Abstract
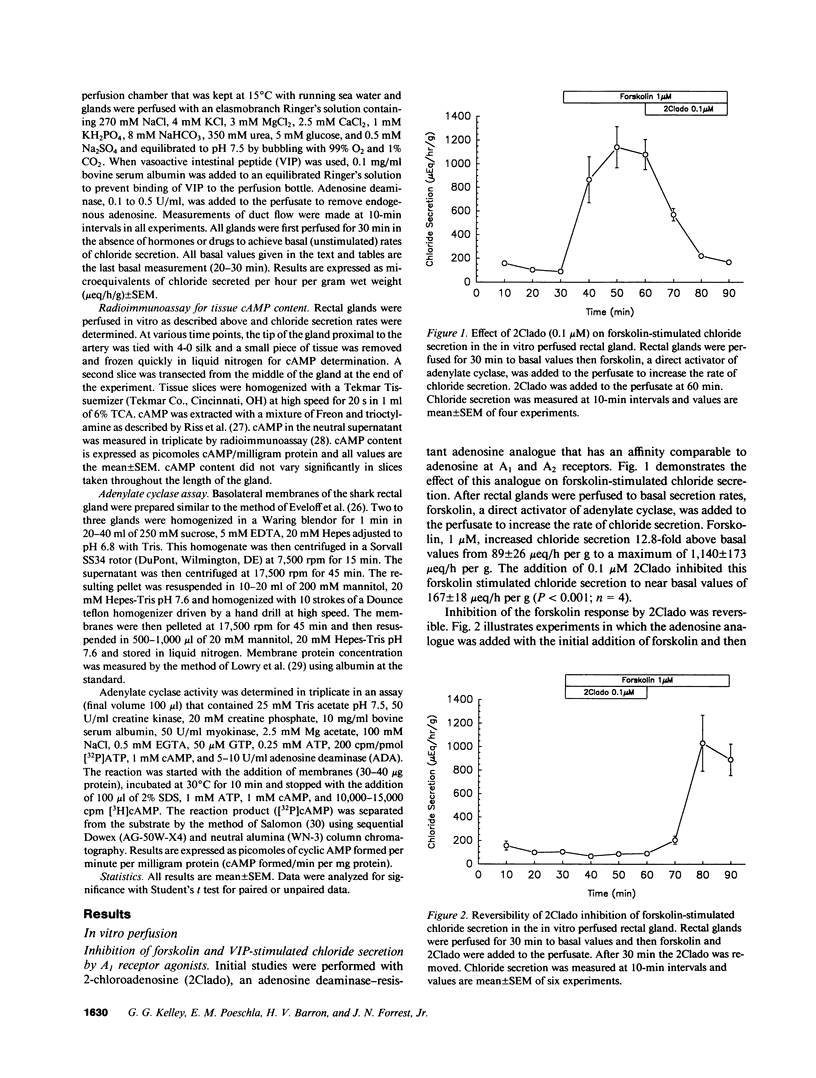
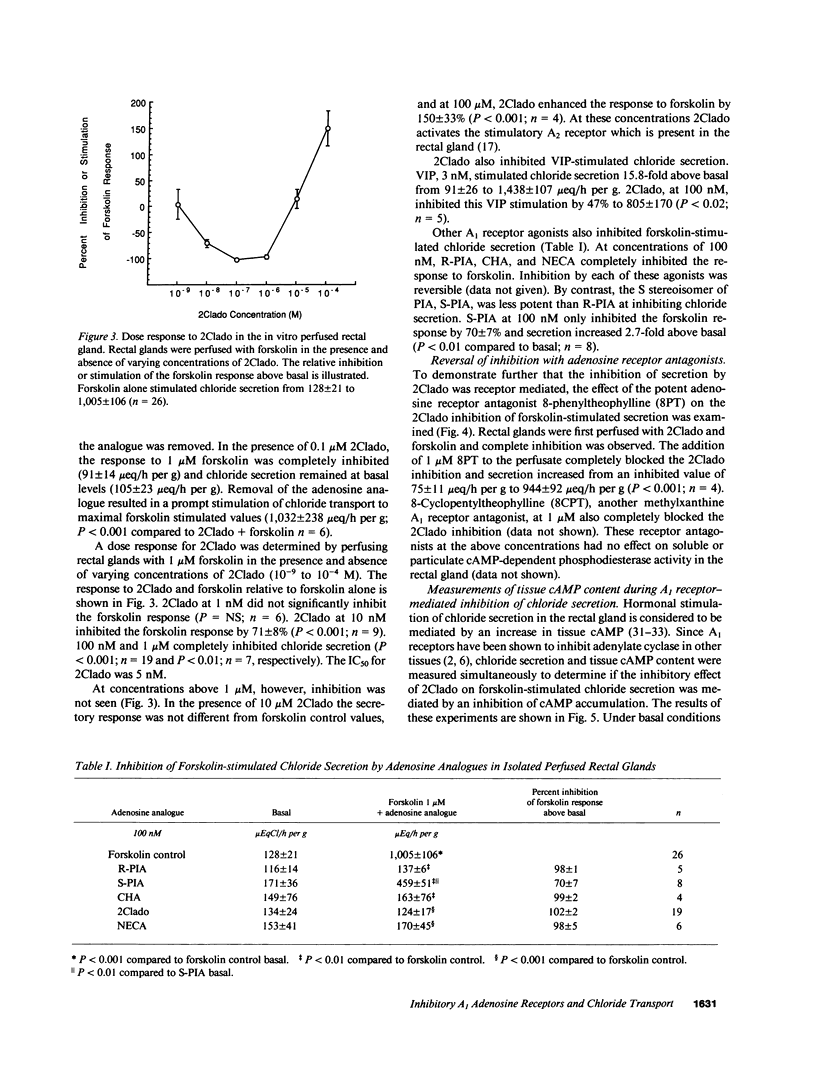
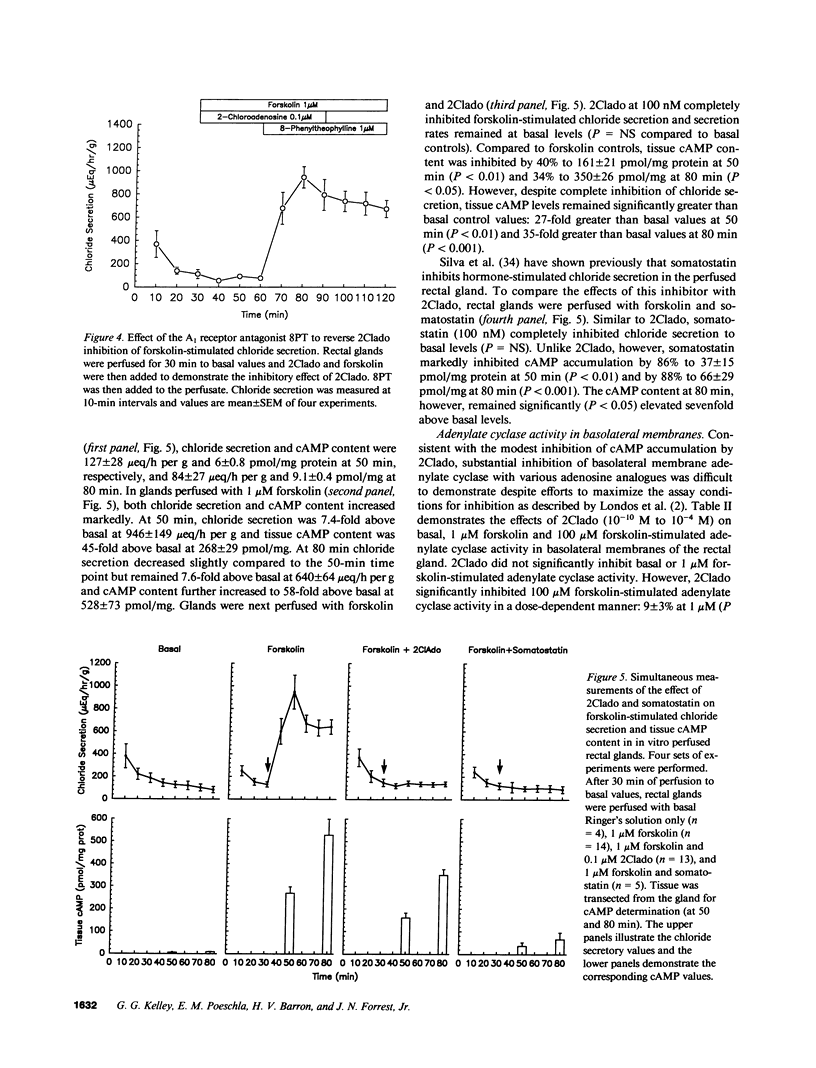
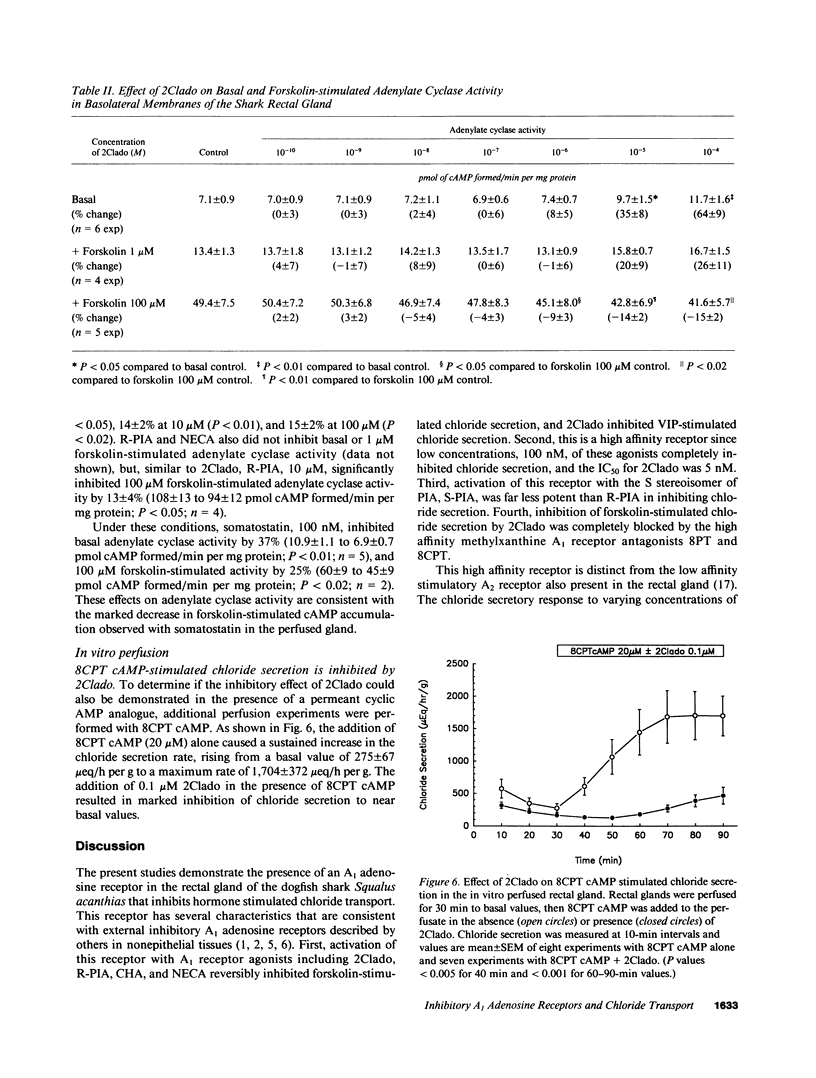
In the in vitro perfused rectal gland of the dogfish shark (Squalus acanthias), the adenosine analogue 2-chloroadenosine (2Clado) completely and reversibly inhibited forskolin-stimulated chloride secretion with an IC50 of 5 nM. Other A1 receptor agonists including cyclohexyladenosine (CHA), N-ethylcarboxamideadenosine (NECA) and R-phenylisopropyl-adenosine (R-PIA) also completely inhibited forskolin stimulated chloride secretion. The "S" stereoisomer of PIA (S-PIA) was a less potent inhibitor of forskolin stimulated chloride secretion, consistent with the affinity profile of PIA stereoisomers for an A1 receptor. The adenosine receptor antagonists 8-phenyltheophylline and 8-cyclopentyltheophylline completely blocked the effect of 2Clado to inhibit forskolin-stimulated chloride secretion. When chloride secretion and tissue cyclic (c)AMP content were determined simultaneously in perfused glands, 2Clado completely inhibited secretion but only inhibited forskolin stimulated cAMP accumulation by 34-40%, indicating that the mechanism of inhibition of secretion by 2Clado is at least partially cAMP independent. Consistent with these results, A1 receptor agonists only modestly inhibited (9-15%) forskolin stimulated adenylate cyclase activity and 2Clado markedly inhibited chloride secretion stimulated by a permeant cAMP analogue, 8-chlorophenylthio cAMP (8CPT cAMP). These findings provide the first evidence for a high affinity A1 adenosine receptor that inhibits