Introduction
FK 506 (Prograf) is a novel macrolide antibiotic isolated from the soil fungus Streptomyces tsukubaensis [24]. Although it is totally distinct in molecular structure from cyclosporin (CsA) (Sandimmune), a cyclic endecapeptide extracted from the fungus Tolypocladium inflatum (Fig. 1), the two drugs share a remarkably similar, selective inhibitory action on the activation and proliferation of CD4+ T helper (TH) lymphocytes [25, 41, 50, 51, 56]. These cells play an essential, central role both in antigen recognition and as the sources of soluble, hormone-like mediators (cytokines) of the cascade of events leading to the expression of immune reactivity. By inhibiting the activation of CD4+ TH cells, FK 506, like CsA, exerts a wide-range of immunosuppressive activities. It is recognized that both drugs prolong solid-organ allograft survival in experimental animals and in man. FK 506, however, is considerably more powerful as an antilymphocytic agent than CsA, as evidenced by the superior potency of the former drug in inhibiting antigen-driven T cell activation, cytokine production and lymphocyte proliferation in vitro [50]. Moreover, the systemic levels of FK 506 required to induce and maintain immune suppression are approximately 100-fold lower than are the blood levels of CsA to achieve the same effect. The immunosuppressive efficacy of CsA in man (in renal transplant recipients and patients receiving bone marrow transplants) was first reported in 1978; in 1989, the first account of the ability of FK 506 to prevent or reverse organ allograft rejection was published [44]. Data obtained over the last 3 years provide good clinical evidence that FK 506 exhibits a narrower range of side effects than does CsA and that, as compared with CsA, FK 506 has greater steroid-sparing activity [45, 46]. Whilst the potential benefits of FK 506 for the prophylaxis and reversal of organ allograft rejection (in particular liver transplant rejection) are becoming recognized, the value of the drug in the treatment of autoimmune disorders is now also beginning to be assessed. In this article (1) a rationale for the use of FK 506 in autoimmune disease, (2) a description of its molecular action and immunosuppressive activities, (3) a consideration of the biological and pharmacological properties of FK 506, (4) a review of its capacity to inhibit a wide variety of experimental autoimmune disorders, and (5) a report on the early clinical experience with FK 506 in the clinical management of a panoply of autoimmune disease seen at the University of Pittsburgh Medical Center (UPMC) will be presented. Moreover, a brief outline of laboratory investigations utilized to monitor the status of T lymphocytes in these patients and a discussion of the side effects of FK 506 will be presented. Throughout, we shall draw upon comparisons between FK 506 and CsA which have been documented in the literature.
Fig. 1.
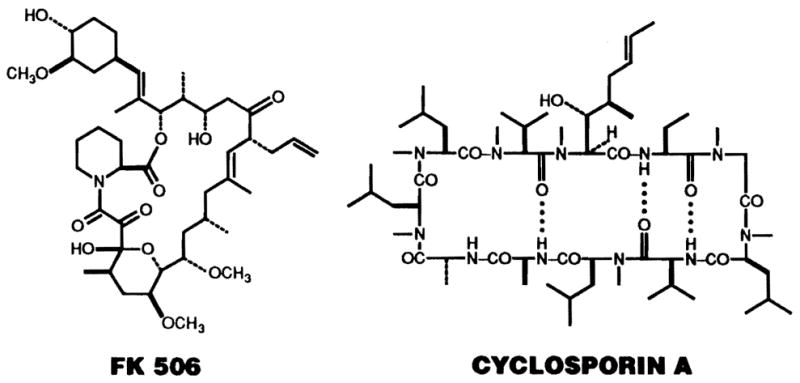
The molecular structure of the immunosuppressive macrolide FK 506 (mol. wt. 822 daltons) and of the less powerful, but similarly acting cyclic endecapeptide cyclosporin A (mol. wt. 1203 daltons)
Rationale for the use of FK 506 in autoimmune diseases
The role of T cells in autoimmunity
The therapeutic use of FK 506 in the treatment of autoimmune disease is based on the premise that all of these disorders are T cell driven [39]. It is, therefore, important to examine the evidence that activated CD4+ TH cells and their cytokine products are important both in the induction and maintenance of various diseases such as psoriasis, uveitis, insulin-dependent type-1 diabetes, chronic active hepatitis-autoimmune (CAH-A), rheumatoid arthritis and multiple sclerosis – diseases that are currently being treated with FK 506. In uveitis [11], type-1 diabetes [6], multiple sclerosis [16] and psoriasis [3] for example, T cells are believed to play an important pathogenic role. Much of the evidence to support this view comes from studies in experimental animal models and from in vitro investigations of the adverse or destructive interactions between T cells, antigen-stimulated cytokines and the target tissue affected by the disease process. In the autoimmune liver diseases [26], CAH-A and primary biliary cirrhosis (PBC), and in rheumatoid arthritis [9], there is abundant evidence for the involvement of T cells in the pathogenesis of each disease and, therefore, a rationale for the use of FK 506 in each exists. In recent years the therapeutic efficacy of CsA in uveitis, psoriasis, PBC, CAH-A, and rheumatoid arthritis has been demonstrated [52]. Moreover, CsA has been shown to alter the natural history of type-1 diabetes [4]. The drug has not, however, made a significant impact upon the clinical management of patients with most of these diseases.
In autoimmune diseases such as systemic lupus erythematosus (SLE) or the nephrotic syndrome, the rationale for the use of CsA or FK 506 is less clear. Thus, in SLE, humoral immunity appears to be more important than cellular immunity in the pathogenesis of the disease, and in idiopathic nephrotic syndrome the pathogenic mechanisms responsible for the disease process are far from clear. Nevertheless, in nephrotic syndrome, T cell dysfunction, recruitment of B cells, immunoglobulin deposition within the kidney and a central role for lymphokines have been implicated by various authors [7, 8, 18, 54]. Moreover, CsA has been shown to be very effective in the steroid-sensitive nephrotic syndrome, although less so in steroid-resistant patients [52].
A spectrum of autoimmune disorders is shown in Table 1. The predicted efficacy of FK 506 in their treatment is based on the assumption that the role of T cells in these various disease processes is central, and also on experience in animal models of these diseases with either CsA or FK 506. Account is also taken of clinical experience with CsA in these autoimmune disorders.
Table 1.
Possible mechanisms of autoimmune diseases and predicted responses to FK 506a
| Disease | Implicated mechanismb | Predicted efficacy of FK 506a |
|---|---|---|
| Autoimmune uveitis | T cells | + + + |
| Type-1 diabetes | T cells/Absc | + + + |
| Psoriasis | T cells/non-immunological | + + + |
| Chronic active hepatitis (autoimmune) | T cells and cytotoxic Abs | + |
| Primary biliary cirrhosis | T cells + Abs | + + |
| Rheumatoid arthritis | T cells + Abs? | + + |
| SLE | Abs | + |
| Myasthenia gravis | Abs | ± |
| Immune cytopenias | Abs | ± |
| Nephrotic syndrome | T cells/non-immunological? | + |
Dependent on timing of instigation of therapy in relation to disease progression
Oversimplification for purposes of concept formation
Antibodies (Abs) to insulin and islet cells believed to be epiphenomena
Evidence that autoimmune diseases are T cell driven
There is a large body of additional experimental data which provides supportive evidence to the thesis that autoimmune diseases are driven by T cells and their cytokine products. In addition to the proven efficacy of CsA or FK 506 in many experimental autoimmune diseases, antibodies directed against CD4+ T cells or against the interleukin 2 receptor (IL-2R; expressed on activated T cells) have been shown to be effective therapeutic agents in these animal models. When stimulated with appropriate antigen or monoclonal antibody, T cell clones derived from lesional tissue or peripheral blood secrete cytokines which effect the pathological changes observed in target tissue (e.g., fibroblasts in scleroderma, keratinocytes in psoriasis or islet cells in type-1 diabetes) that are relevant to the disease process observed in vivo. Such antigen-stimulated T cell clones can induce disease when transferred to healthy recipients (e.g., induction of type-1 diabetes, experimental arthritis or allergic encephalomyelitis). Furthermore, in many experimental models of autoimmunity, it can be shown that neonatal thymectomy has a pronounced beneficial effect in preventing development of the disease. For references and discussion see [40].
