Abstract
INTRODUCTION
Non-occlusive small bowel necrosis (NOSBN) has been associated with early postoperative enteral feeding. The purpose of this study was to determine the incidence of this complication in an elective upper gastrointestinal (GI) surgical patient population and the influence of both patient selection and type of feeding jejunostomy (FJ) inserted, based on the experience of two surgical units in affiliated hospitals.
PATIENTS AND METHODS
The records were reviewed of 524 consecutive patients who underwent elective upper GI operations with insertion of a FJ for benign or malignant disease between 1997 and 2006. One unit routinely inserted needle catheter jejunostomies (NCJ), whilst the other selectively inserted tube jejunostomies (TJ).
RESULTS
Six cases of NOSBN were identified over 120 months in 524 patients (1.15%), with no difference in incidence between routine NCJ (n = 5; 1.16%) and selective TJ (n = 1; 1.06%). Median rate of feeding at time of diagnosis was 105 ml/h (range, 75–125 ml/h), and diagnosis was made at a median of 6 days (range, 4–18 days) postoperatively. All patients developed abdominal distension, hypotension and tachycardia in the 24 h before re-exploratory laparotomy. Five patients died and one patient survived.
CONCLUSIONS
The understanding of the pathophysiology of NOSBN is still rudimentary; nevertheless, its 1% incidence in the present study does call into question its routine postoperative use especially in those at high risk with an open abdomen, planned repeat laparotomies or marked bowel oedema. Patients should be fully resuscitated before initiating any enteral feeding, and feeding should be interrupted if there is any evidence of feed intolerance.
Keywords: Jejunostomy, Enteral feeding, Small bowel necrosis
An increasing body of evidence indicates benefit from early postoperative enteral feeding. Maintenance of immune barrier integrity has been demonstrated in animal studies,1,2 as have decreases in major postoperative infections in trauma patients.3,4 In addition, patients undergoing complex upper gastrointestinal (GI) operations often require prolonged nutritional support due to frequent complications that can restrict oral intake for an appreciable period postoperatively. Nutrition support, when feasible via the GI tract, is recommended in patients who are unable to have an oral intake for 5 days or more.5 Malnutrition and reduced dietary intake are known to increase morbidity and mortality in postoperative patients. The functional benefits of medium-term enteral feeding in some patients make a powerful argument for providing enteral access at operation and using it at an early stage.
Several techniques have been described for postoperative enteral feeding after upper GI operations, including the nasojejunal route and percutaneous feeding jejunostomy (FJ). The main attraction of percutaneous FJ is that the tube is inserted under direct vision downstream to the most distal anastomosis and can be firmly secured in position, thus avoiding displacement by postoperative vomiting. Depending on local expertise and preference, the type of FJ employed may be a commercially-available needle catheter jejunostomy (NCJ) or a tube jejunostomy (TJ) using red rubber catheters, silicone drain tubing,6 or soft latex T-tubes.7
Universal use of a FJ in this context has been limited by its potential complications which, although occasional, are potentially fatal.
There has been a disturbing number of case reports of non-occlusive small bowel necrosis (NOSBN) occurring in surgical patients receiving enteral nutrition. Many of these reports have concerned patients with multiple trauma and may not be representative of those undergoing elective upper GI procedures.6,8–13 The present study aimed to determine the incidence of this complication after such procedures plus the influence of both patient selection and type of FJ inserted.
Patients and Methods
The records were reviewed of 524 consecutive patients who received a FJ after an elective upper GI operation for either benign or malignant disease between 1997 and 2006 in two affiliated surgical units. At least one of the authors was involved in every operation. Only patients with confirmed NOSBN at laparotomy were included in the study. During this time, one unit routinely inserted a NCJ as an adjunctive procedure at all operations, whilst the other unit selectively placed a T-tube TJ in patients deemed to have poor nutritional status, based on pre-operative assessment by the surgical team and dietician.14 Patients were selected for enteral feeding if there was intra-abdominal sepsis, poor pre-operative nutrition or anticipated delay in gastric emptying.
