Abstract
In LEW rats treated daily with variable doses of FK506 for 14 days and weekly thereafter, successful intestinal transplantation from fully allogeneic BN donors never was complicated by fatal GVHD. In contrast, with LEW-to-BN transplantation, rejection was difficult to control and GVHD developed after the end of the daily treatment. However, FK506 in high daily doses continued after the initial 14-day course could prevent this GVHD or even reverse it after allowing its onset. Further experiments did not clarify why the BN rat was an “easy” donor and “difficult” recipient. In unaltered animals the lymphocyte population of normal LEW rats had a higher proportion of T cells, fewer B cells, and a lower CD4:CD8 ratio than normal BN rats. However, one-way MLR reactions of the BN and LEW combinations were generally similar in either direction and not affected differently by the addition of FK506 to the medium. The two-way lymphocyte traffic from graft to host lymphoid organs and vice versa also was similar with BN-to-LEW and LEW-to-BN models. The BN rat may be a useful tool to investigate inadequately explained mechanisms of GVHD.
The prerequisites defined by Billingham (1) for the development of graft-versus-host disease are the presence of mature immunologically competent cells in the graft, sufficient time for these cells to react before they are rejected by the host, and important histocompatibility antigens in the recipient that are lacking in the transplant. Because of the large lymphoid component of the intestine, histopathologic findings in recipients of canine intestine (2, 3) and multivisceral grafts that included intestine (4) were thought but not proved to be explained by GVHD. Subsequently, GVHD and its consequences were delineated clearly by Monchik and Russell (5) in genetically controlled rat experiments in which semiallogeneic small bowel transplantation was from parent strain to F1 hybrid offspring who were incapable of rejecting the grafts but subject to their attack. Although the rat models emphasized the peril of a host-graft immunologic imbalance if this favored the graft, such laboratory experiments overstated the clinical threat of GVHD, which in patients has been minor compared with rejection (6–8)
A commonly used rat strain combination for experimental multivisceral and intestinal transplantation has been the fully allogeneic Brown Norway–to–Lewis model, in which the dominant immunologic response was rejection, either without (9) or with immunosuppression (10). Under FK506, chronic survival was the rule. In these rats (10) and humans (8, 11), the lymphoreticular cells of the intestine and its mesentery were largely replaced with those of the recipient, usually without GVHD. However, if the direction of the BN-LEW rat transplantation is reversed, using the LEW strain as donor instead of recipient, we have observed that both rejection and GVHD are resistant to immunosuppression, making it difficult to achieve survival or graft acceptance (12). In an effort to explain this difference, we have examined LEW -to-BN intestinal transplantation in greater detail, with particular attention to the distribution of the cells that have been shown in rats (10, 12), swine (13), and humans (7, 8, 11) to emigrate from intestinal grafts.
MATERIALS AND METHODS
Inbred male LEW (RT1l) and BN (RT1n) rats weighing 200–300 gm were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN), and were maintained in conventional animal facilities with standard diet.
In Vitro Immunologic Assessment (Unaltered Animals)
To see if there were obvious differences in the cellular immune apparatus of the BN and LEW strains, in vitro studies were made of lymphocytes from the various lymphoid organs (Table 1) of sacrificed unaltered animals. The Peyer’s patches were individually dissected and excised from the ileum and jejunum. The lymphoid tissues were minced into RPMI-1640 (Gibco, Grand Island, NY) followed by vigorous mechanical agitation and filtration. Peripheral blood mononuclear cells were isolated by centrifugation over a Ficoll-Hypaque gradient.
TABLE 1.
Lymphocyte subsets (% total) in different tissues of normal LEW and BN rats
| Strain | Tissue | HBSS | W3/25 (CDA) | OX 8 (CD8) | OX 19 | B cell (IgG) |
|---|---|---|---|---|---|---|
| LEW (n = 5) | Thymus | 0.72±1.07 | 91.02±4.33 | 88.06±8.06 | 96.30±2.23 | 2.98±0.74 |
| Spleen | 4.15±1.72 | 35.10±5.61 | 19.10±2.97 | 48.26±10.09 | 42.88±5.54 | |
| Cervical LN | 0.70±0.52 | 49.32±5.54 | 21.70±3.25 | 72.50±6.06 | 28.18±6.49 | |
| Mesenteric LN | 0.56±0.32 | 50.32±4.57 | 15.88±3.39 | 69.64±5.93 | 32.28±1.63 | |
| Blood | 0.78±0.31 | 50.62±1.72 | 21.78±2.94 | 66.00±3.86 | 21.60±5.21 | |
| Peyer’s patches | 0.96±0.90 | 7.65±2.07 | 3.85±2.33 | 10.43±2.52 | 81.45±2.47 | |
| BN (n = 5) | Thymus | 0.6±0.8 | 92.90±0.47 | 87.78±2.02 | 95.70±2.09 | 3.92±1.28 |
| Spleen | 5.66±3.38 | 29.38±3.38 | 11.00±3.84* | 30..20±2.36* | 64.88±2.29* | |
| Cervical LN | 1.20±0.27 | 58.80±5.19 | 10.20±1.86* | 63.00±3.92 | 36.63±3.06 | |
| Mesenteric LN | 1.10±0.96 | 49.00±2.37 | 6.98±3.64* | 53.08±2.90* | 46.13±3.03* | |
| Blood | 1.80±1.59 | 44.98±6.58 | 6.42±2.89* | 47.76±5.98* | 44.23±5.75* | |
| Peyer’s patches | 1.30±1.06 | 13.96±5.35 | 2.60±0.28 | 15.93±6.55 | 77.03±5.52 |
P < 0.01 versus LEW (Student’s t test).