hormone stimulated ion transport in a model epithelia. A major portion of this inhibition occurs by a mechanism that is independent of the cAMP messenger system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend L. J., Burnatowska-Hledin M. A., Spielman W. S. Adenosine receptor-mediated calcium mobilization in cortical collecting tubule cells. Am J Physiol. 1988 Nov;255(5 Pt 1):C581–C588. doi: 10.1152/ajpcell.1988.255.5.C581. [DOI] [PubMed] [Google Scholar]
- Arend L. J., Sonnenburg W. K., Smith W. L., Spielman W. S. A1 and A2 adenosine receptors in rabbit cortical collecting tubule cells. Modulation of hormone-stimulated cAMP. J Clin Invest. 1987 Mar;79(3):710–714. doi: 10.1172/JCI112875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983 May;244(5):H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Forskolin, adenylate cyclase, and cell physiology: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:81–89. [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Seamon K. B. Activation of cyclic AMP-generating systems in brain membranes and slices by the diterpene forskolin: augmentation of receptor-mediated responses. J Neurochem. 1982 Feb;38(2):532–544. doi: 10.1111/j.1471-4159.1982.tb08660.x. [DOI] [PubMed] [Google Scholar]
- Dillingham M. A., Anderson R. J. Purinergic regulation of basal and arginine vasopressin-stimulated hydraulic conductivity in rabbit cortical collecting tubule. J Membr Biol. 1985;88(3):277–281. doi: 10.1007/BF01871091. [DOI] [PubMed] [Google Scholar]