All FJs were inserted via a stab incision in the abdominal wall and an enterotomy on the antimesenteric border of a segment of jejunum approximately 20 cm downstream to the most distal anastomosis. Needle catheter jejunostomies were performed by a standard technique, in which a 9-Fr catheter (Freka, Fresenius Ltd, Warrington, UK) was inserted into a submucosal tunnel for 8 cm and then into the jejunal lumen for a distance of 20 cm. Tube jejunostomies were performed by inserting a 14-Fr latex T-tube. All FJs were secured with an absorbable purse string suture; the jejunostomy site was then sutured to the peritoneal lining of the abdominal wall to exclude the enterotomy site from the peritoneal cavity.
Postoperative enteral feeding was started as soon as the patient was haemodynamically stable and fully resuscitated, typically within 24 h of operation. Feeding was commenced at 30 ml/h via a pump, using a standard protocol based on the Schofield equation15 to provide an intake of 1931 ± 241 kcal/day, and was then increased as tolerated. Feeds contained a combination of partially hydrolysed protein, carbohydrate and fat in the form of medium-chain triglycerides.
Results
Six cases of NOSBN were identified over 120 months in 524 patients fed enterally via FJ following elective upper GI surgery, giving an overall incidence of 1.15% (Table 1). There were four men and two women of median age 60 years (range, 39–76 years; Table 2). In the routine NCJ unit, NOSBN developed in three patients with total gastrectomy (two for gastric carcinoma and one for non-Hodgkin's lymphoma) and two patients with pylorus-preserving proximal pancreatoduodenectomy or PPPP (one for carcinoma of the head of pancreas and one for intraductal papillary mucinous tumour [IPMT]), giving an incidence of 1.16% among 430 patients. In the selective TJ unit, the only patient with NOSBN had undergone PPPP for carcinoma of the head of pancreas, giving an incidence of 1.06% among 94 patients.
Table 1.
Upper gastrointestinal operations performed
| Operation | No. patients | |
|---|---|---|
| Routine insertion of needle catheter jejunostomy | Selective insertion of T-tube jejunostomy | |
| Gastrectomy | 48 | 8 |
| Oesophagectomy | 182 | 7 |
| PPPP | 190 | 61 |
| Whipple's operation | 3 | 3 |
| Total pancreatectomy | 4 | 3 |
| Distal pancreatectomy | – | 3 |
| Pancreatic cystojejunostomy | – | 1 |
| Debridement of pancreatic necrosis | – | 1 |
| PSDD | 1 | 2 |
| Biliary & gastric bypass | 2 | 4 |
| Ampullectomy | – | 1 |
| Total | 430 | 94 |
| Cases of NOSBN | 5 (1.16%) | 1 (1.06%) |
PPPP, pylorus-preserving proximal pancreatoduodenectomy; PSDD, pancreas-sparing distal duodenectomy; NOSBN, non-occlusive small bowel necrosis.
Table 2.
Clinical details of patients with non-occlusive small bowel necrosis
| Patient | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Age (years) | 76 | 65 | 55 | 72 | 39 | 55 |
| Sex | Male | Female | Male | Male | Male | Female |
| Diagnosis | Carcinoma stomach | Carcinoma stomach | Non-Hodgkin's lymphoma stomach | Carcinoma ampulla | IPMT of pancreas | Carcinoma head of pancreas |
| Operation | Total gastrectomy | Total gastrectomy | Total gastrectomy | PPPP | PPPP | PPPP |
| Feeding jejunostomy | NCJ | NCJ | NCJ | NCJ | NCJ | T-tube |
| Enteral feed | Jevity | Jevity | Jevity | Jevity | Jevity | Perative |
| Maximum rate (ml/h) | 125 (day 3) | 75 (day 5) | 125 (day 6) | 75 (day 5) | 125 (day 3) | 85 (day 6) |
| Predisposing factors | Noradrenaline, dopamine, AF | Nil | Nil | Nil | Nil | Hypotension secondary to haemorrhage day 10 |
| Clinical signs | Abdominal distension, hypotension, tachycardia | Abdominal distension, hypotension, tachycardia | Abdominal distension, hypotension, tachycardia | Abdominal distension, hypotension, tachycardia | Abdominal distension, abdominal pain, tachycardia | Abdominal distension, hypotension, tachycardia |
| Laparotomy (postoperative day) | 4 | 5 | 18 | 5 | 7 | 13 |
| Pathology | Necrosis proximal jejunum | Necrosis entire small bowel | Patchy necrosis entire small bowel, 2 small bowel perforations | Patchy necrosis entire small bowel & ascending colon | Focal full thickness infarction of segment of small bowel | Patchy necrosis entire small bowel |
| Procedure | Nil | Nil | Small bowel resection | Nil | Small bowel resection | Nil |
| Outcome | Death | Death | Death day 23 | Death | Multiple enteric fistulas | Death |
PPPP, pylorus-preserving proximal pancreatoduodenectomy; NCJ, needle catheter jejunostomy; IPMT, intraductal papillary mucinous tumour; AF, atrial fibrillation.
