Nephrotic syndrome developing after renal transplantation has many etiologies. Recurrent focal sclerosing glomerulonephritis is one of the leading causes.1 Other etiologies include: (1) transplant glomerulopathy; (2) chronic rejection; (3) de novo membranous glomerulonephritis (GN); and (4) other recurrent glomerulonephropathies (membranoproliferative glomerulonephritis [MPGN], systemic lupus erythematosus [SLE], diabetes, etc).2 The important treatment goals for these patients are to control the metabolic consequences of nephrotic syndrome and delay or prevent progression of renal failure. None of these diseases has been successfully treated after transplantation with the therapies conventionally used for native kidney GN. After a dramatic improvement in proteinuria in a patient with de novo focal segmental glomerulosclerosis (FSGS), we embarked on a limited pilot trial to determine if FK 506 might be helpful in managing these patients.3 Because this was a probing effort, no patient with posttransplant nephrotic syndrome was eliminated from consideration. This diverse group of histological lesions might reproduce the immunologic mediators of the primary disease that developed in the patient’s native kidneys or initiate a wholly different set of immune processes that result in a totally different form of GN.4 Posttransplant glomerulopathies may provide a unique opportunity to investigate the determinants of glomerular injury.
PATIENTS AND METHODS
From March 1990 until May 1992, 24 patients with posttransplant nephrotic syndrome were converted from cyclosporine A (CyA) to FK 506. Approximately half the patients were initially transplanted at the University of Pittsburgh; the remainder were referred to the University of Pittsburgh specifically for management of the nephrotic syndrome with FK 506. The criteria for entry into this study were serum creatinine (Scr) <6.0 and the presence of nephrotic range proteinuria. Baseline graft biopsies were performed in all cases.
Immunosuppression Protocols
All patients were receiving CyA and prednisone immunosuppression; most also received imuran. CyA was stopped 24 hours or the evening prior (12 hours) to conversion if no rejection was noted on baseline biopsies. The initial conversion FK 506 dose was .3 mg/kg/d given in two divided doses. After approximately six patients, the dose of FK 506 was lowered to .2 mg/kg/d in divided doses. This reduction in dosage was primarily due to significant nephrotoxicity and other symptoms of excessive FK 506 therapy. The starting dose was later lowered to .15 mg/kg/d twice per day if rejection was absent at the time of conversion. If moderate rejection was present on pretreatment biopsies, a dose of .2 to .3 mg/kg/d given twice was used. One patient with moderate rejection received intravenous solumedrol at the time of conversion.
Imuran was discontinued in the first cases but continued in the most recent patients in an attempt to reduce the dose of FK 506 required and the accompanying nephrotoxicity. The prednisone dose was initially reduced by approximately one half at the time of conversion in the early cases. The prednisone dose is now continued unchanged unless rejection is detected on pretreatment biopsies.
Patient Groups by Pretreatment Graft Biopsy
Based upon pretreatment biopsies, patients were classified into four groups: (1) FSGS (10 patients); (2) recurrent lupus nephropathy (2 patients); (3) chronic rejection (11 patients); (4) de novo membranous GN (1 patient). One of the patients with FSGS had de novo FSGS; his native kidney disease was polycystic kidney disease. One patient with chronic rejection also had evidence for transplant glomerulopathy, but the latter was not felt to be the predominant lesion.
Definition of Outcomes
Patients were considered improved if 24-hour protein excretion was reduced by at least 50% and SCr was <1.5 mg/dL greater than baseline values. Patients were considered to have failed therapy if 24-hour protein excretion had not declined by at least 50%. If SCr increased >2.0 mg/dL or was progressively increasing, patients were considered to have failed. Any patient requiring dialysis or retransplantation was considered to have failed. Differences between means were determined by Student’s t test or analysis of variance when appropriate.
