppressive effect seen in NACC.
Interpretation
Examination of the total operant session data (i.e., 60 min) show that opioid receptors in the NACC regulate ethanol- but not saccharin-maintained responding. In contrast, antagonism of opioid receptors in the VTA appears to produce non-specific suppressive effects on operant responding.
Time course analysis of responding at the six 10-min intervals of the 60-min operant session
Time-course analyses are particularly important in making inferences as to whether a compound interferes with the acquisition versus the maintenance of ethanol responding. Typically, compounds that interfere with acquisition reduce ethanol-motivated behaviors during the initial 5- or 10-min intervals. In contrast, compounds that interfere with maintenance will reduce responding during later periods in the operant session (e.g., 20- to 30-min intervals). Nonselective suppression is often indicated when suppression is observed during earlier intervals (e.g., 5- or 10-min intervals) for both ethanol and the alternative solution (e.g., saccharin, sucrose). However, it should be noted that often it is very difficult to unequivocally evaluate whether a compound reduces ethanol responding during the earlier versus later intervals. This difficulty stems from the fact that many variables may interact in determining the time course of a drug on ethanol-maintained responding across an experimental session. Some of these may include the following: (1) the half-life of a compound, (2) the CNS site of injection, (3) whether the CNS infusion is unilateral or bilateral, (4) the alternative reinforcer being used, and (5) the rat line employed. Despite these factors, when complete dose-response evaluations are performed and the controls indicated in this unit are employed, optimal interpretations can be made regarding the time course effects of most compounds.
NACC as an experimental target region
Figure 9.12.6 illustrates cumulative time-course graphs for the ethanol and saccharin responding, respectively, from the data depicted in Figure 9.12.5. A two-way ANOVA with repeated measures over both time interval and dose was performed. Again, for ease of interpretation, separate analyses were performed on the ethanol and saccharin data. The cumulative 6 × 5 analysis was as follows for ethanol: interval, [F(5,48) = 12.72, p < 0.0001]; dose, [F(5,240) = 136.72, p < 0.0001]; interaction [F(25,240) = 9.16, p < 0.0001]. The cumulative 6 × 5 analysis was as follows for saccharin: interval, [F(5,48) = 6.76, p < 0.0001]; dose, [F(5,240) = 17.62, p < 0.0001]; interaction [F(25,240) = 6.34, p < 0.0001]. Post-hoc Newman Keuls tests were conducted against the aCSF and BL control conditions. Figure 9.12.6A shows unilateral microinjection of nalmefene (10 to 60 ug) suppressed ethanol-maintained responding primarily during the 20- to 30-min intervals (p ≤0.05), and did not appear to suppress responding during the initial 10-min interval (p > 0.05), except at the highest dose (e.g., 60 ug; p < 0.05). The suppression was sustained throughout the remainder of the 60-min interval with all three doses. Figure 9.12.6B shows that unlike the time course profile seen for ethanol, none of the nalmefene doses suppressed saccharin-maintained responding across the six intervals (p > 0.05). However, elevations were seen across the 60-min session with the 40- μg dose, albeit, only the 20-through 60-min intervals were statistically significant relative to the control conditions (p ≤0.05).
Figure 9.12.6.
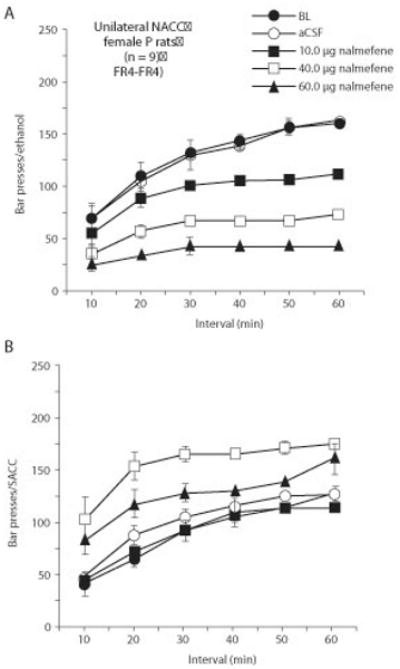
Cumulative response profiles in the NACC rats (n = 9) for (A) ethanol- and (B) saccharin-maintained responding for the nalmefene treatment conditions (e.g., 10 to 60 ug) when compared with the control conditions (e.g., aCSF, BL). The data are redrawn from Figure 9.12.3. Asterisks are omitted from the cumulative time course figures for clarity.