Lymphocyte subsets (flow cytometry)
After washing with RPMI, the lymphocytes were resuspended in Hank’s balanced salt solution (Gibco) with 1.0% bovine serum albumin and 0.1 % NaN3 at a concentration of 10×106/ml. The purified lymphocytes were studied with a panel of monoclonal antibodies (Sera Laboratory, Westbury, NY) that included W3/25 (CD4, 1:400), OX8 (CD8, 1:100), OX19 (pan T cell, 1:400), and antirat IgG (B cell, 1:75). Lymphocytes from Peyer’s patches, spleen, thymus, mesenteric LN, cervical LN, and peripheral blood were stained with the diluted monoclonal antibodies using FITC-conjugated goat antimouse IgG1 (1:400, Sera Laboratory) as a secondary antibody. The samples were analyzed on a FACScan Flow Cytometer and the percentage of cells staining positive with each monoclonal antibody was recorded.
Mixed lymphocyte reaction
One-way MLR was performed (BN stimulator → LEW responder or LEW → BN) with mesenteric node lymphocytes. Triplicate cultures of 1.75×105 responder cells and 3×105 (irradiated (2000 rad) stimulator cells were cultured in a final volume of 0.2 ml RPMI supplemented with 25 mM HEPES buffer, 5×10−5 M 2-mercaptoethanol, 2 mM L-glutamine, penicillin (50 U/ml), streptomycin (50 μg/ml), and 10% heat-inactivated normal rat serum. Cultures were incubated in a humidified atmosphere of 5% CO2 in air for 5 days at 37°C. One μCi of 3H-thymidine was added to each well 16 hr before the termination of the culture. Cultures were harvested with multiple sample harvester and 3H-thymidine uptake was determined by liquid scintillation spectrometry.
In addition, the effect on the MLR response of varying concentrations of FK506 in the culture was tested. The results were expressed as percentage of inhibition by using the following formula:
Experimental and Therapeutic Procedures
Orthotopic small intestine transplantation with portal drainage was performed with a previously described technique (12) from BN to LEW and from LEW to BN. The entire donor small intestine from the ligament of Treitz to the ileocecal valve was isolated with a vascular pedicle consisting of the superior mesenteric artery on a segment of aorta and the superior mesenteric vein in continuity with the portal vein. After donor heparinization (300 U), the graft was perfused with 10 ml of cold lactated Ringer’s solution via the aorta. The lumen was washed with cold neomycin sulfate solution. End-to-side vascular anastomoses were performed with 10-0 Novafil suture between graft aorta and recipient infrarenal aorta, and between graft portal vein and recipient portal vein. The entire recipient small intestine was resected. Intestinal continuity was restored by proximal and distal intestinal anastomoses using 6-0 continuous or interrupted silk suture.
The animals were given 20 mg/day cefamandole nafate for 3 postoperative days. Regular rat chow was started from the day after transplantation. Body weight and activity were checked every day for the first 2 weeks and then twice or more a week. For immunosuppression, intramuscular FK506, dissolved in HCO-60 and D-mannitol carrier solvent (gift from Fujisawa Pharmaceutical Co., Osaka, Japan), was diluted in normal saline and injected into the thigh daily starting on the day of operation and continuing for at least 13 more days. In selected groups, variable doses were continued or started thereafter.
Pathologic Studies
For frozen and formalin fixation, mesenteric lymph nodes and Peyer’s patches from the jejunum and ileum were obtained from the grafts. Recipient liver, spleen, mesenteric lymph nodes, duodenum, thymus, peripheral lymph nodes, and skin (ear and tongue) were obtained at autopsy or sacrifice. The tissues were stained with H&E, and L-21-6 mouse anti-LEW class II monoclonal antibody, kindly provided by Dr. A. Yagihashi and Dr. Y. Iwaki (Departments of Surgery and Pathology, University of Pittsburgh) as described before (10).
In addition, bits of fresh tissue were obtained from the graft and recipient lymphoid organs for removal and purification of lymphocytes as was described for unaltered animals. Donor and recipient cells were identified with affinity-purified biotinylated rat monoclonal antibody 163 (rat IgG2b), which is specific for the RT1.Al antigen on LEW, or monoclonal antibody 42 (rat IgG2a), which is specific for the RT1.An antigen on BN (14) (kindly provided by Dr. H. W. Kunz, University of Pittsburgh, Department of Pathology). The monoclonal antibodies were diluted (1:200) and mixed with 50 μl of lymphocyte suspension and incubated for 45 min at 4°C. The suspensions were washed once with HBSS with BSA and NaN3. Then, FITC-conjugated streptavidin (1:100, Pharmingen, San Diego, CA) was added for another 10 min at 4°C. Finally, cells were washed twice and fixed in 0.3 ml saline containing 1.0% paraformaldehyde. The samples were analyzed using a FACScan Flow Cytometer. The percentage of cells stained positive with each monoclonal antibody was determined.