The central role of CD4+ T cells
There are two general prerequisites for the induction of autoimmunity. First, self-reactive T cells must be present in the immunological repertoire and second, self antigen must be presented in conjunction with major histocompatibility complex (MHC) class II determinants on a competent, antigen-presenting cell (APC) (Fig. 2). IL-2 is produced in response to the signal generated by processed antigen presented on the APC surface in association with MHC class II molecules interacting with the antigen-specific T cell receptor (TCR). Secreted IL-2 interacts with specific cell surface IL-2R to induce clonal T lymphocyte proliferation. The CD4+ T cell population contains subsets (TH1 and TH2 cells), which have been characterized in murine systems and have been shown to differ in the mixture of cytokines that they secrete [34]. Thus, TH1 cells can be shown to be the source of IL-2 and interferon (IFN)-γ and promote cell-mediated immune responses, whilst TH2 cells secrete IL-4, IL-5, IL-6 and IL-10 which direct the differentiation of (self-reactive) B cells. It appears likely that cross-regulation of T cell function exists between the TH1 and TH2 cell populations and that this effect is modulated by cytokines (see Fig. 2). The cytokine products of activated CD4+ T cells direct the production and recruitment of effectors, which include MHC class I-restricted cytotoxic (CD8+) T cells that directly attack antigen-bearing cells (such as islet cells in type-1 diabetes or thyrocytes in experimental autoimmune thyroiditis). Effectors also include the lymphokines tumor necrosis factor (TNF)-α and -β, which damage surrounding cells, and IFN-γ, which increases production of natural killer (NK) cells and increases MHC class II expression both on epithelial and endothelial cells. This in turn may promote localization of CD4+ T cells. Up-regulation of adhesion molecule expression on vascular endothelium by IFN-γ may also promote the homing of lymphocytes and neutrophils to affected tissues.
Fig. 2.

The central role of CD4+ T helper (TH) lymphocytes and their cytokine products in the generation of effector mechanisms in autoimmunity. Additional mechanisms (not shown) include cytokine-driven activation (via IFN-γ) of macrophages leading to tissue lysis and via (IL-2) of natural killer cells and (via IL-6) of vascular endothelium. Most autoimmune diseases are probably a combination of all of these pathogenetic mechanisms. The site of action of FK 506 on the early event of antigen-induced CD4+ TH cell activation is depicted by the open arrow
In a number of autoimmune diseases (e.g., SLE), the primary pathology is attributable to antigen-antibody complexes which localize in capillary beds, activate complement and attract leukocytes that inflict tissue damage. In another important group of autoimmune diseases, autoantibodies directed against physiological receptors on the cell surface are responsible for disease activity. Such antibodies include autoantibodies directed against the acetylcholine receptor in myasthenia gravis and autoantibodies to the thyroid-stimulating hormone receptor in Graves’ disease. Most autoimmune diseases, however, are probably caused by a combination of various cell, antibody, complement and cytokine-mediated pathogenic mechanisms.
Given the foregoing considerations, it is likely that FK 506 will have a greater efficacy in the diseases in which T cells have been shown to have a major role in inducing and maintaining disease pathology than will CsA (Table 1).
The molecular action of FK 506
The molecular actions of FK 506 and CsA have recently been reviewed by Schreiber and Crabtree [42] and Sigal and Dumont [43]. FK 506 inhibits CD4+ T cell activation mediated by the TCR-CD3 complex and via the cell surface molecule, CD2. IL-2 production, T cell proliferation, and apoptosis (programmed cell death) are all sensitive to FK 506, as they are to CsA. Inhibition of T cell activation is dependent on exposure to the drug within the first few hours of antigenic stimulation. Effective concentrations of FK 506 are 100-fold lower than those of CsA. FK 506 does not appear to directly affect the function of accessory cells. Functions of other leukocytes, however, may be influenced by FK506. Thus FK 506 (like CsA) inhibits proinflammatory mediator release from human basophils and rat mast cells, as well as the transcription of several cytokine genes, including IL-3 and IL-5. These latter actions may contribute to some of the drug’s therapeutic anti-inflammatory effects.
In T cells FK 506 acts at a step distal to the cell membrane receptors and second messengers but proximal to the transcriptional activation of early genes. Both FK 506 and CsA strongly and specifically inhibit the expression of early activation genes encoding IL-2, IL-3, IL-4, IFN-γ, granulocyte/macrophage-colony-stimulating factor and c-myc [51]. Two subsets of murine CD4+ T cells (TH1 and TH2) have been identified on the basis of the cytokines they secrete and their functional profiles [34]. There is some evidence that such functional TH subsets may also exist in humans. TH1 cells produce IL-2, IFN-γ, and TNF-β. TH2 cells secrete IL-4, IL-5, and IL-10, but not IL-2 or IFN-γ. It appears that in vitro, at least, FK 506 may spare IL-10 gene transcription by TH2 cells, while inhibiting concomitant IL-4 mRNA production [53]. Thus, differential interference with cytokine gene transcription and cross-regulation of IL-2 and IFN-γ production by TH1 cells may be an important mechanism whereby FK 506 acts to inhibit induction of immune reactivity and maintain immunosuppression.
Both FK 506 and CsA bind to specific, intracellular cytosolic receptors or “immunophilins,” designated FK 506-binding protein (FKBP) and cyclophilin, respectively [42, 43]. Both molecules exist in a number of isoforms. These “immunophilins” are peptidyl-prolyl cis-trans isomerases (PPIases) which catalyze cis-trans isomerization of Ala-Pro bonds in oligopeptides and accelerate protein folding. Binding of each drug to its respective immunophilin inhibits isomerase activity. Recent data indicate that inhibition of isomerase activity is not the critical site of action of either FK 506 or CsA. Instead, the immunosuppressive effect of both drugs results from the formation of active complexes between the drug and its respective isomerase. Complexes of FK 506 and FKBP and of CsA and cyclophilin bind specifically to three polypeptides, calmodulin, and the two subunits of a Ca2+ activated, Ser-Thr protein phosphate calcineurin [27]. In each instance, FK 506 or CsA promotes the interaction of the normally non-interacting immunophilin and calcineurin, resulting in inhibition of the phosphatase activity of calcineurin. Neither FK 506, FKBP, CsA, nor cyclophilin alone inhibits the enzymic activity of calcineurin.
A further important property of the drug-immunophilin complexes is that they block Ca2+ -dependent assembly of the IL-2 gene transcription activator nuclear factor of activated T cells (NF-AT). This is achieved by inhibition of the translocation of the preexisting cytoplasmic component of NF-AT (NF-ATc) from the cytoplasm to the nucleus [10]. The nuclear component of NF-AT (NF-ATn), which is induced by signals from the TCR, is transcriptionally inactive in all cells other than activated T lymphocytes. Its appearance is not blocked by FK 506 or CsA. It appears, however, that FK 506 and CsA block the calcineurin-mediated dephosphorylation of NF-ATc which is required for its translocation to the nucleus. In the absence of both NF-ATc and NF-ATn transcriptional activation of the IL-2 gene and other genes is suppressed. For further details of the molecular action of FK 506 and CsA the reader is referred to the recent literature [10, 27, 42, 43].