VTA as a control target region
The cumulative response profiles for ethanol- and saccharin-maintained responding (not shown) were also examined following unilateral infusion of nalmefene into the VTA. A two-way ANOVA with repeated measures over both time interval and dose was performed. Except for the 20-min interval, the 40-μg dose of nalmefene suppressed ethanol-maintained responding throughout the 60-min session (p ≤0.05), while the 30-μg dose suppressed responding only from the 40- through 60-min intervals (p ≤0.05) relative to the BL condition. The 40-μg dose suppressed saccharin-maintained responding from the 30- through 60-min interval compared with the two control conditions (p ≤0.05). No other treatment effects altered saccharin-maintained responding.
Pharmacological Manipulation (Example 2)
Analysis of binge-like operant ethanol responding
Establishing binge-like operant self-administration
Binge-like ethanol self-administration is defined by NIAAA as an excessive pattern of alcohol drinking that produces BACs greater than 0.08 g% within a two-hour period. Before undertaking any pharmacological testing in rats, it is important to confirm that rats are achieving the criterion BAC level. In the rats described by Figure 9.12.7, tail blood samples were collected and BACs determined on days 13 and 16 of the baseline period. Eight of the 12 animals in the alcohol binge group achieved BACs of greater than 0.08 g% on at least one of those two days (data not shown). The mean BAC for all 12 animals in the alcohol binge group across two days was 0.084 g%, which is higher than the BAC criterion for binge drinking in humans. Figure 9.12.7 illustrates binge-like ethanol self-administration following systemic injection with a selective serotonin/norepinephrine reuptake inhibitor (SSNRI; duloxetine), an opioid receptor antagonist (naltrexone), and a CRF1-receptor antagonist (MPZP). SSRIs are often used to treat depression, and low serotonin function is related to impulsivity in humans; naltrexone suppresses consumption of alcohol and other positive reinforcers in humans and rats; and CRF1-receptor antagonists selectively suppress alcohol drinking in dependent populations, presumably via anxiety-reducing actions. Therefore, it was hypothesized that duloxetine and naltrexone would suppress binge consumption of ethanol and perhaps also of sweet solutions, whereas MPZP would not affect consumption of either fluid.
Figure 9.12.7.
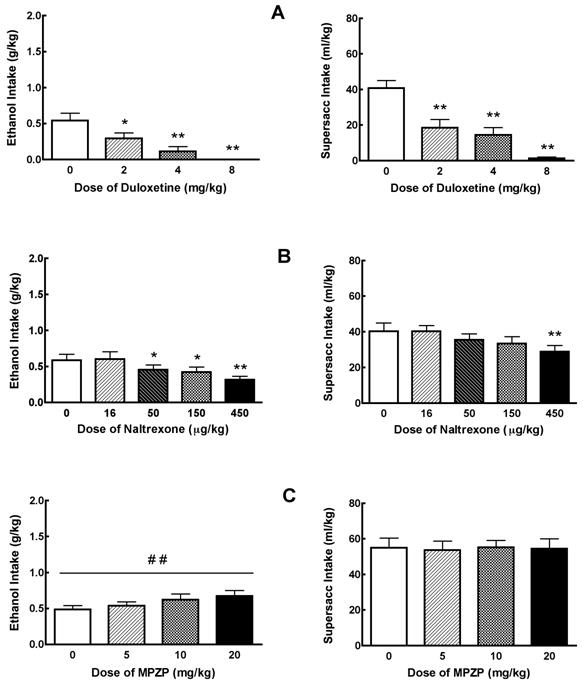
Mean ±SEM alcohol intake (g/kg) by animals in the alcohol binge group (n=11; left panel) and supersac intake (ml/kg) by supersac controls (n=12; right panel) following pretreatment with (A) one of four doses (0, 2, 4, 8 mg/kg) of duloxetine (i.p. injection 40 min prior to drinking session), (B) one of five doses (0, 16, 50, 150, 450 μg/kg) of naltrexone (s.c. injection 30 min prior to drinking session), or (C) one of four doses (0, 5, 10, 20 mg/kg) of MPZP (s.c. injection 60 min prior to drinking session). *p< 0.05, **p< 0.01 significant difference from vehicle condition.
Effects of duloxetine on operant binge-like drinking
Duloxetine pretreatment dose-dependently reduced intake of sweetened alcohol solution by animals in the alcohol binge group, F (3,33) = 12.62, p<0.01 (Figure 9.12.7A; left panel). Post hoc analysis revealed that all doses significantly suppressed binge-like alcohol intake (p<0.05 in all cases). Duloxetine also suppressed supersac intake by controls F(3,33) = 28.54, p<0.01. Post hoc analysis revealed that all doses significantly suppressed supersac intake (p<0.01 in all cases) in those rats (Figure 9.12.7A; right panel). Water intake was not affected in either of the two groups (data not shown).