Statistical Analysis
Results were expressed as mean ± SD. Results were compared using an unpaired Student’s t test. A value of less than 0.01 was considered significant.
RESULTS
In Vitro Studies (Unaltered Animals)
Lymphocyte subsets
The thymus in both rat strains was similar. The contribution of T cells (OX19) to the lymphocyte population was smaller in the BN rats than in LEW in all of the individual lymphoid organs except Peyer’s patches, in which it was greater (Table 1). The differences were significant in the mesenteric lymph nodes, spleen, and blood. CD4 (W3/25) was similar in both strains and the disparity in T cells was primarily of the CD8 (OX8) specificity. B lymphocytes were commensurately increased throughout the lymphoid organs except the thymus and Peyer’s patches in BN compared with LEW (Table 1).
MLR
The one-way MLR was not different with BN-to-LEW than with LEW-to-BN (data not shown). The inhibition of MLR by FK506 was not significantly different in the two strain directions except at a concentration of 0.5 ng/ml (Fig. 1), where the proliferative response of BN-to-LEW stimulator cells was more suppressed than the response of LEW-to-BN stimulator cells although not quite to the level of significance (P=0.0184).
Figure 1.
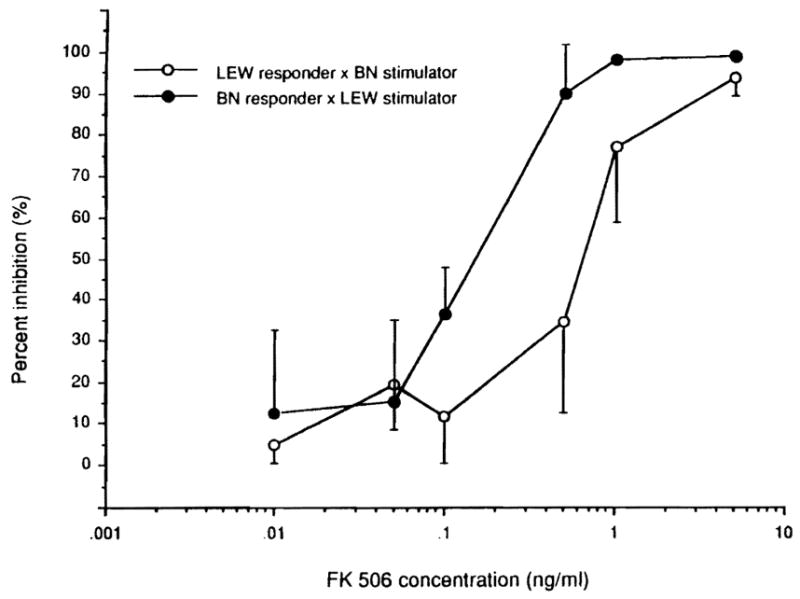
Inhibition of one-way MLR (using mesenteric lymphocytes) with different concentrations of FK506. LEW responder–BN stimulator (open circle); BN responder–LEW stimulator (closed circle).
Studies After Transplantation
BN-to-LEW: no treatment
It was previously reported that untreated animals died of rejection after a median survival of 10.5 days (12).
Low-dose FK506
Median survival was increased to 42 days with 0.15 mg/kg/day for 14 days, but the animals developed the skin flush of GVHD (biopsy-confirmed) in the ears and face (especially periorbital) after 6 and 9 days. The GVHD was self-resolving and disappeared in 4 to 5 days (Table 2). Graft rejection was the ultimate cause of death.
TABLE 2.
Survival after small bowel transplantation from BN to LEW and from LEW to BN
| Group | Donor | Recipient | FK506 treatment | N | Survival (days)a | Median | Ileus | Temporary GVHD | Fatal GVHD |
|---|---|---|---|---|---|---|---|---|---|
| 1) (12) | BN | LEW | — | 6 | 9, 9, 10, 11, 12, 14 | 10.5 | 0 | 0 | 0 |
| 2 | BN | LEW | 0.15 mg/kg × 14 | 8 | 9,b 10,b 36,41, 43, 44, 53, >100 | 42.0 | 2/8 | 5/8 | 0 |
| 3) (12) | BN | LEW | 0.64 mg/kg × 14 | 9 | >100 × 9 | >100 | 0 | 0 | 0 |
| 4) (10) | BN | LEW | 0.64 mg/kg × 14, then weekly | 4 | >100 × 4 | >100 | 0 | 0 | 0 |
| 5) (12) | LEW | BN | — | 3 | 11,12,13 | 12.0 | 0 | 3/3 | 0 |
| 6 | LEW | EN | 0.15 mg/kg × 14 | 6 | 14,b 20, 22, 23, 32, 32 | 22.5 | 1/6 | 2/6 | 3/6 |
| 7) (12) | LEW | BN | 0.64 mg/kg × 14 | 8 | 6,b 27, 27, 28, 29, 30, 36, 36 | 28.5 | 1/8 | 0 | 7/8 |
| 8 | LEW | BN | 1.0 mg/kg × 14 | 8 | 8,b 10,b 10,b 40, 40, 43, 44, 53 | 40.0 | 3/8 | 0 | 4/8 |
| 9 | LEW | BN | 1.0 mg/kg × 14, then weekly | 8 | 8,b 23,b 35, 42, 44, 47, 49, 54 | 43.0 | 2/8 | 0 | 6/8 |
| 10 | LEW | BN | 0.64 mg/kg × 14, then rescue with 1.28 mg/kg × 7 and 0.64 mg/kg there after | 7 | 27, 30, 34, 44, 62,b 88, 97 | 1/7 | 0 | 6/7 | |
| 11 | LEW | BN | 0.64 mg/kg continuously | 7 | 33,b 57,b 83,b >91, >100 × 3 | 3/7 | 0 | 0 |
)previously reported.