Biological and pharmacological properties of FK 506 in comparison to CsA
Although quite distinct in molecular structure, FK 506 and CsA share many biological and pharmacological properties (e.g., binding to intracellular immunophilins, selective interference with CD4+ T cell function, rate of systemic absorption, plasma half-lives, etc.). The similar properties of the two immunosuppressants are listed in Table 2. Properties of FK 506 and CsA which are known to differ are shown in Table 3. Of special significance, in relation to the potential value of the drugs in autoimmune diseases, is the superior capacity of FK 506, compared with CsA, to reverse cell-mediated immunity (e.g., allograft rejection or graft-versus-host disease).
Table 2.
Biological and pharmacological properties of FK 506 and cyclosporin A (CsA): similarities
|
FK BP, FK 506-binding protein
Table 3.
Biological and pharmacological properties of FK 506 and CsA: differences
|
GVHD, Graft-versus-host disease
The influence of FK 506 on experimental autoimmune diseases
FK 506 is effective in inhibiting a wide variety of experimental autoimmune disorders in rodents and in larger laboratory animals (Table 4). The autoimmune diseases in which the immunosuppressive efficacy of FK 506 has been proved include uveitis, type-1 diabetes, thyroiditis, autoimmune renal disorders, experimental allergic encephalomyelitis (a correlate of multiple sclerosis) and autoimmune myocarditis. An example of the inhibitory effect of FK 506 on experimental autoimmune disease is shown in Fig. 3. PVG/c female rats which have been neonatally thymectomized and sublethally irradiated subsequently develop autoimmune thyroiditis. The development of the disease is inhibited by a 3-week course of FK 506 starting after demonstration of serological evidence of autoantibody (anti-thyroglobulin antibody) production. Some untreated disease control animals also develop insulitis. This can be prevented by FK 506 administration.
Table 4.
Experimental autoimmune diseases suppressed by FK 506
| Disorder | Species | FK 506 dose (mg/kg per day unless specified) | [Reference] |
|---|---|---|---|
| Arthritis (type II collagen-induced) | Rat (Lewis) | 0.32a,b | [20] |
| Rat (outbred) | 2.5c | [2] | |
| Mouse (DBA/1) | 2.0 | [48] | |
| Type-1 diabetes | NOD mouse | 2.0 mg/kg per 48 ha | [32] |
| Cyclophosphamide-treated NOD mouse | 0.2, 1. 2 | [5] | |
| BB rat | 1.0a | [35] | |
| BB rat | 25 μg i.m.d | [36] | |
| Uveoretinitis | Rat (Lewis) | 1.0a,e | [23] |
| Rhesus & cynomolgus monkeys | 0.5f | [12] | |
| Thyroiditis | Rat (PVG) | 2.0g | [49] |
| Lupus (SLE) | MRL/lpr/lpr mouse | 2 mgh | [55] |
| NZB/NZW F1 mouse | 2.5 mg/kg per 48 hi | [47] | |
| Glomerulonephritis (Nephrotoxic antiserum nephritis) | Rat (Wistar) | 0.3 mgi | [15] |
| Rat (Wistar) | 0.64 | [38] | |
| Heymann nephritis | Rat (Wistar) | 0.64a | [38] |
| Rat (Lewis) | 1.0k | [28] | |
| Allergic encephalomyelitis | Rat (Lewis) | 1.0j | [19] |
| Autoimmune myocarditis | Rat (Lewis) | 0, 1 0.32, 1 | [14] |
| Experimental allergic contact dermatitis | Farm pig | 0.04, 0.4% topical | [31] |
| Murine (coxsackie B3) myocarditis | Mice (C3H/He) | 2.5 | [17] |
Suppresses induction of disease
Partially effective during efferent phase of response
On day of immunization
Administered daily from 27–120 days of age
Effective only in induction phase
Administered from 3 weeks after immunization
Administered for 3 weeks following induction of disease
Administered from 8 weeks of age
From time of immunization
Administered 5 days per week after immunization
Administered from day 0–13 or 56–69
Fig. 3.

a–d. Prevention of the development of experimental autoimmune thyroiditis and insulitis in thymectomized, x-irradiated female PVG/c rats treated with FK 506 starting after serological evidence of autoantibody (anti-thyroglobulin) production. a Lymphocytic thyroiditis with obliteration of thyroid follicles in untreated, control animals; b inhibition of thyroiditis by FK 506; c severe insulitis, which was evident in 30% of untreated disease control animals, and d prevention of insulitis by FK 506
Early clinical experience with FK 506 at the University of Pittsburgh (Table 5)
Table 5.
Protocols for treatment of autoimmune diseases utilizing FK 506 at the University of Pittsburgh Medical Center (September 1992)a
| Eye and ear diseases |
| Uveitis (2); scleritis (1) |
| Ocular cicatrical pemphigoid (1) |
| Autoimmune inner ear disease (4) |
| Neurological |
| Multiple sclerosis (57); amyotrophic lateral sclerosis (4) |
| Renal |
| Nephrotic syndrome (32) |
| Gastrointestinal |
| Ulcerative colitis (19) |
| Crohn’s disease (17) |
| Chronic active hepatitis (autoimmune) (21) |
| Primary sclerosing cholangitis (11) |
| Primary biliary cirrhosis (16) |
| Sprue (4) |
| Skin |
| Psoriasis (16) |
| Pyoderma gangrenosum (6) |
| Epidermolysis bullosum (1) |
| Endocrine |
| New onset type-1 diabetes (11) |
| Collagen vascular disease/vasculitis |
| Behçet’s disease (2) |
| Scleroderma (5) |
| Polymyositis/dermatomyositis (2) |
| Wegener’s granulomatosis (1) |
Figures in parentheses denote numbers of patients being treated
Primary sclerosing cholangitis
A total of 11 cases of primary sclerosing cholangitis (PSC) have been treated with FK 506 in the autoimmune clinic at UPMC. Most of these cases have also had ulcerative colitis. A few have either had Crohn’s colitis (n=4) or no colonic disease at all (n = 4). The biochemical responses of a who responded patient with PSC and ulcerative colitis to FK 506 therapy is shown in Fig. 4. A dramatic decline in total bilirubin and alkaline phosphatase occurred early, with a later reduction in the ALT and AST levels.
Fig. 4.
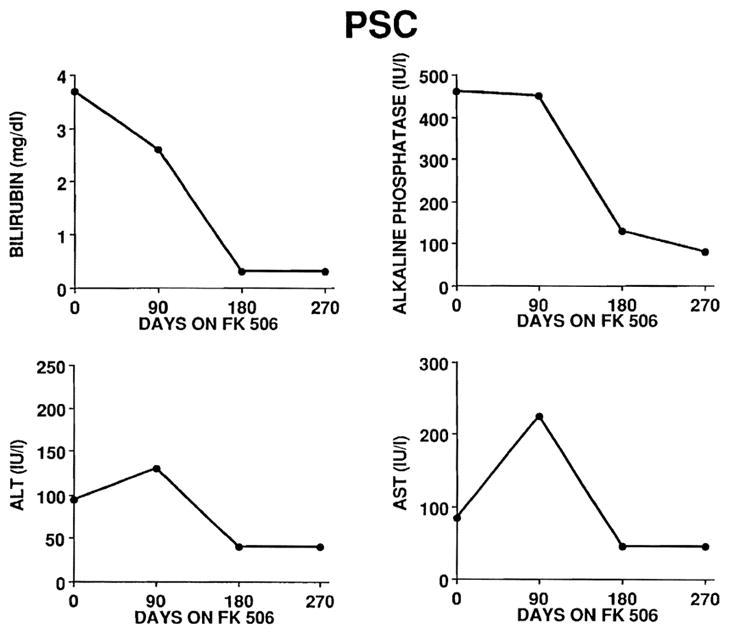
Biochemical responses (serum total bilirubin, alkaline phosphatase, ALT and AST levels) in a representative responder with primary sclerosis cholangitis (PSC) treated with FK 506
Primary biliary cirrhosis
A total of 16 patients with PBC have been treated with FK 506 in the autoimmune clinic of UPMC. The majority of these cases have been women. Most but not all have shown a response to FK 506 therapy as illustrated in Fig. 5, with a marked reduction in the serum bilirubin and alkaline phosphatase levels and with modest reductions into the normal range for serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels.