All six patients who developed NOSBN had begun enteral feeding with a polymeric formulation on the first postoperative day (Table 2). Median rate of feeding at time of diagnosis was 105 ml/h (range, 75–125 ml/h); two patients failed to reach maximal feeding rates as a result of minor feed-related problems (abdominal discomfort and nausea). The diagnosis of NOSBN was made at a median of 6 days (range, 4–18 days) postoperatively, four patients being diagnosed within 7 days and two at approximately 2 weeks. Two patients had potential precipitating causes: one had an episode of hypotension secondary to haemorrhage from a displaced abdominal drain 3 days before diagnosis, and the other patient was receiving inotropic support (noradrenaline and dopamine) at the time of diagnosis.
All patients developed abdominal distension, hypotension and tachycardia within the 24 h before re-exploratory laparotomy. Three patients were investigated with computed tomography (CT) before laparotomy. One patient who developed symptoms at 5 days demonstrated extensive portal venous and intramural bowel gas (Fig. 1A,B), while the scans of two patients who developed symptoms at 2 weeks were essentially normal. The decision to re-operate was always made on clinical grounds. At operation, one patient had patchy necrosis of the small bowel and ascending colon, two patients had patchy necrosis of the small bowel, one had confluent necrosis of the entire small bowel and two patients had necrosis of the proximal jejunum only. There was no evidence of kinking of the intestine from adhesions or of occlusion of the superior mesenteric artery or vein, nor was there evidence of displacement or erosion of any FJs. Four patients had no further procedure and died within 24 h. Two patients had small bowel resections (one extensive, the other limited) with ileostomies. One died 5 days later, the other survived with residual multiple enteric fistulas. Histological examination of the resected specimens revealed foci of ischaemic changes with sloughing and ulceration of mucosal surfaces, foci of transmural necrosis and multiple perforations.
Figure 1.
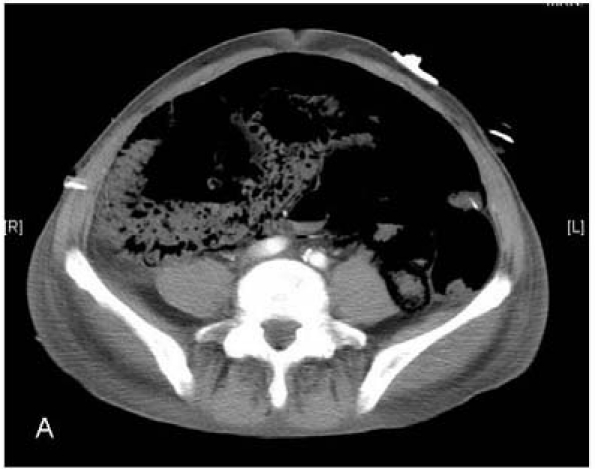
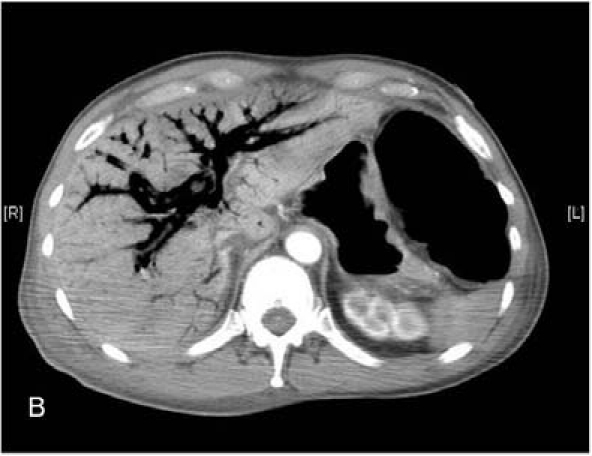
Computed tomography showing (A) extensive intramural bowel gas and (B) extensive portal venous gas.