Bold type: temporary GVHD. Underlining: fatal GVHD.
Volvulus, distal anastomosis obstruction.
High-dose FK506
With 0.64 mg/kg/day for 14 days, all animals survived more than 100 days without GVHD (Table 2). Although allowing long survival, this 2-week treatment has been shown before to result eventually in chronic graft rejection (12). When the 14-day treatment was supplemented with weekly injections of the same dose, it was previously shown that all animals survived without chronic rejection (Table 2).
LEW-to-BN: no treatment
Unmodified BN recipients (group 5) rejected LEW intestine (histopathologically proved) in 11 to 13 days (Table 2). These animals had signs of temporary GVHD after 6 to 9 days, similar to LEW recipients of BN under low-dose FK506 (group 2). Four additional animals were sacrificed for flow cytometry studies, 2 at 5 and 2 at 9 days. At 5 days, lymphocytes of donor phenotype were detectable in the recipients’ blood and lymphoid organs, but in the 2 animals killed at 9 days, these were not detectable (Table 3). The cytometry findings were concordant with the appearance and spontaneous disappearance of clinical GVHD in the untreated animals studied for survival.
TABLE 3.
Flow cytometry analysis of donor LEW cells in recipient BN tissue during acute rejection in untreated rats and during late GVHD in rats treated with FK506
| Treatment | Days after transplantation | Lymphocytes | Recipient phenotype | Donor phenotype |
|---|---|---|---|---|
| None | 5 (n = 2) | Spleen | 93.30±0.71 | 5.35±1.06 |
| Blood | 91.05±1.63 | 8.25±1.06 | ||
| MLN | 94.25±0.49 | 4.30±0.71 | ||
| 9 (n = 2) | Spleen | 97.35±0.35 | 0.10±0.14* | |
| Blood | 98.15±0.07 | 0.55±0.35* | ||
| MLN | 96.40±0.14 | 0.90±0.14* | ||
| FK506 (1.0 mg/kg/day × 14 plus weekly) | 44, 54 (n = 2) (GVHD) | Spleen | 95.75±1.77 | 4.55±3.04 |
| Blood | 95.95±2.65 | 2.65±1.20 | ||
| MLN | 96.50±1.98 | 2.85±2.62 |
Positive staining was not detectable with mAb 163.
Low-dose FK506
When FK506 was given at 0.15 mg/kg/day for 14 days (Table 2, group 6), FK506 increased median survival to 22.5 days, but 5 of 6 of these BN recipients of LEW grafts showed clinical GVHD. The GVHD was temporary (beginning after 8 to 12 days) in 2 animals who died ultimately of rejection at 32 days. In the other 3 (Table 2) the GVHD was fatal after 20 to 23 days; their intestinal grafts were essentially normal. The sixth animal died of volvulus and distal anastomotic obstruction. This was thought to be an immunologic complication rather than technical because it was commonly seen in lightly treated animals in BN-to-LEW and especially in LEW-to-BN transplantation (Table 2).
High-dose FK506
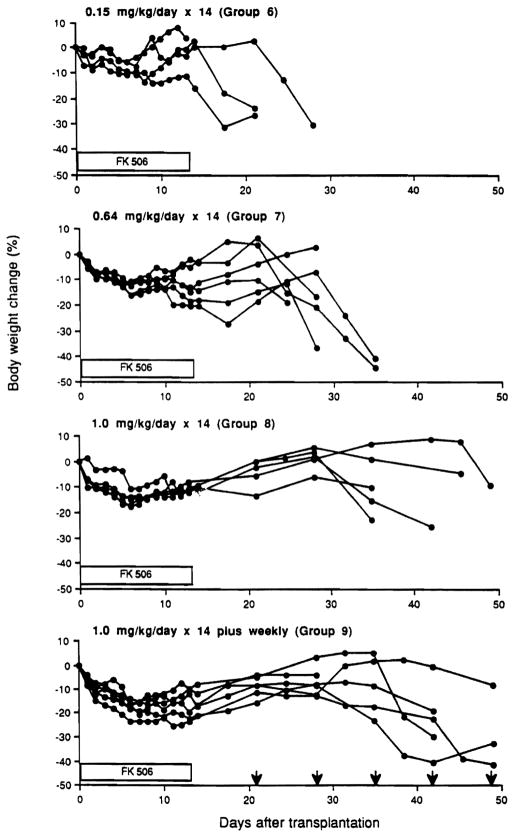
At FK506 doses of .64 mg/kg/day or 1.0 mg/kg/day for 14 days with or without supplemental weekly doses, 17 of 24 rats developed fatal GVHD (groups 7–9, Table 2). This began with a generalized skin flush at variable times after stopping the daily FK506, whether or not weekly treatment was continued (Table 2). Compared with the mean time of 1.3 days for onset of GVHD after stopping daily treatment with 0.15 mg/kg FK506, the interval was 24 days with the highest FK506 dose plus weekly supplementation. The postoperative weight loss in the treated animals was delayed with each increment of therapy, but eventually affected all groups (Fig. 2).