- Dobbins J. W., Laurenson J. P., Forrest J. N., Jr Adenosine and adenosine analogues stimulate adenosine cyclic 3', 5'-monophosphate-dependent chloride secretion in the mammalian ileum. J Clin Invest. 1984 Sep;74(3):929–935. doi: 10.1172/JCI111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks M. L., Jackson M., Manganiello V. C., Vaughan M. Effect of N6-(L-2-phenylisopropyl)adenosine and insulin on cAMP metabolism in 3T3-L1 adipocytes. Am J Physiol. 1987 Mar;252(3 Pt 1):C342–C348. doi: 10.1152/ajpcell.1987.252.3.C342. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Kinne R., Kinne-Saffran E., Murer H., Silva P., Epstein F. H., Stoff J., Kinter W. B. Coupled sodium and chloride transport into plasma membrane vesicles prepared from dogfish rectal gland. Pflugers Arch. 1978 Dec 28;378(2):87–92. doi: 10.1007/BF00584439. [DOI] [PubMed] [Google Scholar]
- Forrest J. N., Jr, Wang F., Beyenbach K. W. Perfusion of isolated tubules of the shark rectal gland. Electrical characteristics and response to hormones. J Clin Invest. 1983 Sep;72(3):1163–1167. doi: 10.1172/JCI111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasl M., Turnheim K. Stimulation of electrolyte secretion in rabbit colon by adenosine. J Physiol. 1984 Jan;346:93–110. doi: 10.1113/jphysiol.1984.sp015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Cl- -channels in the apical cell membrane of the rectal gland "induced" by cAMP. Pflugers Arch. 1985 Apr;403(4):446–448. doi: 10.1007/BF00589260. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Wang F., Forrest J. N., Jr Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). III. Effects of stimulation of secretion by cyclic AMP. Pflugers Arch. 1984 Dec;402(4):376–384. doi: 10.1007/BF00583938. [DOI] [PubMed] [Google Scholar]