Effects of naltrexone on binge-like drinking
Naltrexone pretreatment dose-dependently reduced intake of sweetened alcohol solution by animals in the alcohol binge group, F (4,40) = 7.95, p<0.01 (Figure 9.12.7B; left panel). Post hoc analysis revealed that 50, 150, and 450 μg/kg significantly suppressed binge-like alcohol intake (p<0.05 in all cases). Naltrexone also suppressed supersac intake by controls F (4,44) = 4.43, p<0.01, though only at the highest dose; post hoc analysis revealed that only the 450 μg/kg dose suppressed supersac intake (p<0.05) in those rats (Figure 9.12.7B; right panel). Water intake was not affected in either of the two groups (data not shown).
Effects of MPZP on binge-like drinking
Analyses of variance revealed no effect of MPZP pretreatment on consumption of sweetened alcohol solution, supersac or water by either of the two groups (Figure 9.12.7C). However, the analysis did reveal a significant upward linear trend of MPZP dose (Figure 9.12.7C) on binge-like alcohol drinking, F (3,43) = 2.69, p<0.01, but not on supersac drinking (p>0.05). Water intake was not affected in either of the two groups (data not shown).
Interpretation
Duloxetine suppressed operant responding for both ethanol and supersac; naltrexone did the same although much lower doses of the drug affected ethanol drinking relative to supersac drinking; MPZP did not affect consumption of ethanol or supersac. Therefore, this model of binge-like ethanol self-administration is highly sensitive to compounds that suppress drinking via opioidergic (naltrexone) and serotonergic (duloxetine) mechanisms, but not sensitive to compounds that suppress drinking via decreases in CRF activity (MPZP). The fact that duloxetine and naltrexone also suppressed supersac consumption suggests that both agents are blocking positive reinforcing effects of the drinking solutions, and the heightened sensitivity to naltrexone in binge-like ethanol drinkers suggests neural plasticity in those animals.
Additional Measures (optional)
Cumulative record dose-response profiles
To aid in data interpretation, a computer record of the cumulative dose-response data can be generated to evaluate the effects of a drug pretreatment during Basic Protocols 1 to 4. The cumulative dose-response record software can be purchased from Coulbourn or Med Associates. Analyses of ethanol, saccharin, sucrose, or cocktail response profiles should permit an evaluation of how a pretreatment alters the slope of the cumulative record in comparison with no injection control or saline control conditions. Slopes can be determined for the various treatment conditions using standard regression equations. A cumulative dose-response record is particularly helpful in interpreting selectivity of a drug treatment when animals are being tested on a concurrent schedule (see June et al., 1998b). For example, if a drug treatment produces a dose-dependent reduction in the slope of the cumulative record for ethanol and a concomitant dose-dependent increase in the slope for saccharin responding (e.g., see Basic Protocol 3), it is probable that the drug is selectively reducing ethanol-maintained responding. Selectivity on ethanol responding can also be hypothesized if an agent produces a dose-dependent reduction in the slope of the cumulative record for the ethanol-saccharin cocktail (e.g., see Basic Protocol 4), while simultaneously increasing the slope for the sucrose reinforcer.
Evaluation of nonspecific locomotor effects on ethanol-reinforced responding
In addition to evaluating whether a pretreatment reduces responding for the control fluid (e.g., saccharin, sucrose), nonspecific effects of a test agent can also be evaluated on locomotion (UNIT 8.1) in the operant chamber (Coulbourn Instruments), motor coordination (UNIT 8.12), or open field (Ascu Scan Electronics). An evaluation of locomotion in the operant box may have advantages over its evaluation in the open field in that data are collected while animals are performing the operant. In addition, since many of the test agents used by investigators are research compounds, and are not synthesized in large quantities, evaluation of locomotor activity while the agents are being assessed for effects on operant responding would preclude their evaluation in the open field.
Time Considerations
Basic Protocol 1
The minimum amount of time (excluding the 1-week handling period) needed to successfully train rats to lever press for 10% ethanol is ∼35 to 40 days (however, see Critical Parameters). This period includes full stabilization to the point where rats are ready to be used in neuropharmacological research studies. The number of rats that can be trained in a single experiment will depend on the number of available operant chambers and the number of investigators assigned to the operant experiments. Because experimental sessions are only 30 or 60 min, several squads of rats can be tested in a single day. It is strongly recommended that during the acquisition phase, rats undergo training in the operant chamber for at least 5 to 6 days a week. Once the rats are fully trained (which also includes stabilization), this can be reduced to 4 or 5 days a week; however, during drug treatment periods, rats should be tested in the operant chambers daily. This additional time is needed to assure the investigator that the residual effects of a drug treatment have dissipated prior to a second treatment. In most operant experiments, animals are tested with a pharmacological treatment at least two to three times a week, with the washout periods ranging between 48 and 72 hr. If an agent has a rather long half-life, rats may be tested only once a week. To accurately interpret the effects of a drug manipulation in any of the protocols discussed in this unit, it is imperative that dose-response investigations be conducted. This also applies to microinfusion studies (see Figures 9.12.5 and 9.12.6) where only a limited number of infusions can be performed so that the CNS tissue within the area of the guide cannula remains viable.