Discussion
Patients undergoing oesophageal, gastric and pancreatic surgery pose two particular challenges: they are frequently malnourished at the time of operation, and they may require prolonged nutritional support because of postoperative complications that preclude early oral feeding. Insertion of a FJ both anticipates and treats these problems.
Non-occlusive small bowel necrosis is now recognised as a rare, but often fatal, complication of enteral tube feeding after elective upper GI or trauma surgery. It has been suggested that the incidence of major complications is lower in those with elective operations who do not have the deleterious systemic effects of multiple trauma.7 However, the overall incidence of NOSBN among our elective upper GI surgical population (1.15%) is comparable not only to the incidence reported in other upper GI series (0.15–5.88%) but also to the incidence reported in trauma patients (0.23–4.65%).6,8–10,12,13 There was no obvious difference in incidence between the unit routinely inserting NCJs and the unit selectively inserting TJs (1.16 versus 1.06%). This finding is in line with other series: the reported incidence after routine insertion of an FJ has ranged from 0% among 262 patients reported by Sica and colleagues16 to 0.23% (10 of 4311 patients) reported by Marvin and associates,12 and from 0.15% (3 of 2022 patients)17 to 1.35% (3 of 222 patients)6 after selective insertion of an FJ. Only two reports have compared NCJ with TJ in the general surgical population. In one report, Haun and Thompson18 reviewed 90 patients (55 NCJ, 35 TJ) and found equivalent major FJ-related complication rates, but no cases of NOSBN. By contrast, Holmes and colleagues6 reviewed 222 patients (171 NCJ, 51 TJ) and found that those with major FJ-related complications (including three with
It has been suggested that NOSBN is the result of early aggressive enteral feeding. Tube feeding was commenced within 24 h and increased in a step-wise fashion as tolerated in all our patients, although two failed to reach maximal feeding rates as a result of minor feed-related problems. The type of enteral formula could play a role.12 All our patients received a polymeric formula. Perhaps a more refined elemental diet would have been better tolerated, yet it may not avoid NOSBN (Table 3).
Table 3.
Previous reports of non-occlusive small bowel necrosis in patients undergoing elective upper gastrointestinal surgery and insertion of feeding jejunostomy
| First author | Year | Cases (n) | Incidence (%) | FJ | Formula | Site of necrosis | Mortality (%) |
|---|---|---|---|---|---|---|---|
| *Gaddy7 | 1986 | 1 | ? | NCJ | Elemental | ? | 100 |
| *Smith-Choban8 | 1988 | 1 | 1.72 | NCJ | Elemental | ? | 100 |
| Schunn19 | 1995 | 4 | 0.3 | TJ | Polymeric | Jejunum | 50 |
| Myers20 | 2000 | 3 | 0.15 | NCJ | ? | Entire SB 2. Patchy entire SB 1 | 66 |
| Jorba21 | 2000 | 1 | ? | NCJ | ? | Jejunum | 100 |
| Zetti22 | 2002 | 1 | ? | NCJ | ? | Ileum | 100 |
| Thaler23 | 2005 | 2 | 5.88 | NCJ | ? | Jejunum | 0 |
| Present series | 2006 | 1 | 1.06 | TJ | Polymeric | Patchy entire SB 4 | 83 |
| 5 | 1.16 | NCJ | Polymeric | Entire SB 1. Jejunum 1 |
NCJ, needle catheter jejunostomy; TJ, tube jejunostomy; SB, small bowel.
Both reports from the same group including the same case.