Figure 2.
Body weight changes after LEW-to-BN transplantation with different dose schedules. FK506 was given for 14 days after transplantation in groups 6–9. In group 9, subsequent weekly injections were given of FK506 (1 mg/kg: arrow).
The flushing of GVHD was followed by skin erosion and hyperkeratosis, and was reflected in loss of body weight. However, the severity of the GVHD and the clinical course from its onset to death were not predictably related to the treatment dosages in individual experiments. Once GVHD was diagnosed, the time to death was 2 to 12 days.
Correlation of GVHD with flow cytometry
Two animals in Group 9 (1.0 mg/kg/day FK506 for 2 weeks and weekly thereafter) were sacrificed at 44 and 54 days with obvious GVHD. Flow cytometry studies showed donor cells in the blood, spleen, and host mesenteric lymph nodes similar to those found in untreated intestinal recipients (Table 3).
Rescue from GVHD
With resumption of delayed daily treatment of BN recipients with 1.28 mg/kg FK506 for 7 days followed by reduced daily doses of .64 mg/kg, it was possible to reverse the established GVHD (confirmed by ear biopsy) that developed after an initial 14-day course of .64 mg/kg/day (group 10, Table 2). After the development of GVHD and following the loss of more than 15% of their body weight, 3 of 7 animals had secondary weight gain (Fig. 3), decrease of the skin rash, and restoration of hair growth. The remissions lasted for 25 to 30 days, but the GVHD recurred with a second decrease of body weight when the FK506 dose was halved to .64 mg/kg/day, causing death 57 and 65 days after rescue.
Figure 3.
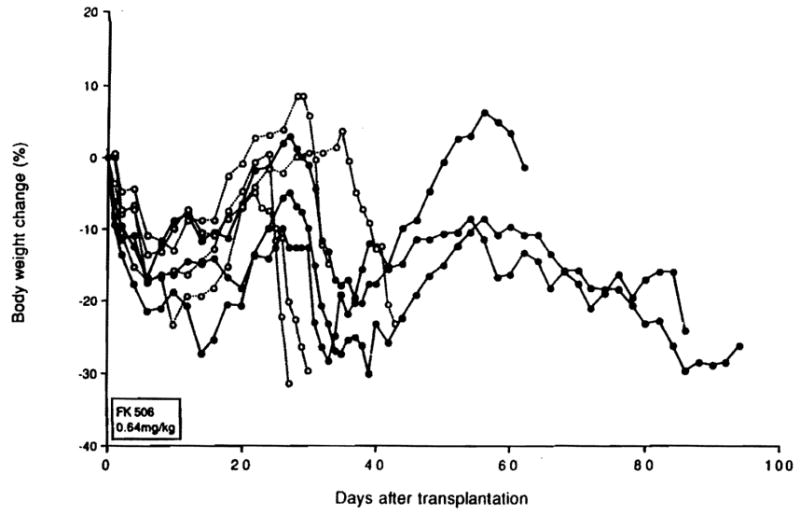
Body weight change of BN recipients of LEW intestine (group 10) after GVHD was diagnosed, whose original FK506 treatment was 0.64 mg/kg for 14 days. When GVHD developed with a 15% loss of weight, FK506 treatment was resumed with 1.28 mg/kg/day for 7 days, then 0.64 mg/kg/day thereafter.
Prophylaxis of GVHD
When primary daily treatment with 0.64 FK506 was continued for 100 days instead of being stopped at 14 days, none of the animals showed any clinical sign of GVHD or rejection during treatment (group 11, Table 2). However, 3 of the 7 rats died under treatment after 33 to 83 days from volvulus or ileus.
Cell migration and repopulation
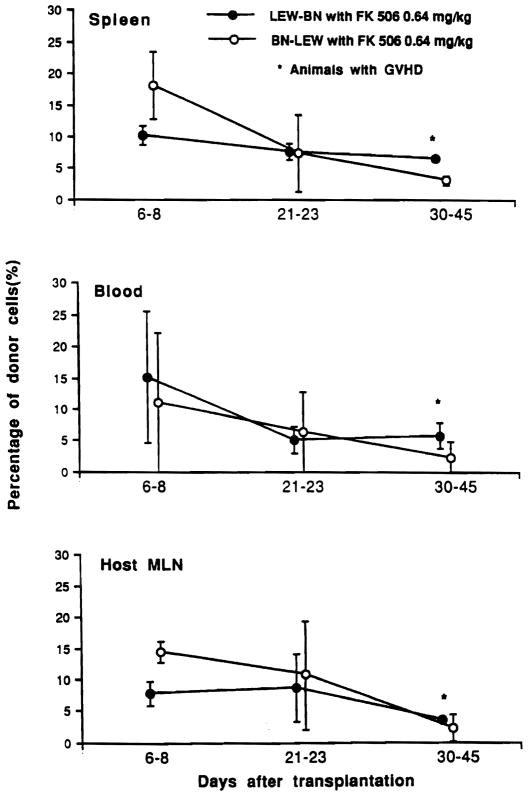
A formal comparison of the cell repopulation after BN-to-LEW versus LEW-to-BN intestinal transplantation was made in 15 additional animals treated for 2 weeks with 0.64 mg/kg/day. Almost all of the lymphocytes found in the graft mesenteric lymph nodes could be phenotyped. At the end of the first week (during treatment) and for the rest of the observation period out to 30–45 days, the lymphocytes in the graft were predominantly of recipient phenotype no matter in which strain direction the transplants were performed (Fig. 4). The percentage of donor lymphocytes in recipient blood and lymphoid tissues also was similar in the two strain directions (Fig. 5). The greatest donor representation in the grafts as well as in the recipient blood, spleen, and lymph nodes was in the 1-week specimens followed by a gradual decline thereafter. However, there was a tendency with the LEW-BN combination for greater LEW (donor) representation in all of these locations in the specimens collected at 30–45 days (Figs. 4 and 5).