RESULTS
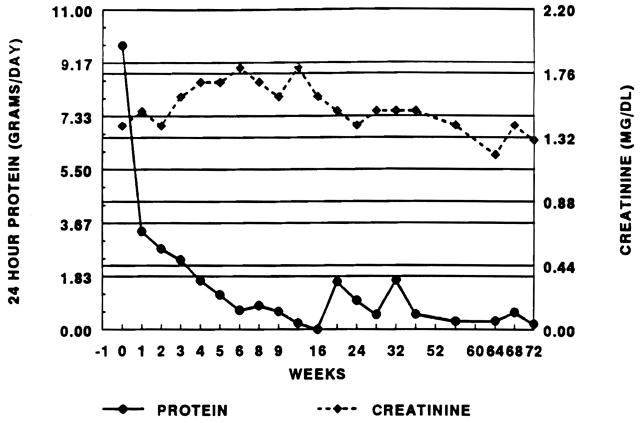
Our experience in converting renal transplant patients from CyA to FK 506 was encouraged by the clinical outcome of our first patient; his clinical course is illustrated in Fig 1. This patient, with a history of polycystic kidney disease, developed de novo FSGS with significant mesangial proliferation and mild acute cellular rejection (ACR) on a pretreatment biopsy. Simple conversion from CyA to FK 506 resulted in a rapid and sustained fall in proteinuria without deterioration in renal function. These encouraging results led us to explore a potential role for this drug in managing nephrotic syndrome after renal transplantation.
Fig 1.
Clinical course of the first patient treated converted from CyA to FK 506 for nephrotic syndrome.
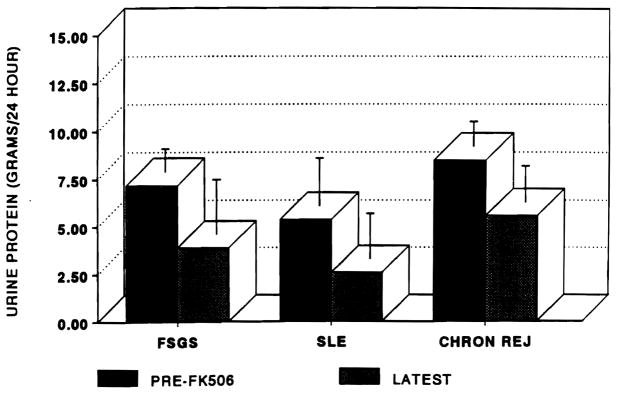
The clinical outcomes in all patients by pretreatment renal histology are illustrated in Fig 2. Twenty-four-hour protein excretions were: FSGS (7.2 ± 1.2 g pretreatment to 3.9 ± 2.9); recurrent SLE (5.4 ± 2.5 pre and 2.6 ± 2.4 post); and chronic rejection (8.5 ± 1.3 pre and 5.6 ± 1.9 post). There was no significant difference in pretreatment or latest 24-hour protein excretion between groups although patient numbers were small. Only 1 of the 11 patients with FSGS was found to improve, 6 of 10 patients with FSGS. Neither of the two patients with SLE improved and the patient with de novo membranous GN has experienced a 50% fall in proteinuria after 2 months of therapy.
Fig 2.
Urinary protein excretion by pretreatment allograft biopsy. Error bars are ± SEM.
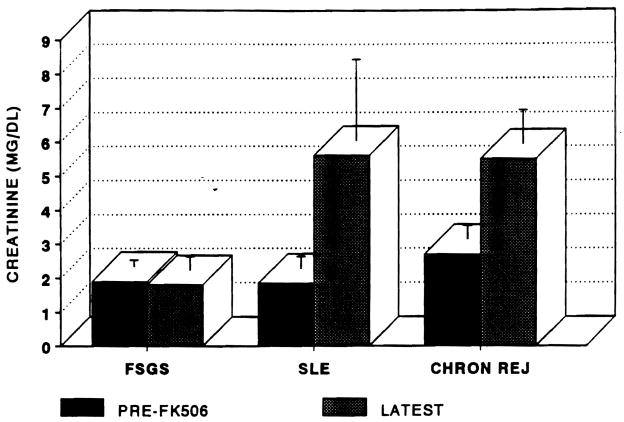
The changes in renal function by pretreatment histology are demonstrated in Fig 3. Pretreatment and latest SCr were: FSGS (1.9 ± 0.2 to 1.8 ± 0.4 mg/dL); SLE (1.85 ± 0.35 to 5.6 ± 2.4 mg/dL); and chronic rejection (2.7 ± 0.4 to 5.5 ± 1.0 mg/dL). P < .05 FSGS pre and post vs chronic rejection. The 10 patients with chronic rejection have progressed to require dialysis or retransplantation. The sole patient with improved protein excretion died of a subarachnoid hemorrhage after 3 months of therapy. Both of the patients with SLE are awaiting retransplantation although neither has started dialysis to date. Four of the six patients with FSGS have been treated for approximately 2 months. Two of the patients who failed had been converted after rapid recurrence of FSGS after transplantation. Institution of FK 506 therapy had no beneficial effect on the patients’ proteinuria and both ultimately required nephrectomy.