The mechanism of NOSBN in surgical patients may be multifactorial. Although systemic hypoperfusion is commonly believed to predispose to intestinal necrosis, NOSBN is extremely unusual in unfed patients who survive resuscitation from haemorrhagic shock. Nevertheless, one of our patients had an episode of transient hypotension 3 days before diagnosis. Several factors permit the gut to survive short periods of profound hypoperfusion. Thus when reduction in intestinal perfusion pressure is modest, autoregulation and a compensatory increase in oxygen extraction will prevent mucosal injury. In addition, increased sympathetic nerve stimulation shifts the autoregulation curve to the right (i.e. tissue blood flow occurs at higher pressures).24 Factors that reduce mesenteric blood flow in response to feeding – low cardiac output, incomplete fluid resuscitation, hyptension, atherosclerotic vascular diseas and congestive heart failure – may contribute to NOSBN. One of our patients was on ionotropes, and noradrenaline and dopamine both cause α1-adrenergic stimulation. As there are dense adrenergic plexuses in submucosal arteries and arterioles, vasconstrictive α1-adrenergic stimulation will reduce intestinal mucosal blood flow and thus, theoretically, predispose to NOSBN.25
Many clinical and pathological features of NOSBN are similar to neonatal necrotising enterocolitis, and several theories attempt to explain both conditions. The obligatory absorption of intraluminal nutrients may adversely increase energy demands in metabolically stressed enterocytes; splanchnic blood flow can increase by 150% after meals.26 By increasing splanchnic flow with continuous feeding, the threshold for bowel ischaemia in the presence of hypoperfusion or inadequate resuscitation may be lowered. Alternatively, enteral nutrition administered on the background of an ileus allows bacterial overgrowth, and intraluminal gas production causing distension could impair mucosal perfusion.12 Enteral nutrition might aggravate this process by generating intraluminal toxins that injure the mucosa.
To avoid a feeding enterostomy, patients could either be kept nil-by-mouth with its attendant catabolic consequences or receive total parenteral nutrition despite the risks of catheter sepsis. Short of abandoning the use of FJs with their potential benefits, it is unlikely that NOSBN can be completely prevented. Its incidence in the present study certainly suggests that it should be withheld in those patients deemed to be at high risk with an open abdomen, planned repeat laparotomies or marked bowel oedema.6 In addition, feeding should be interrupted in the face of haemodynamic instability, marked abdominal distension or increased pain.
If postoperative jejunostomy feeding is to be used, it should probably be delayed for 48 h to allow full assessment of the risk factors for small bowel necrosis. Feeds with a low osmolarity should be used, proceeding at a low rate. Any evidence that enteral feed tolerance is inadequate should be an indication for parental nutrition. TPN should be given to patients in whom there is enteral feed intolerance or evidence of inadequate gut function.
References
- 1.Luyer MD, Buurman WA, Hadfoune M, Jacobs JA, Konstantinov SR, et al. Pretreatment with high-fat enteral nutrition reduces endotoxin and tumour necrosis factor-alpha and preserves gut barrier function early after hemorrhagic shock. Shock. 2004;21:65–71. doi: 10.1097/01.shk.0000101671.49265.cf. [DOI] [PubMed] [Google Scholar]
- 2.Zonta S, Doni M, Alessiani M, Lovisetto F, Vigano J, et al. Elemental enteral nutrition preserves the mucosal barrier and improves the trophism of the villi after small bowel transplantation in piglets. Transplant Proc. 2007;39:2024–7. doi: 10.1016/j.transproceed.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 3.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma – a prospective, randomized study. J Trauma. 1986;26:874–81. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–11. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Acute Care. Nutrition support in adults: oral nutrition support, enteral feeding and parental nutrition. London: National Collaborating Centre for Acute Care; 2006. < www.rcseng.ac.uk>. [PubMed] [Google Scholar]