Fig. 5.
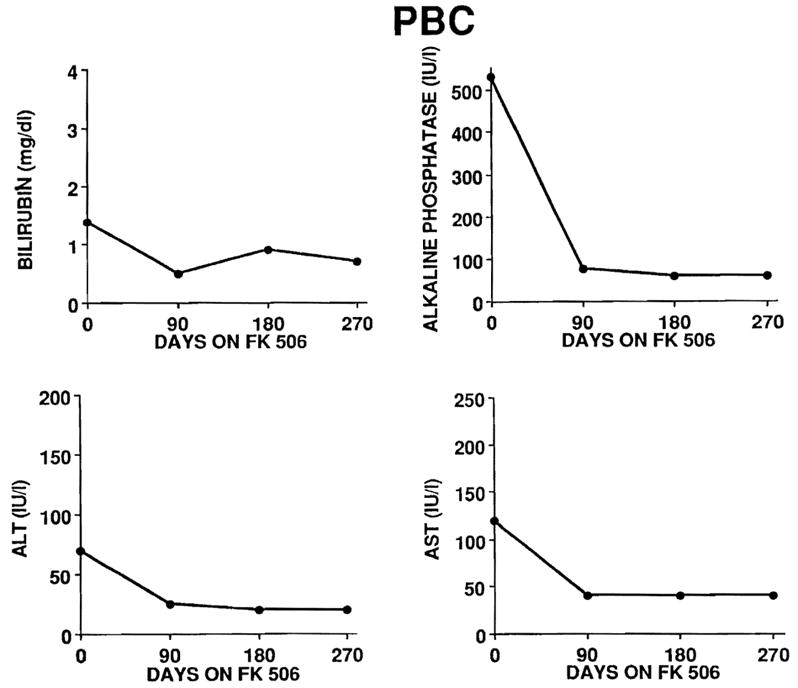
Biochemical responses (serum total bilirubin, alkaline phosphatase, ALT and AST levels) in a representative responder with primary biliary cirrhosis (PBC) treated with FK 506
Autoimmune chronic active hepatitis
A total of 21 cases of autoimmune chronic active hepatitis have been treated with FK 506 at UPMC. The majority have been women. Half had failed to respond to glucocorticoids alone or used in combination with azathioprine. One failed with CsA. In responders, elevated bilirubin and transaminase levels declined into the normal range with the institution of FK 506 (Fig. 6). Of the three types of hepatic disease that have been treated with FK 506, this group with CAH-A appears to respond best and most consistently. Patients with well-established, advanced cirrhosis are unlikely to respond as shown in Figs. 4–6.
Fig. 6.
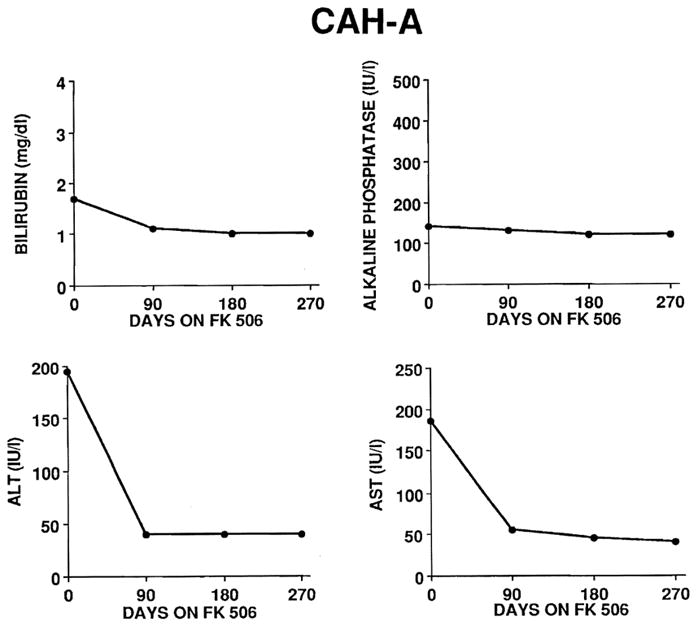
Biochemical responses (serum total bilirubin, alkaline phosphatase, ALT and AST levels) in a representative responder with chronic active hepatitis-autoimmune (CAH-A) treated with FK 506
Crohn’s disease
A total of 17 patients with Crohn’s disease have been treated with FK 506 at UPMC. Most have had complicated diseases, involving the colon or colon and small bowel with multiple perianal, vaginal, vesicular or cutaneous fistulae. FK 506 treatment has, in all cases, been followed by a reduction in the amount of drainage and in most cases, by drying up of fistulous tracts. Enteric disease has not been eradicated under FK 506 but the complications have resolved such that surgical repair has become possible. It may well be that the ideal role for FK 506 in this disease process may be in the preoperative management of cases. Often, an apparently inoperable case can be converted to an easily resectable case with the use of FK 506. Moreover, its use preoperatively enables the patient to go to surgery without the use of steroids that complicate healing and foster infection in the early post-operative period.
Ulcerative colitis
A total of 19 cases of ulcerative colitis have been treated with FK 506. Of these 11 also have PSC, while 6 have ulcerative colitis without confounding PSc. The response of ulcerative colitis to FK 506 treatment has not been particularly impressive. Nonetheless, it has enabled several of the non-PSC cases to stabilize their disease activity and then to undergo one or another form of surgery to treat a complication of the disease, usually a colonic stricture. The role of FK 506 in the management of ulcerative colitis appears to be less clear than its role in the treatment of Crohn’s disease and related complications.
Sprue (celiac disease)
A total of four patients with sprue documented by (i) the presence of gluten sensitivity, (ii) an atrophic small bowel mucosa on intestinal biopsy, and (iii) the presence of detectable reticulum antibodies have been treated with FK 506. One of these four cases had failed gluten withdrawal and had required total parenteral nutrition (TPN) for several years prior to the use of FK 506. All patients are able to eat a normal diet containing gluten without the development of symptoms while taking FK 506. D-Xylose absorption has improved in all four subjects but remains abnormal in the single patient who had required TPN for several years prior to the institution of FK 506. On FK 506 treatment the appearance of intestinal biopsies has improved in all cases; the improvement has been rather small, however, in the single case that had required TPN to maintain her nutritional status prior to the use of FK 506. Despite still requiring TPN, the extraintestinal consequences of her disease (hypogonadism, cheilitis, hair loss, fatigue and seizures related to hypocalcemia) have all resolved or improved while on FK 506.
Uveitis/scleritis (n=3)
FK 506 appears to be of benefit in these conditions [33]. One patient with scleritis who was refractory to CsA has responded to FK 506. Preliminary data appear to show that steroid use will still be required in these conditions, although at a reduced dose.