The incorporation of dependence induction procedures (Support Protocol 3) or changes in operant response requirements (Support Protocol 2) impose additional time requirements on ethanol self-administration protocols. Once rats are made dependent on alcohol, testing occurs either at acute withdrawal time points when BACs are approaching zero, or at protracted abstinence time points, well after physical signs of withdrawal have dissipated. In these situations, the frequency of testing will be determined by experimental parameters, but should not exceed three tests per week. Tests for the effects of pharmacological agents on PR operant responding generally follow a timeline similar to that used for FR protocols. The support protocols in this unit often extend the overall time course of an experiment, and as such might introduce new considerations into the experiment, for example, attrition of subjects due to age-related health complications.
Basic Protocol 2
The minimum amount of time needed to successfully train rats to lever press for 0.025% to 0.10% saccharin is ∼14 to 21 days (however, see Critical Parameters). This period includes full stabilization to the point where the rats are ready to be used in neuropharmacological research studies. Unlike ethanol-maintained responding, most animals (i.e., outbred rats) eagerly lever press for palatable solutions such as saccharin and sucrose, especially when presented as the only available solution in the operant chamber. The time considerations for Basic Protocol 1 are also applicable to Basic Protocol 2.
Basic Protocol 3
The minimum amount of time needed to successfully train rats to lever press for a concurrent presentation of 10% ethanol and 0.025% saccharin is ∼60 days (however, see Critical Parameters). This period includes full stabilization to the point where rats are ready to be used in neuropharmacological research studies. Unlike responding for ethanol or saccharin alone, training a large number of rats to respond for concurrently presented reinforcers at similar rates can be very time-consuming. This primarily reflects the fact that equating reward efficacy (i.e., strength of two qualitatively different reinforcers) can be difficult for some rats to achieve. The additional information discussed under the time consideration section of Basic Protocol 1 is also applicable to Basic Protocol 3.
Basic Protocol 4
The minimum amount of time needed to successfully train rats to lever press for a concurrent presentation of an isocaloric 10% ethanol and 14.2% sucrose solution is ∼48 days (however, see Critical Parameters). This period includes full stabilization to the point where rats are ready to be used in neuropharmacological research studies. Unlike responding for the concurrent solutions of Basic Protocol 3, training rats to respond for the concurrent presentations of ethanol and sucrose requires considerably less effort and time. This primarily reflects the fact that it is not necessary to train the rats to lever press at similar rates to obtain the two reinforcers. The additional information discussed under the time consideration section of Basic Protocol 1 is also applicable here for Basic Protocol 4.
Basic Protocol 5
At the beginning of the initiation training, all rats are typically given 3 days access to supersac in a single-lever situation, followed by 3 days access to supersac vs. water in a two-lever situation. At that point, rats are assigned to alcohol binge or control binge groups and subsequently administered daily operant sessions until responding for the experimental solution has stabilized. This process typically takes about 15 sessions. A peripheral but important advantage of this procedure is the very short time period needed to train and stabilize rats relative to other ethanol self-administration procedures.
Contributor Information
Harry L. June, Email: hjune@psych.umaryland.edu.
Nicholas W. Gilpin, Email: nwgilpin@gmail.com.