Fig 3.
Serum creatinine after conversion to FK 506. Error bars are ± SEM.
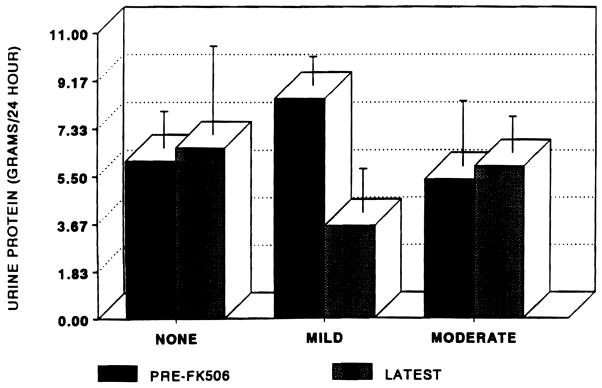
The contribution of graft rejection on pretreatment biopsies to protein excretion is depicted in Fig 4. Patients with mild ACR experienced the greatest fall in protein excretion. Patients with moderate ACR or none had insignificant changes in proteinuria. Pretreatment and latest SCr by degree of rejection were: no ACR (2.54 ± 0.8 to 3.2 ± 1.0 mg/dL); mild ACR (2.1 ± 0.26 to 3.7 ± 0.9 mg/dL); and moderate ACR (2.6 ± 0.8 to 9.0 ± 1.0 mg/dL).
Fig 4.
Urinary protein excretion by degree of ACR on pretreatment allograft biopsies. Error bars are ± SEM.
DISCUSSION
Our growing experience with FK 506 in posttransplant nephrotic syndrome has revealed the limitations of this therapy and further defined the characteristics of the group of patients who are likely to benefit. Patients with chronic rejection of any degree on preconversion biopsies universally fail to benefit and progress to end-stage renal disease or retransplantation. The presence of chronic rejection is probably an absolute contraindication for conversion to FK 506. These patients usually have advanced renal disease and apparently possess a nonimmunologic pathophysiological process that is irreversible. The speculated role of interleukin (IL)-1 in this process may also explain the lack of effectiveness of an agent that is primarily an IL-2 inhibitor.5 Neither rapidly recurrent FSGS nor recurrent SLE improved with FK 506. The lack of responsiveness of these lesions is probably due to persistent immunologic factors, such as cytokine activation and persistent antibody production, that are not under the control of CyA or FK 506.
More encouraging results have been demonstrated in patients with de novo FSGS after transplantation. FSGS has been associated with increased IL-1, IL-2, and IL-8 production that may, at least in part, be inhibited by FK 506 in the early developmental stages.6–8 Mild ACR associated with mesangial proliferation appears to be the most promising setting for FK 506 conversion. Interestingly, the absence of ACR or moderate ACR carried a worse prognosis.
The use of FK 506 after renal transplantation is likely to be helpful in managing the nephrotic syndrome in a well-defined population of patients. Investigation of the determinants of responsiveness to FK 506 offers the opportunity to more fully uncover the causes of nephrotic syndrome both after transplantation and in native kidney glomerulonephritis.
References
- 1.Senggutuvan P, Cameron JS, Hartley RB, et al. Pediat Nephrol. 1990;4:21. doi: 10.1007/BF00858431. [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ, Adler SG, Ward HJ, et al. In: The Kidney. 4. Brenner BM, Rector FC, editors. Philadelphia: W.B. Saunders; 1991. p. 1233. [Google Scholar]
- 3.McCauley J, Shapiro R, Scantlebury V, et al. Transplant Proc. 1991;23:3354. [PMC free article] [PubMed] [Google Scholar]
- 4.Wardle EN. Nephron. 1991. p. 257. [DOI] [PubMed] [Google Scholar]
- 5.Braun W. Contemp Issues Nephrol. 1989;19:45. [Google Scholar]
- 6.Fukatsu A, Matsuo S, Tamai H, et al. Lab Invest. 1991;65:61. [PubMed] [Google Scholar]
- 7.Torbohm I, Berger B, Schoenermark M, et al. Clin Exp Immunol. 1989;75:427. [PMC free article] [PubMed] [Google Scholar]
- 8.Lovett DH, Larsen A. J Clin Invest. 1988;82:115. doi: 10.1172/JCI113558. [DOI] [PMC free article] [PubMed] [Google Scholar]