Figure 4.
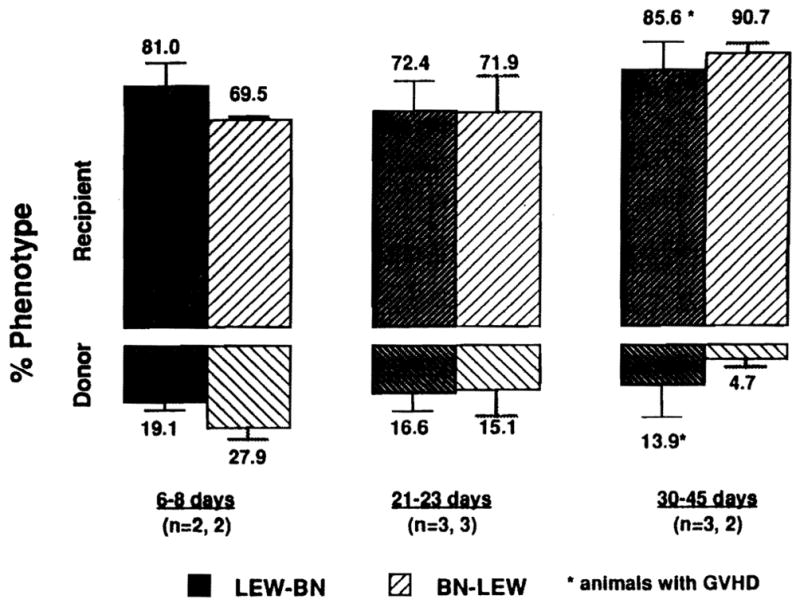
Donor and recipient phenotypes of graft mesenteric lymph node lymphocytes at different times after LEW-to-BN and BN-to-LEW transplantation under FK506 treatment (0.64 mg/kg/day for 14 days).
Figure 5.
Percentage of donor cells in recipient peripheral blood, spleen, and mesenteric lymph nodes after BN-to-LEW and LEW-to-BN intestinal transplantation.
Other histopathologic studies (LEW-to-BN)
The skin of BN recipients with clinical GVHD showed apoptosis and lymphocyte infiltration and vacuolization in the basal layer similar to the findings in GVHD after bone marrow transplantation (15). Phenotypic analysis of the dermal infiltrates revealed many donor class II–positive (L-21-6+) cells. Similar donor cells were also detected after 30–45 days in the spleen, host mesenteric lymph nodes, and thymus. Donor class II–positive cells in the recipient thymus were rare early in the course of GVHD and were largely located in the medulla. The number of donor cells in the recipient thymus increased as GVHD worsened, but remained small in number. These latter observations were reported elsewhere (12).
DISCUSSION
We have described previously the ease with which BN (RT1n) multivisceral and intestinal grafts can be transplanted to LEW (RT1l) recipients using a brief induction course of FK506 (10, 16–18). The difficulty in controlling intestinal rejection in the opposite strain direction also was reported before (12) and was confirmed in the present experiments.
A particularly striking example of the ease of BN-to-LEW transplantation is with liver transplantation, in which permanent graft survival can be obtained routinely without any treatment (9, 19); the same is true with BN-to-ACI and BN-to-PVG liver transplants (unpublished observations). It is noteworthy that the control of rejection and achievement of heart graft acceptance under immunosuppression also is easier with the BN-to-LEW model than the other way around (unpublished data). BN may have “universal donor” qualities for heart transplantation in other strain combinations such as BN-to-WAG (easy), whereas WAG-to-BN is difficult (20). Why BN should be a universal donor is not known.
An interesting clue is that monoclonal antibody L-21-6, which was used in our phenotyping experiments, is anti-LEW class II and has been identified as an mAb against the invariant chain of class II (unpublished observations). L-21-6 stains many rat strains (LEW, ACI, W/F, PVG, and F344) but not BN, which may be due to its absence or a change in antigenic composition. The invariant chain is involved with intracellular transport, assembly, and sorting of MHC class II molecules. It is believed that invariant chain and MHC class II molecules are assembled after insertion into endoplasmic reticulum of antigen-presenting cells (APC), an arrangement that prevents the binding of endogenous peptides to these molecules. After intracellular transport to the endosomal compartment, the invariant chain is degraded and detached from class II molecules. Subsequently, class II polypeptides are charged with processed antigen and undergo passage to the cell surface (21, 22). Thus, the absence or alteration of invariant chain in BN rats could explain an ineffective endogenous and exogenous antigen presentation pathway through APCs across a wide spectrum of recipient strain diversity.