Literature Cited
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biol. 2006;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Carmona G, May SA. Modifying drug-reinforced behavior by altering the economic conditions of the drug and a non-drug reinforcer. J Exp Anal Behav. 1991;18:361–376. doi: 10.1901/jeab.1991.56-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res. 2008;32:1688–96. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Oldfather CM. Inelastic preference for ethanol in rats: An analysis of ethanol's reinforcing effects. Psychol Sci. 1992;3:122–130. [Google Scholar]
- Hubbell CL, Reid LD. Opioids modulate rat's intake of alcoholic beverages. In: Reid LD, editor. Opioids, Bulimia and Alcohol Abuse and Alcoholism. Springer-Verlag; New York: 1990. pp. 145–191. [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2000;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABAA antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a CRF-1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Hughes RW, Spurlock KR, Domangue KR, Lewis ML. Ethanol self-administration in freely-feeding rats: Effects of Ro15-4513 alone, and in combination with flumazenil (Ro15-1788) Psychopharmacology. 1994;115:332–339. doi: 10.1007/BF02245074. [DOI] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Lawrence A, Thomas A, Cummings R, Williams L, McCane SL, Durr LF, Mason D. The opioid receptor antagonist nalmefene reduces alcohol motivated behaviors: Preclinical studies in alcohol preferring (P) and outbred Wistar rats. Alcohol Clin Exp Res. 1998a;22:2174–2185. [PubMed] [Google Scholar]
- June HL, Torres L, Cason CR, Hwang BH, Braun MR, Murphy JM. The novel benzodiazepine inverse agonist RO19-4603 antagonizes ethanol motivated behaviors: Neuropharmacological studies. Brain Res. 1998b;784:256–275. doi: 10.1016/s0006-8993(97)01380-2. [DOI] [PubMed] [Google Scholar]
- June HL, McCane S, Zink RW, Portoghese P, Li TK, Froehlich JC. The delta 2-opioid receptor antagonist naltriben reduces motivated responding for ethanol. Psychopharmacology. 1999;147:81–89. doi: 10.1007/s002130051145. [DOI] [PubMed] [Google Scholar]
- June HL, Harvey SC, Foster KL, McKay PF, Cummings RC, Garcia M, Mason D, Grey C, McCane S, Williams L, Johnson TB, He X, Rock S, Cook JM. GABAA-receptors containing α5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: An extended ethanol reward circuitry. J Neurosci. 2001;21:2166–2177. doi: 10.1523/JNEUROSCI.21-06-02166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng L, Murphy JM, McBride WJ, Li TK. Genetic influences on alcohol preference in animals. In: Begleiter H, Kissin B, editors. The Genetics of Alcoholism. Oxford University Press; New York: 1995. pp. 165–201. [Google Scholar]
- Linseman MA. Alcohol consumption in freely feeding rats: Procedural, genetic and pharmacokinetic factors. Psychopharmacology. 1987;92:254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McCullough DE, Mosemiller AK, Zhou FC, Portoghese PS, Froehlich JC. Infusion of a delta opioid antagonist into the ventral tegmental area attenuates alcohol drinking in P rats. Alcohol Clin Exp Res. 1998;22:45. [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABAA receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacol. 1998;139:108–116. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Petry NM, Heyman GM. Behavioral economic analysis of concurrent ethanol/sucrose and sucrose reinforcement in the rat: Effects of altering variable-ratio requirements. J Exp Anal Behav. 1995;64:331–359. doi: 10.1901/jeab.1995.64-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol. 1997;5:183–194. [PubMed] [Google Scholar]
- Ragnauth A, Ruegg H, Bodnar RJ. Evaluation of opioid receptor subtype antagonist effects in the ventral tegmental area upon food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1997;767:8–16. doi: 10.1016/s0006-8993(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Campbell UC, Cosgrove KP, Carroll ME. Naltrexone pretreatment decreases the reinforcing efficacy of ethanol and saccharin but not PCP or food under concurrent progressive-ratio schedules in rhesus monkeys. Psychopharmacology. 1999;147:81–89. doi: 10.1007/s002130050854. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol-maintained behavior: A comparison of animal models and their implication to human drinking. In: Thompson T, Dews PB, Barret J, editors. Neurobehavioral Pharmacology: Advances in Behavioral Pharmacology. Vol. 6. Lawrence Erlbaum Associates; New Jersey: 1987. pp. 221–248. [Google Scholar]
- Samson HH, Grant KA. Chlordiazepoxide effects on ethanol self-administration: Dependence on concurrent conditions. J Exp Anal Behav. 1985;43:353–364. doi: 10.1901/jeab.1985.43-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Haraguchi M, Tolliver GA, Sadeghi KG. Antagonism of ethanol-reinforced behavior by the benzodiazepine inverse agonists RO15-4513 and FG 7142: Relationship to sucrose reinforcement. Pharm Biochem Behav. 1989;33:601–608. doi: 10.1016/0091-3057(89)90395-x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW. Neurobehavioral regulation of ethanol intake. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous Systems. CRC Press; New York: 1996. pp. 203–226. [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Koob GF. The neuropharmacology of ethanol self-administration. In: Meyer RF, Koob GF, Lewis MJ, Paul S, editors. Neuropharmacology of Ethanol. Birkhauser; Boston: 1991. pp. 125–162. [Google Scholar]