Psoriasis
The results of treatment of psoriasis with FK 506 (n = 16) have been quite rewarding [21] (Fig. 7). All patients have shown a dramatic response to treatment, some with rapid clearing and others who take months to show clearing of the disease. Disease remission is associated with reduction in activated T cells within lesional skin (Fig. 8). The renal toxicity of the drug appears to limit the ability to achieve remissions in some patients, especially in those who have received prior treatment with methotrexate. Combination therapy may be required in some of these subjects.
Fig. 7.

a, b. Appearance of patient with severe, recalcitrant, chronic plaque psoriasis a before and b 4 weeks after start of treatment with FK 506
Fig. 8.
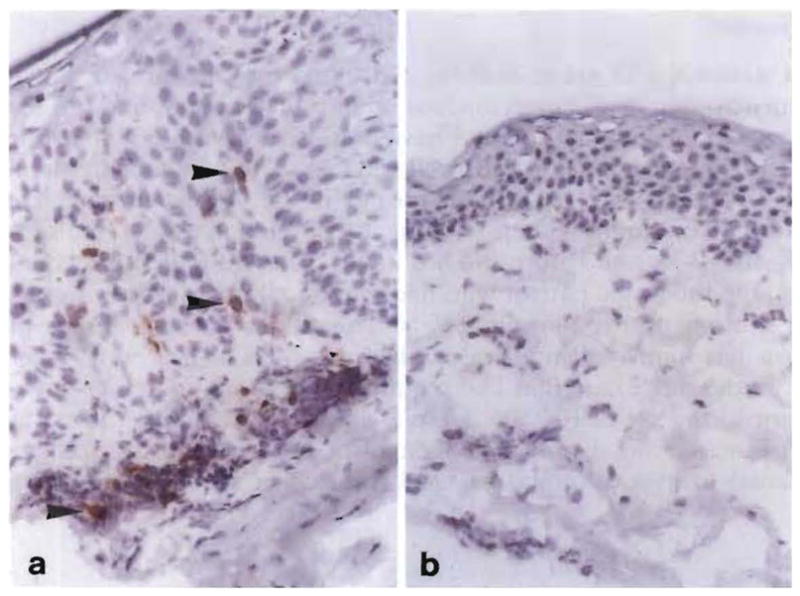
a, b. Effect of FK 506 treatment on psoriatic skin lesions. a IL-2 receptor α (IL-2Rα) chain-positive (CD25+) lymphocytes within the upper dermis in untreated active disease and b absence of IL-2Rα+ cells in lesional skin of the same patient, 4 weeks after start of systemic FK 506 therapy. Note also the marked reduction in epidermal keratinocyte proliferation
Pyoderma gangrenosum
The results of treatment of pyoderma gangrenosum (n = 6) with FK 506 are promising [1]. One patient who was chronically steroid dependent since childhood, with multiple steroid-induced complications, was able to suspend the use of steroids for the first time in his life. Four out of six patients achieved total remissions and one significant partial remission was obtained. One patient was refractory to FK 506.
Multiple sclerosis
In the last few months, we have begun to treat patients (n = 57) with FK 506 and it is too early to make concrete statements. Patients with severe and mild disabilities have been treated, so the group is heterogenous. Some patients who received FK 506 for transplants and who coincidentally, had multiple sclerosis, appear to have shown objective improvements over time. The neurotoxicity of the drug seems to be more limiting in this condition than in other autoimmune disorders.
Type-1 insulin-dependent diabetes
To date, 11 patients with new onset type-1 diabetes have been treated with FK 506. Here we describe the experience with one case.
A 20-year-old male who was well until sustaining a cartilage injury to the right knee noted the sudden onset of polyuria, polydipsia and nocturia 1 day after the injury. There was no weight loss or blurred vision. He was noted to have glycosuria in the orthopedic surgeon’s office and a blood sugar of 300 mg/100 ml with ketonuria. He was started on 15 units of insulin daily and was referred to the UPMC autoimmune clinic for consideration in the FK 506 trial. The physical examination was unremarkable and the patient was started on FK 506 (10 mg twice daily). His HLA type was A1, A2, B8, B27, DR4 and islet cell antibodies were positive.
A 6 calorie/kg Sustacal stimulation was done to assess endogenous C-peptide at trial entry and at 3-monthly intervals. The results of the stimulation tests performed over time are shown in Fig. 9. At 3 months, his insulin dose had decreased to 10 units/day. His glycosylated hemoglobin was 6.4% (normal up to 6.4%). His starting creatinine was 1.0 and at 3 months was 1.2 mg/100 ml with a glycosylated hemoglobin of 6.6%. His FK 506 dose was continued at 10 mg twice daily since he had no associated toxicities. Levels of FK 506 in serum were 0.4−0.7 ng/ml. At 6 months, his insulin dose was 5 units/day. His serum creatinine was 1.1 mg/100 ml. His glycosylated hemoglobin was 6.6% and he had no toxicities. The FK 506 was continued. He was able to suspend the use of exogenous insulin 8 months after entry and 6 weeks after suspending insulin, a 75 g oral glucose tolerance test showed impaired glucose tolerance (2-h plasma glucose 285 mg/100 ml) with no intervening value ≥200 mg/100 ml. He is being maintained on 10 mg FK 506 twice daily with no toxicity.
Fig. 9.
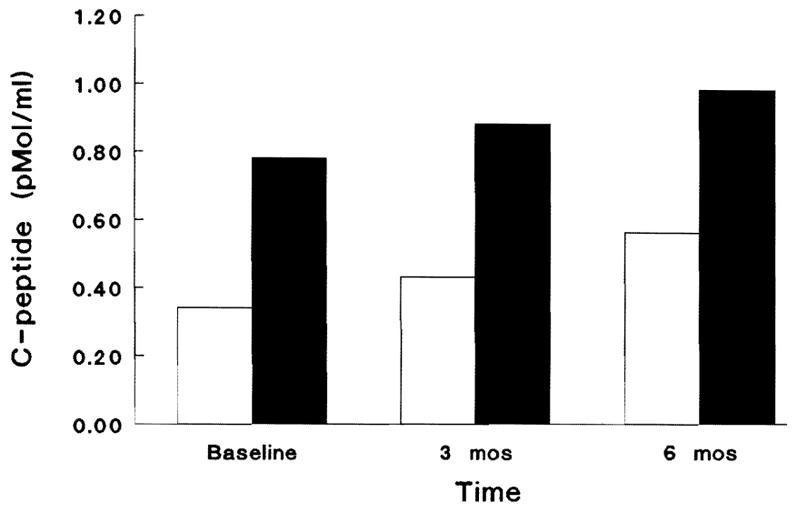
The influence of FK 506 therapy in a case of type-1 diabetes (described in the text). The figure shows serial results of a 6 ca/kg sustacal stimulation test (max 360 ml). Bloods were drawn at −5, 0, 15, 30, 60, 120, 180, and 300 min. The figure shows the basal and maximally stimulated C-peptide levels that reflect endogenous insulin secretion over time after start of FK 506 treatment. The patient is currently off insulin in a complete remission. □ Basal; ■ stimulated
Nephrotic syndrome
Our experience in treating steroid-resistant nephrotic syndrome (n = 32) with FK 506 monotherapy has demonstrated three distinct patterns [29, 30] (Fig. 10). These three groups are approximately equally divided. Some patients (e.g., patient no. 1 in Fig. 10) experienced a rapid reduction in proteinuria to essentially normal values within weeks of initiating therapy. Others (e.g., patient no. 2) responded partially to FK 506 alone, reducing protein excretion to approximately 50% of pretreatment values. The last group (e.g., patient no. 3) have demonstrated no change in protein excretion and have progressed to end-stage kidney disease. The majority of the patients treated to date have had focal sclerosing glomerulonephritis (GN) as the cause of steroid-resistant nephrotic syndrome. This, and the other histological lesions treated in our study, tend to be resistant to essentially all forms of therapy and tend to progress to end-stage renal disease
Fig. 10.