- 6.Holmes JH, Brundage SI, Yuen P, Hall RA, Maier RV, Jurkovich GV. Complications of surgical feeding jejunostomy in trauma patients. J Trauma. 1999;47:1009–12. doi: 10.1097/00005373-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Thodiyil PA, El-Masry NS, Peake H, Williamson RCN. T-tube jejunostomy feeding after pancreatic surgery: a safe adjunct. Asian J Surg. 2004;27:80–4. doi: 10.1016/S1015-9584(09)60318-3. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JS. Pneumatosis intestinalis and needle catheter jejunostomy: a word of caution. JPEN J Parenter Enteral Nutr. 1983;7:495. doi: 10.1177/0148607183007005495. [DOI] [PubMed] [Google Scholar]
- 9.Gaddy MC, Max MH, Schwab CW, Kauder D. Small bowel ischemia: a consequence of feeding jejunostomy? South Med J. 1986;79:180–2. doi: 10.1097/00007611-198602000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Smith-Choban P, Max MH. Feeding jejunostomy: a small bowel stress test? Am J Surg. 1988;155:112–7. doi: 10.1016/s0002-9610(88)80267-8. [DOI] [PubMed] [Google Scholar]
- 11.Munshi IA, Steingrub JS, Wolpert L. Small bowel necrosis associated with early postoperative jejunal tube feeding in a trauma patient. J Trauma. 2000;49:163–5. doi: 10.1097/00005373-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Marvin RG, McKinley BA, McQuiggan M, Cocanour CS, Moore FA. Nonocclusive bowel necrosis occurring in critically ill trauma patients receiving enteral nutrition manifests no reliable clinical signs for early detection. Am J Surg. 2000;179:7–12. doi: 10.1016/s0002-9610(99)00261-5. [DOI] [PubMed] [Google Scholar]
- 13.Frey C, Takala J, Krahenbuhl L. Non-occlusive small bowel necrosis during gastric tube feeding: a case report. Intensive Care Med. 2001;27:1422–5. doi: 10.1007/s001340101013. [DOI] [PubMed] [Google Scholar]
- 14.Baker JP, Detsky AS, Wesson DE, Wolman SL, Stewart S, et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982;306:969–72. doi: 10.1056/NEJM198204223061606. [DOI] [PubMed] [Google Scholar]
- 15.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 16.Sica GS, Sujendran V, Wheeler J, Soin B, Maynard N. Needle catheter jejunostomy at esophagectomy for cancer. J Surg Oncol. 2005;91:276–9. doi: 10.1002/jso.20314. [DOI] [PubMed] [Google Scholar]
- 17.Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma. 1986;26:882–91. doi: 10.1097/00005373-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Haun JL, Thompson JS. Comparison of needle catheter versus standard tube jejunostomy. Am Surg. 1985;51:466–9. [PubMed] [Google Scholar]
- 19.Schunn CD, Daly JM. Small bowel necrosis associated with post-operative jejunal tube feeding. J Am Coll Surg. 1995;180:410–6. [PubMed] [Google Scholar]
- 20.Myers JG, Page CP, Stewart RM, Schwesinger WH, Sirinek KR, Aust JB. Complications of needle catheter jejunostomy in 2,022 consecutive applications. Am J Surg. 1995;170:547–50. doi: 10.1016/s0002-9610(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 21.Jorba R, Fabregat J, Borobia FG, Torras J, Poves I, Jaurrieta E. Small bowel necrosis in association with early postoperative enteral feeding after pancreatic resection. Surgery. 2000;128:111–2. doi: 10.1067/msy.2000.104119. [DOI] [PubMed] [Google Scholar]
- 22.Zetti G, Tagliabue F, Barabino M, Fontana S, Ceppi M, Samori G. Small bowel necrosis associated with postoperative enteral feeding. Chir Ital. 2002;54:555–8. [PubMed] [Google Scholar]
- 23.Thaler K, Garreau J, Hansen PD. Non-occlusive small bowel necrosis during enteral feeding after pancreatico-duodenectomy. Dig Surg. 2005;22:375–7. doi: 10.1159/000090997. [DOI] [PubMed] [Google Scholar]
- 24.Reilly PM, Bulkley GB. Vasoactive mediators and splanchnic perfusion. Crit Care Med. 1993;21:S55–68. doi: 10.1097/00003246-199302001-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hinds CJ, Shock Watson D. In: Intensive Care: a Concise Textbook. 2nd edn. Hinds CJ, Watson D, editors. London: Saunders; 1999. p. 92. [Google Scholar]
- 26.Gatt M, MacFie J, Anderson AD, Howell G, Reddy BS, et al. Changes in superior mesenteric artery blood flow after oral, enteral and parenteral feeding in humans. Crit Care Med. 2009;37:171–6. doi: 10.1097/CCM.0b013e318192fb44. [DOI] [PubMed] [Google Scholar]