- Isom L. L., Cragoe E. J., Jr, Limbird L. E. Multiple receptors linked to inhibition of adenylate cyclase accelerate Na+/H+ exchange in neuroblastoma x glioma cells via a mechanism other than decreased cAMP accumulation. J Biol Chem. 1987 Dec 25;262(36):17504–17509. [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang M. A., Preston A. S., Handler J. S., Forrest J. N., Jr Adenosine stimulates sodium transport in kidney A6 epithelia in culture. Am J Physiol. 1985 Sep;249(3 Pt 1):C330–C336. doi: 10.1152/ajpcell.1985.249.3.C330. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. FASEB J. 1988 Aug;2(11):2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. L., Skerritt J. H., Werz M. A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J Physiol. 1986 Jan;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken D. E. An analysis of glomerular dynamics in rat, dog, and man. Kidney Int. 1982 Aug;22(2):136–145. doi: 10.1038/ki.1982.145. [DOI] [PubMed] [Google Scholar]
- Pratt A. D., Clancy G., Welsh M. J. Mucosal adenosine stimulates chloride secretion in canine tracheal epithelium. Am J Physiol. 1986 Aug;251(2 Pt 1):C167–C174. doi: 10.1152/ajpcell.1986.251.2.C167. [DOI] [PubMed] [Google Scholar]
- Rosenthal W., Hescheler J., Trautwein W., Schultz G. Control of voltage-dependent Ca2+ channels by G protein-coupled receptors. FASEB J. 1988 Sep;2(12):2784–2790. doi: 10.1096/fasebj.2.12.2457531. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Silva P., Stoff J. S., Leone D. R., Epstein F. H. Mode of action of somatostatin to inhibit secretion by shark rectal gland. Am J Physiol. 1985 Sep;249(3 Pt 2):R329–R334. doi: 10.1152/ajpregu.1985.249.3.R329. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Spielman W. S., Thompson C. I. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol. 1982 May;242(5):F423–F435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- Spinowitz B. S., Zadunaisky J. A. Action of adenosine on chloride active transport of isolated frog cornea. Am J Physiol. 1979 Aug;237(2):F121–F127. doi: 10.1152/ajprenal.1979.237.2.F121. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stoff J. S., Rosa R., Hallac R., Silva P., Epstein F. H. Hormonal regulation of active chloride transport in the dogfish rectal gland. Am J Physiol. 1979 Aug;237(2):F138–F144. doi: 10.1152/ajprenal.1979.237.2.F138. [DOI] [PubMed] [Google Scholar]
- Stoff J. S., Silva P., Field M., Forrest J., Stevens A., Epstein F. H. Cyclic AMP regulation of active chloride transport in the rectal gland of marine elasmobranchs. J Exp Zool. 1977 Mar;199(3):443–448. doi: 10.1002/jez.1401990319. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Sekura R. D., Birnbaumer L., Brown A. M. Reconstitution of somatostatin and muscarinic receptor mediated stimulation of K+ channels by isolated GK protein in clonal rat anterior pituitary cell membranes. Mol Endocrinol. 1987 Apr;1(4):283–289. doi: 10.1210/mend-1-4-283. [DOI] [PubMed] [Google Scholar]
- de Mazancourt P., Giudicelli Y. Guanine nucleotide- and GTP-dependent N6-phenylisopropyladenosine stimulation of the membrane-bound cyclic AMP high affinity phosphodiesterase in rat brain. FEBS Lett. 1984 Feb 13;167(1):142–146. doi: 10.1016/0014-5793(84)80849-2. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