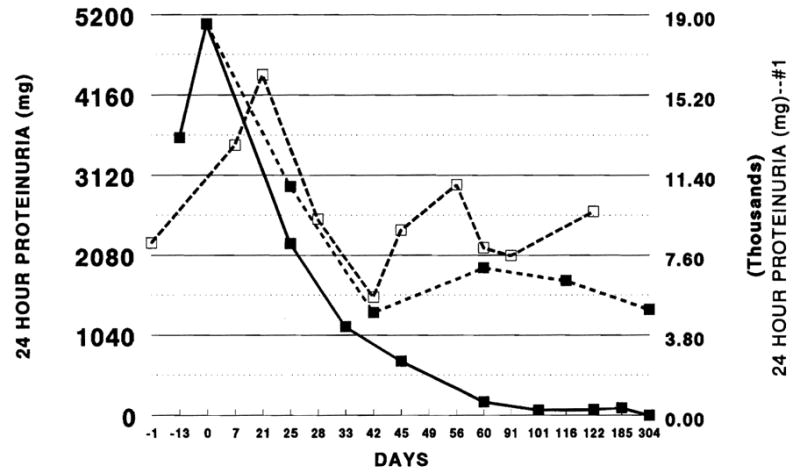
Three patterns of response to FK 506 in nephrotic syndrome, illustrated by individual patients (nos 1–3). For a fuller description see the text.—■—#1;––#2; #3
#3
Previous studies have demonstrated elevated IL-2 and IL-2R levels in some forms of GN [22, 37]. Figure 11 illustrates the change in IL-2R in a patient (no. 1 of Fig. 10), a 21 year old female with steroid resistant nephrotic syndrome, who developed a complete remission of nephrotic syndrome and dramatic improvement in renal function. The patient’s proteinuria fell from 18000 mg/24 h to non-detectible levels within 3 months. IL-2R levels were elevated at all times relative to controls but fell with the introduction of FK 506. Although IL-2R levels rose slightly after 96 days of therapy, there was no associated increase in proteinuria or deterioration of renal function. This patient had, importantly, presented with a sudden onset of proteinuria which was treated within weeks of its perceived induction time. These three patterns of response may indicate variations in the basic pathogenesis of the disease in which cytokine production plays greater or lesser parts. Another possible explanation might be the existence of different pathogenic mechanisms in the induction and maintenance of the various forms of GN. Data from experimental Heymann’s nephritis clearly suggest an evolution from a major involvement of T cells to later recruitment of other immune effector elements in the cascade illustrated in Fig. 2. Partial and total unresponsiveness to FK 506 may reflect that mediation of GN in these patients lies distal to IL-2 activation. In these cases, addition of other immunosuppressive agents which affect the more distal elements might improve the efficacy of FK 506. The use of anti-B cell agents, such as cyclophosphamide, would seem to be a rational choice, as would the use of small doses of prednisone in patients refractory to FK 506 alone.
Fig. 11.

Serum IL-2R and FK 506 levels in a patient with steroid-resistant nephrotic syndrome who showed a dramatic improvement in a renal function following the start of FK 506 therapy. For additional details, see the text. IL-2R Normal;—●— IL-2R Patient; —◆–– FK 506 level
The nephrotoxicity of FK 506 has been the primary limiting factor in the treatment of these diseases. As with CsA, FK 506 causes acute rises in serum creatinine which are dose dependent. There is improvement of renal function with dosage reduction. We have reduced the starting dose of FK 506 progressively from 0.15 mg/kg/day given twice to approximately one-third of this dose. Whether this approach will lessen the efficacy of FK 506 is yet to be determined. We have seen no evidence of chronic FK 506 toxicity in any of the patients treated for nephrotic syndrome, even at the highest dose.
Laboratory investigations to monitor the status of TH cells in FK 506-treated patients
We are presently monitoring systemic CD4+ T cell activation status in autoimmune disease patients receiving FK 506 therapy. These studies are aimed at determining the influence of FK 506 on those markers of peripheral blood T cell function which may be affected by disease activity. In addition to quantitative one- and two-color immunofluorescence analysis for functional T cell subsets (Table 6), we are evaluating the influence of FK 506 treatment on cytokine gene expression by peripheral blood mononuclear cells and determining, where possible, proliferative responses of T cells to disease-associated antigens (e.g., myelin basic protein in multiple sclerosis). Representative preliminary results in patients with nephrotic syndrome and PBC (Fig. 12) show reductions both in CD4+ and CD8+ T cells expressing the IL-2R α chain (CD25), which is a functional marker of T cell activation, in response to FK 506 treatment.
Table 6.
Investigations on peripheral blood lymphocytes of autoimmune disease patients
| Quantitative flow cytometric immunophenotypic analysis |
| One-color |
| CD4 |
| CD8 |
| Two-color |
| CD4: IL-2Rα; IL-2Rβ 4B4 (helper-inducer); 2H4 (suppressor-inducer) |
| CD8: IL-2Rα; IL-2Rβ |
| Cytokine gene expression and cytokine production |
| PCR analysis (mRNA) and ELISA (secreted protein) for: |
| TH1 cytokines (IL-2; IFN-γ) |
| TH2 cytokines (IL-4; IL-10) |
| Proliferative responses |
| Con A |
| Anti-CD3 |
| rIL-2 |
| Relevant antigen (e.g., MBP, GAD) |
IL, Interleukin; PCR, polymerase chain reaction, ELISA, enzyme-linked immunosorbent assay;
TH, T helper (cell). IFN, interferon; Con A, concanavalin A; r, recombinant; MBP, myelin basic protein; GAD, glutamic acid decarboxylase
Fig. 12.
Influence of FK 506 treatment on the mean incidences of IL-2Rα+ CD4+ and CD8+ T cells in the peripheral blood of patients with nephrotic syndrome or PBC treated for various times with FK 506. Values (means ± 1 SD) obtained in normal healthy adults are also shown. Number of patients in parentheses
The side effects of FK 506 in humans
Side effects of FK 506 and CsA observed both in transplant and autoimmune disease patients are shown in Table 7. Adverse effects associated with the use of FK 506 in transplant patients have recently been reviewed [13]. Adverse reactions requiring treatment or FK 506 dose reduction are impairment of renal function (due in part to decreased glomerular blood flow), alterations in glucose homeoastasis (a 15% incidence of new onset diabetes has been observed in FK 506-treated transplant patients but none in autoimmune disease patients) and neurotoxicity. Amongst the benefits of FK 506 compared with CsA are a lower incidence of de novo hypertension, and the absence of gum hypertrophy and hirsutism. A 1.6% incidence of post-transplant lymphoproliferative disorders has been observed in UPMC FK 506-treated transplant patients. No lymphoproliferative disorders have been reported from the FK 506 treated autoimmune disease patient population (mean follow up time > 24 months).
Table 7.