In addition to donor antigen differences and their presentation, rejection depends on the recipient’s genetically determined ability to respond to these differences. Certain rat strains (LEW [RT1l], W/F [RT1u]) have been classed as high responders and others have been characterized low responders (DA [RT1a], PVG [RT1c]) according to their ability upon stimulation to produce antibody and CTL (23). Efforts to equate recipient responder status to the CD4 population in rats (24–27) and mice (28) or to variable dependence of CD8+ cell activation on CD4+ cells have provided confusing results. In our studies of unaltered animals the lymphoid tissues of LEW rats had significantly more T lymphocytes and more CD8+ and fewer B cells than the BN rats. The CD4+ population was equal. However, both strains were well endowed with all subpopulations. The one-way MLR with lymphocytes from these animals was similar in each direction with or without media enrichment with FK506. Thus, our supporting studies in cells from unaltered BN and LEW or of cultured cells exposed to FK506 did not explain convincingly the disparity of rejection in LEW-to-BN compared with BN-to-LEW transplantation.
Even more puzzling was the propensity of intestinal LEW grafts to cause GVHD. DeBruin et al. (29) have described analogous findings under cyclosporine after WAG (RT1u)–to–BN but not BN-to-WAG intestinal transplantation. The paradox in our experiments was that the LEW intestinal lymphoid component was intensely hostile to the whole BN recipient, whereas the intact total LEW immune apparatus responded benignly to BN intestinal grafts. The severity of the resulting GVHD was not what might have been predicted from strict extrapolation from Billingham’s rules.
The geographic pattern of graft lymphoid repopulation and lymphoid migration from the LEW intestine into the BN host could not be used to explain either GVHD or graft rejection in the LEW-BN experiments, because it was essentially the same as after BN-to-LEW intestinal or multivisceral transplantation under FK506 in which neither rejection nor GVHD occur. In either strain direction with or without FK506 immunosuppression, a shower of intestinal lymphocytes left the graft as they were replaced by recipient lymphocytes, accounting peripherally for 2 to 10% of the lymphocytes in the blood and major lymphoid organs of the host. The migration excepted only the thymus, where donor lymphocytes were rare and largely confined to the medulla. The consequent well-tolerated composite grafts after BN-to-LEW (10) and human (11) intestinal transplantation, which are made up of donor parenchymal and recipient lymphoreticular components, have been described as local organ chimeras.
It would be inappropriate to think of the intestinal chimerism as “local” if in fact the donor lymphoreticular cells remain permanently viable, as seems likely. Ildstad and Sachs have demonstrated a state of systemic “mixed” allogeneic (30) and xenogeneic (31, 32) chimerism in which rodents conditioned with total body or total lymphoid irradiation have been permanently constituted with bone marrow grafts of combined syngeneic and allogeneic (or xenogeneic) cells. Because these stable mixed chimeras accept other tissues and organs from the bone marrow donor strains and are highly resistant to GVHD, this work has reawakened hope of successful tolerance induction.
In view of the lymphoreticular migration and cell repopulation that follows intestinal transplantation, our hypothesis is that stable mixed allogeneic chimerism is frequently and accidentally achieved after intestinal or multivisceral transplantation under immunosuppression both in animals and in man. LEW rats with apparent permanent survival of BN intestinal or multivisceral grafts are currently being studied to see if systemic chimerism exists. If so, it will remain to be explained why this state is so easy to achieve with some experimental and clinical donor-recipient combinations and so difficult with others.
The circumstances predicting success are unclear. In the successful human intestinal transplantations performed so far under FK506 or cyclosporine, GVHD has been rare in spite of the fact that HLA matching has been random and uniformly poor (7, 8). How to identify and avoid bad clinical donor-recipient combinations analogous to the LEW-to-BN model is not evident. Nor is it certain, as has been assumed until recently, that iatrogenic efforts to alter the host-graft immunologic balance with antigraft lymphoid procedures will be fruitful; there may be unexpected penalties, including the development of lymphoproliferative lesions (33). Finally, it was shown in the experiments herein reported that even the relatively intractable GVHD of LEW-to-BN transplantation could be controlled or even reversed with heavy FK506 immunosuppression, which must be continuous. The reversal with FK506 of established GVHD also has been demonstrated after bone marrow splenocyte transplantation (34). This suggests that eventual graft acceptance can be achieved even with “bad” donor-recipient combinations.