Side effects of FK 506 and CsA
| Similarities |
| Decreased glomerular blood flow |
| Elevated serum postassium (? reduced aldosterone sensitivity of renal tubules) |
| De novo hypertension (CsA > FK 506) |
| Lymphoproliferative diseasea |
| Nephrotoxicity |
| Neurotoxicity |
| Diabetogenicity |
| Decreased bone mineralizationb (in vitro) |
| Steroid spearing (FK 506 > CsA) |
| Differences |
| Hirsutism (CsA; not FK 506) |
| Gum hypertrophy (CsA; not FK 506) |
| Hyperlipidemia (CsA) |
| Hypocholesterolemia (FK 506) |
| Elevated 1,25-(OH)2 Dc levels (CsA; not FK 506) |
Reported in transplant patients
FK 506 data based on in vitro observations only
Serum, 1,25-dihydroxyvitamin D
Prospects for drug combination therapies
In addition to FK 506 monotherapy, the potential exists for drug combination therapies in autoimmune disease using FK 506 in combination with other novel (see Table 8) or well-established classes of immunosuppressive agents which act by mechanisms different from FK 506 or CsA. Experimental combination therapies which are currently envisaged (e.g., for psoriasis or rheumatoid arthritis) include FK 506 together with an antiproliferative agent, such as methotrexate, cyclophosphamide or the purine biosynthesis inhibitor RS 61443 (mycophenolate mofetil).
Table 8.
Modes of action of new immunosuppressive drugs which offer opportunities for drug combination therapy in autoimmune disease
| Inhibitors of cytokine synthesis | |
| CsA | |
| FK506 | |
| Inhibitor of cytokine action | |
| Rapamycin | |
| Inhibitors of DNA synthesis | |
| Mizoribine | ↓ purine synthesis |
| Mycophenolic acid (RS61443) | ↓ purine synthesis |
| Brequinar sodium | ↓ pyrimidine synthesis |
| Inhibitors of cell maturation | |
| Deoxyspergualin | |
References
- 1.Abu-Elmagd K, Jegasothy BV, Ackerman CD, Thomson AW, Rilo H, Nikolaidis N, et al. Efficacy of FK 506 in the treatment of recalcitrant pyoderma gangrenosum. Transplant Proc. 1991;23:3328. [PMC free article] [PubMed] [Google Scholar]
- 2.Arita C, Hotokebuchi T, Miyahara H, Arai K, Sugioka Y, Takagishi K, Kaibara N. Inhibition by FK 506 of established lesions of collagen-induced arthritis in rats. Clin Exp Immunol. 1990;832:456. doi: 10.1111/j.1365-2249.1990.tb05471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker BS, Fry L. The immunology of psoriasis. Br J Dermatol. 1992;126:1. doi: 10.1111/j.1365-2133.1992.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 4.Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 year of cyclosporin treatment with enhanced insulin secretion. Diabetes. 1988;37:1574. [PubMed] [Google Scholar]
- 5.Carroll PB, Strasser S, Alejandro R. The effect of FK 506 on cyclophosphamide-induced diabetes in the NOD mouse model. Transplant Proc. 1991;23:3348. [PubMed] [Google Scholar]
- 6.Castano L, Eisenbarth GL. Type 1 diabetes: a chronic autoimmune disease of human, mouse and rat. Annu Rev Immunol. 1990;8:647. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 7.Clark G, Williams DG. Immunology of minimal-change nephropathy. In: Pusey P, editor. Immunology of renal diseases. Kluwer; Boston: 1992. p. 161. [Google Scholar]
- 8.Dohi K, Iwano M, Muraguchi A, Horii Y, et al. The prognostic significance of urinary interleukin 6 in IgA nephropathy. Clin Nephrol. 1991;33:1. [PubMed] [Google Scholar]
- 9.Firestein GS. Mechanisms of tissue destruction and cellular activation in rheumatoid arthritis. Curr Opin Rheumatol. 1992;4:348. doi: 10.1097/00002281-199206000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK 506 and cyclosporin A. Nature. 1991;352:803. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 11.Forrester JY. Uveitis: pathogenesis. Lancet. 1991;338:1498. doi: 10.1016/0140-6736(91)92309-p. [DOI] [PubMed] [Google Scholar]
- 12.Fujino Y, Mochizuki M, Raber J, Kotake S, Gery I, Nussenblatt RB. FK 506 treatment of S-antigen induced uveitis in primates. Invest Ophthalmol Vis Sci. 1990;31:61. doi: 10.3109/02713689109013859. [DOI] [PubMed] [Google Scholar]
- 13.Fung JJ, Alessiani M, Abu-Elmagd K, Todo S, Shapiro R, Tzakis A, et al. Adverse effects associated with the use of FK 506. Transplant Proc. 1991;23:3105. [PMC free article] [PubMed] [Google Scholar]
- 14.Hanawa H, Kodama M, Zhang S, Izumi T, Shibata A. An immunosuppressant compound, FK 506, prevents the progression of autoimmune myocarditis in rats. Clin Immunol Immunopathol. 1992;62:321. doi: 10.1016/0090-1229(92)90110-a. [DOI] [PubMed] [Google Scholar]
- 15.Hara S, Fukatsu A, Suzuki N, Sakamoto N, Matsuo S. The effects of a new immunosuppressive agent, FK 506, on the glomerular injury in rats with accelerated nephrotoxic serum glomerulonephritis. Clin Immunol Immunopathol. 1990;57:351. doi: 10.1016/0090-1229(90)90110-c. [DOI] [PubMed] [Google Scholar]
- 16.Hintzen RQ, Polman CH, Lucas CJ, van Lier RAW. Multiple sclerosis: immunological findings and possible implications for therapy. J Neuroimmunol. 1992;39:1. doi: 10.1016/0165-5728(92)90169-l. [DOI] [PubMed] [Google Scholar]
- 17.Hiraoka Y, Kishimoto C, Kurokawa M, Ochiai H, Sasayama S. The effects of FK 506, a novel and potent immunosuppressant, upon murine coxsackievirus B3 myocarditis. J Pharmacol Exp Ther. 1992;260:1386. [PubMed] [Google Scholar]
- 18.Hisanaga S, Kawagoe H, Yamamoto Y, Kuroki N, Fujimoto S, Tanaka K, Kurokawa M. Nephrotic syndrome associated with recombinant interleukin-2. Nephron. 1990;54:277. doi: 10.1159/000185875. [DOI] [PubMed] [Google Scholar]
- 19.Inamura N, Hashimoto M, Nakahara K, Nakajima Y, Nishio M, Aoki H, Yamaguchi I, Kohsaka M. Immunosuppressive effect of FK 506 on experimental allergic encephalomyelitis in rats. Int J Immunopharmacol. 1988;10:991. doi: 10.1016/0192-0561(88)90046-x. [DOI] [PubMed] [Google Scholar]
- 20.Inamura N, Hashimoto M, Nakahara K, Aoki H, Damaguchi I, Kohsaka M. Immunosuppressive effect of FK 506 on collagen-induced arthritis in rats. Clin Immunol Immunopathol. 1988;46:82. doi: 10.1016/0090-1229(88)90008-6. [DOI] [PubMed] [Google Scholar]
- 21.Jegasothy BV, Ackerman CD, Todo S, Fung JJ, Abu-Elmagd K, Starzl TE. Tacrolimus (FK 506) - A new therapeutic agent for severe psoriasis. Arch Dermatol. 1992;128:781. [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan SC, Querfeld U, Toyoda M, Prehn J. Serum interleukin-2 levels in a patient with focal segmental glomerulosclerosis. Relationship to clinical course and cyclosporin A therapy. Pediatr Nephrol. 1990;4:166. doi: 10.1007/BF00858831. [DOI] [PubMed] [Google Scholar]
- 23.Kawashima H, Fujino Y, Mochizuki M. Effects of a new immunusuppressive agent, FK 506, on experimental autoimmune uveoretinitis in rats. Invest Ophthalmol Vis Sci. 1988;23:1265. [PubMed] [Google Scholar]