Footnotes
This work was supported by Research Grants from the Veterans Administration and by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Billingham RE. Reactions of grafts against their hosts: transplantation immunity works both ways—hosts destroy grafts and grafts may harm hosts. Science. 1959;130:947. doi: 10.1126/science.130.3381.947. [DOI] [PubMed] [Google Scholar]
- 2.Lillehei RC, Manax WG, Lyona GW, Dietzman RH. Transplantation of gastrointestional organs, including small intestine and stomach. Gastroenterology. 1966;51:936. [PubMed] [Google Scholar]
- 3.Cohen Z, MacGregor AB, Moore KTH, Falk RE, Langer B, Cullen JB. Canine small bowel transplantation. Arch Surg. 1976;111:248. doi: 10.1001/archsurg.1976.01360210042008. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693. [PubMed] [Google Scholar]
- 6.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;26:1449. [PMC free article] [PubMed] [Google Scholar]
- 7.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 8.Todo S, Tzakis A, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel–liver transplantation in humans. Transplantation. 1992;53:369. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of the multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 10.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 11.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. 1991;337:818. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murase N, Demetris AJ, Woo J, et al. Lymphocyte traffic and GVHD after fully allogeneic small bowel transplantation. Transplant Proc. 1991;23:3246. [PMC free article] [PubMed] [Google Scholar]
- 13.Arnaud-Battandier F, Salmon H, Vaiman M, et al. Small intestinal allotransplantation in swine with cyclosporine treatment: studies of the intestinal lymphoid populations. Transplant Proc. 1985;17:1440. [Google Scholar]
- 14.Gill TJ, Kunz HW, Misra DN, Hassett ALC. The major histocompatibility complex of the rat. Transplantation. 1987;43:773. [PubMed] [Google Scholar]
- 15.Markus PM, Cai X, Ming W, Demetris AJ, Starzl TE, Fung JJ. Prevention of graft-versus-host disease following allogeneic bone marrow transplantation in rats using FK506. Transplantation. 1991;52:590. doi: 10.1097/00007890-199110000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase N, Kim DG, Todo S, Cramer DV, Fung J, Starzl TE. Induction of liver, heart, and multivisceral graft acceptance with a short course of FK 506. Transplant Proc. 1990;22 (suppl 1):74. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KKW, Stangl MJ, Todo S, Langrehr JM, Starzl TE, Schraut WH. Successful orthotopic small bowel transplantation with short-term FK 506 immunosuppressive therapy. Transplant Proc. 1990;22(suppl 1):78. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman AL, Makowka L, Banner B, et al. The use of FK506 for small intestine allotransplantation: inhibition of acute rejection and prevention of fatal graft-versus-host disease. Transplantation. 1990;49:483. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada N. Experimental liver transplantation. Boca Raton, FL: CRC Press; 1988. p. 55. [Google Scholar]
- 20.Guillaume J, Niessen GJCM, Marquet RL, Bijnen AB, Obertop H, Jeekel JJ. The effect of cyclosporin A and blood transfusions on cardiac allograft survival in rats. Surgery. 1982;91:339. [PubMed] [Google Scholar]
- 21.Teyton L, O’Sullivan D, Dickson PW, et al. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990;348:39. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 22.Koch N, Moldenhauer G, Hofmann WJ, Moller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II–associated invariant chain (CD74) J Immunol. 1991;147:2643. [PubMed] [Google Scholar]
- 23.Butcher GW, Howard JC. Genetic control of transplant rejection. Transplantation. 1982;34:161. [PubMed] [Google Scholar]
- 24.Ilano AL, McConnell MV, Gurley KE, Spinelli A, Pearce NW, Hall BM. Cellular basis of allograft rejection in vivo: V. Examination of the mechanisms responsible for the differing efficacy of monoclonal antibody to CD4+ T cell subsets in low- and high-responder rat strains. J Immunol. 1989;143:2828. [PubMed] [Google Scholar]
- 25.Salomon DR, Cohen DJ, Williams JM, Carpenter CB. T cell synergy in the primary MLR: proliferative kinetic, effector cell generation, and IL2 production. J Immunol. 1984;133:3075. [PubMed] [Google Scholar]
- 26.Mason DW, Pugh CW, Webb M. The rat mixed lymphocyte reaction: role of a dendritic cell in intestinal lymph and T-cell subsets defined by monoclonal antibodies. Immunology. 1981;44:75. [PMC free article] [PubMed] [Google Scholar]
- 27.Hall BM. Cells mediating allograft rejection. Transplantation. 1991;51:1141. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Sprent J, Schaefer M. Antigen-presenting cells for unprimed T cells. Immunol Today. 1989;10:17. doi: 10.1016/0167-5699(89)90060-1. [DOI] [PubMed] [Google Scholar]
- 29.Saat RE, De Bruin RWF, Heineman E, Jeekel J, Marquet RL. The limited efficacy of cyclosporine in preventing rejection and graft-versus-host disease in orthotopic small bowel transplantation in rats. Transplantation. 1990;50:374. doi: 10.1097/00007890-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 31.Ildstad ST, Vacchio MS, Markus PM, Wren SM, Hodes RJ. Cross species transplantation tolerance: rat bone marrow–derived cells can contribute to the ligand for negative selection of mouse TCR-Vβ in chimeras tolerant to xenogeneic antigens (mouse + rat → mouse) J Exp Med. 1992;175:147. doi: 10.1084/jem.175.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ildstad ST, Boggs SS, Vecchini F, et al. Mixed xenogeneic chimeras (rat + mouse → rat): evidence of rat stem cell engraftment, strain-specific transplantation tolerance, and skin-specific antigens. Transplantation. 1992;53:815. [PubMed] [Google Scholar]
- 33.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 34.Markus PM, Cai X, Ming W, Demetris AJ, Fung JJ, Starzl TE. FK 506 reverses acute graft-versus-host disease following allogeneic bone marrow transplantation in rats. Surgery. 1991;110:357. [PMC free article] [PubMed] [Google Scholar]