- 24.Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. FK 506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation and physiochemical and biological characteristics. J Antibiot (Tokyo) 1987;40:1249. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- 25.Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, Goto T, Okuhara M, Kohsaka M, Aoki H, Ochiai T. FK 506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK 506 in vitro. J Antibiot (Tokyo) 1987;40:1256. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 26.Krawitt EL, Wiesner RH. Autoimmune liver diseases. Raven Press; New York: 1991. [Google Scholar]
- 27.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK 506 complexes. Cell. 1991;66:807. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 28.Matsukawa W, Hara S, Yoshida F, Suzuki N, Fukatsu A, Yuzawa Y, Sakamoto N, Matsuo S. Effects of a new immunosuppressive agent, FK 506, in rats with active Heymann nephritis. J Lab Clin Med. 1992;119:116. [PubMed] [Google Scholar]
- 29.McCauley J, Shapiro R, Scantlebury V, Gilboa N, et al. FK 506 in the management of transplant related nephrotic syndrome and steroid resistant nephrotic syndrome. Transplant Proc. 1991;23:3354. [PMC free article] [PubMed] [Google Scholar]
- 30.McCauley J, Shapiro R, Ellis D, Igdal H, et al. A pilot trial of FK 506 in the management of steroid resistant nephrotic syndrome. Nephrol Dial Transplant. 1993 in press. [PMC free article] [PubMed] [Google Scholar]
- 31.Meingassner JG, Stutz A. Immunosuppressive macrolides of the type FK 506: a novel class of topical agents for treatment of skin diseases? J Invest Dermatol. 1992;98:851. doi: 10.1111/1523-1747.ep12456939. [DOI] [PubMed] [Google Scholar]
- 32.Miyagawa J, Yamamoto K, Hanafusa T, Itoh N, Nakagawa C, Otsuka A, Katsura H, Yamagata K, Miyazaki A, Kono N, Tarui S. Preventive effect of a new immunosuppressant FK 506 on insulinitis and diabetes in non-obese diabetic mice. Diabetologia. 1990;33:503. doi: 10.1007/BF00405113. [DOI] [PubMed] [Google Scholar]
- 33.Mochizuki M, Masuda K, Sakane T, Inaba G, Ito K, Kogure M, et al. A multi-centre clinical open trial of FK 506 in refractory uveitis, including Behçet’s disease. Transplant Proc. 1991;23:3343. [PubMed] [Google Scholar]
- 34.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 35.Murase N, Lieberman I, Nalesnik M, Mintz D, Todo S, Drash AL, Starzl TE. FK 506 prevents spontaneous diabetes in the BB rat. Lancet. 1990;II:373. [PMC free article] [PubMed] [Google Scholar]
- 36.Nicoletti F, Meroni PL, Barcellini W, Grasso S, Borghi MO, Lunetta M, Di Marco R, Stefani S, Mughini L. FK 506 prevents diabetes in diabetes-prone BB/Wor rats. Immunopharmacology. 1991;13:1027. doi: 10.1016/0192-0561(91)90057-e. [DOI] [PubMed] [Google Scholar]
- 37.Ohno I, Gomi H, Matsuda H, Nakano H, Matsumoto H, Kodama K. Soluble IL-2 receptor in patients with primary nephrotic syndrome. Nippon Jinzo Gakkai Shi. 1991;33:489. [PubMed] [Google Scholar]
- 38.Okuba Y, Tsukada Y, Maezawa A, Ono K, Ydano S, Naruse T. FK 506, a novel immunosuppressive agent, induces antigen-specific immunotolerance in active Heymann’s nephritis and in the autologous phase of Masugi nephritis. Clin Exp Immunol. 1990;82:450. doi: 10.1111/j.1365-2249.1990.tb05470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roitt IM, Hutchings PR, Dawe KI, Sumar N, Bodman KB, Cooke A. The forces driving autoimmune disease. J Autoimmun. 1992;5(Suppl A):11. doi: 10.1016/0896-8411(92)90015-i. [DOI] [PubMed] [Google Scholar]
- 40.Rose NR. Current concepts of autoimmune disease. In: Kahan BD, editor. Cyclosporine. Applications in autoimmune diseases. Grune & Stratton; Philadelphia: 1988. pp. 3–10. [Google Scholar]
- 41.Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent. FK 506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139:1797. [PubMed] [Google Scholar]
- 42.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK 506. Immunol Today. 1992;13:136. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 43.Sigal NH, Dumont FJ. Cyclosporin A, FK 506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 44.Starzl TE, Fung J, Venkataramanan R, Todo S, Demetris AJ, Jain A. FK 506 for liver, kidney and pancreas transplantation. Lancet. 1989;II:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starzl TE, Todo S, Fung JJ, Groth C, editors. Transplant Proc. Vol. 22. 1990. FK 506. A potential breakthrough in immunosuppression - clinical implications; p. 5. [Google Scholar]
- 46.Starzl TE, Thomson AW, Todo S, Fung JJ, Starzl TE, editors. Transplant Proc. Vol. 23. 1991. Proceedings of the first international congress on FK 506; p. 2709. [Google Scholar]
- 47.Takabayashi K, Koike T, Kurasawa K, Matsumura R, Sato T, Tomioka H, Ito I, Yoshiki T, Yoshida S. Effect of FK 506, a novel immunosuppressive drug on murine systemic lupus erythematosus. Clin Immunol Immunopathol. 1989;51:110. doi: 10.1016/0090-1229(89)90211-0. [DOI] [PubMed] [Google Scholar]
- 48.Takagishi K, Yamamoto M, Nishimura A, Yamasaki G, Kanazawa N, Hotekebuchi T, Kaibara N. Effects of FK 506 on collagen arthritis in mice. Transplant Proc. 1989;21:1053. [PubMed] [Google Scholar]
- 49.Tamura K, Woo J, Murase N, Carrieri G, Nalesnik MA, Thomson AW. Suppression of autoimmune thyroid disease by FK 506: influence on thyroid-infiltrating cells, adhesion molecule expression and anti-thyroglobulin antibody production. Clin Exp Immunol. 1993;91:368. doi: 10.1111/j.1365-2249.1993.tb05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson AW. FK 506. How much potential? Immunol Today. 1989;10:6–9. doi: 10.1016/0167-5699(89)90057-1. [DOI] [PubMed] [Google Scholar]
- 51.Tocci MJ, Matkovich DA, Collier KA, Kwok P, Dumont F, Lin S, Degudicibus S, Siekierka JJ, Chin J, Hutchinson NI. The immunosuppressant FK 506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718. [PubMed] [Google Scholar]
- 52.von Graffenried B, Friend D, Shand N, Scheiss W, Timonen P. Cyclosporin A (Sandimmun) in autoimmune diseases. In: Thomson AW, editor. Cyclosporin. Mode of action and clinical application. Kluwer; Boston: 1989. pp. 213–251. [Google Scholar]
- 53.Wang SC, Zeevi A, Tweardy DJ, Jordan ML, Simmons RL. FK 506, rapamycin and cyclosporine A: effects on IL-4 and IL-10 mRNA levels in a T helper cell line. Transplant Proc. 1991;23:2920. [PubMed] [Google Scholar]
- 54.Wardle E. Cytokine growth factors and glomerulonephritis. Nephron. 1991;57:257. doi: 10.1159/000186272. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto K, Mori A, Nakahama T, Ito M, Okudaira H, Miyamoto T. Experimental treatment of autoimmune MRL/lpr/lpr mice with immunosuppressive compound FK 506. Immunology. 1988;69:222. [PMC free article] [PubMed] [Google Scholar]
- 56.Zeevi A, Duquesnoy R, Eiras G, Rabinowich H, Todo S, Makowka L, Starzl TE. Immunosuppressive effect of FK 506 on in vitro lymphocyte alloactivation: synergism with cyclosporine A. Transplant Proc. 1987;19(Suppl 6):40. [PMC free article] [PubMed] [Google Scholar]



