Abstract
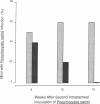
Pulmonary infections with Pneumocystis carinii are an important cause of morbidity and mortality in patients with AIDS. P. carinii infections are seen in patients with decreased numbers of helper T lymphocytes, suggesting that these cells are important in preventing infection. To test this hypothesis, we sought to establish experimental infection with P. carinii in mice selectively depleted of helper T lymphocytes. Weekly injections of a monoclonal anti-CD4 antibody produced sustained depletion of helper T lymphocytes from blood and lymphoid organs. To establish pulmonary infection, lymphocyte-depleted mice were then given intratracheal inoculations of P. carinii organisms derived from the lungs of chronically infected athymic mice. Pulmonary infection with P. carinii was demonstrable in the antibody-treated mice and was centered around the conducting airways. Infection was persistent for up to 3 mo with continued antibody treatments, and yet could be cleared from the lungs if antibody treatments were discontinued. This experimental model of P. carinii infection permits the study of infection associated with a specific immune defect and implicates the helper T lymphocyte as a critical cell in host defense against this pathogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett M. S., Durkin M. M., Jay M. A., Queener S. F., Smith J. W. Sources of rats free of latent Pneumocystis carinii. J Clin Microbiol. 1987 Sep;25(9):1794–1795. doi: 10.1128/jcm.25.9.1794-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. S., Fishman J. A., Queener S. F., Durkin M. M., Jay M. A., Smith J. W. New rat model of Pneumocystis carinii infection. J Clin Microbiol. 1988 Jun;26(6):1100–1102. doi: 10.1128/jcm.26.6.1100-1102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. S., Verbanac P. A., Smith J. W. Cultivation of Pneumocystis carinii with WI-38 cells. J Clin Microbiol. 1979 Dec;10(6):796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld W., Griffiss J. M. In vitro differentiation of human-derived Pneumocystis carinii. J Clin Microbiol. 1989 Mar;27(3):480–485. doi: 10.1128/jcm.27.3.480-485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Coleman D. L., Luce J. M., Wilber J. C., Ferrer J. J., Stephens B. G., Margaretten W., Wagar E. A., Hadley W. K., Pifer L. L., Moss A. R. Antibody to the retrovirus associated with the acquired immunodeficiency syndrome (AIDS). Presence in presumably healthy San Franciscans who died unexpectedly. Arch Intern Med. 1986 Apr;146(4):713–715. [PubMed] [Google Scholar]
- Crocker P. R., Jefferies W. A., Clark S. J., Chung L. P., Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987 Aug 1;166(2):613–618. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Cushion M. T., Ruffolo J. J., Walzer P. D. Analysis of the developmental stages of Pneumocystis carinii, in vitro. Lab Invest. 1988 Mar;58(3):324–331. [PubMed] [Google Scholar]
- Debs R. J., Blumenfeld W., Brunette E. N., Straubinger R. M., Montgomery A. B., Lin E., Agabian N., Papahadjopoulos D. Successful treatment with aerosolized pentamidine of Pneumocystis carinii pneumonia in rats. Antimicrob Agents Chemother. 1987 Jan;31(1):37–41. doi: 10.1128/aac.31.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Eisen S. Decreased yield of Pneumocystis carinii from cortisonized rats. J Parasitol. 1989 Feb;75(1):82–85. [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J. CD4-/CD8-T cells: amplification in spleens of mice following in vivo treatment with monoclonal antibody anti-L3T4. Eur J Immunol. 1988 Feb;18(2):231–235. doi: 10.1002/eji.1830180208. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Macher A. M., Longo D. L., Lane H. C., Rook A. H., Masur H., Gelmann E. P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984 Jan;100(1):92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Schwartz R. H., Pardoll D. M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988 Aug 18;334(6183):620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. C. Immunological studies of Pneumocystis carinii. J Protozool. 1989 Jan-Feb;36(1):60–69. doi: 10.1111/j.1550-7408.1989.tb02700.x. [DOI] [PubMed] [Google Scholar]
- Gutstein N. L., Wofsy D. Administration of F(ab')2 fragments of monoclonal antibody to L3T4 inhibits humoral immunity in mice without depleting L3T4+ cells. J Immunol. 1986 Dec 1;137(11):3414–3419. [PubMed] [Google Scholar]
- Heyworth M. F., Carlson J. R., Ermak T. H. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1743–1748. doi: 10.1084/jem.165.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Smith B. L. Efficacy of diaminodiphenylsulfone and other drugs in murine Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1984 Oct;26(4):436–440. doi: 10.1128/aac.26.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Smith B. Provocation of infection due to Pneumocystis carinii by cyclosporin A. J Infect Dis. 1982 May;145(5):767–767. doi: 10.1093/infdis/145.2.767. [DOI] [PubMed] [Google Scholar]
- Kim C. K., Foy J. M., Cushion M. T., Stanforth D., Linke M. J., Hendrix H. L., Walzer P. D. Comparison of histologic and quantitative techniques in evaluation of therapy for experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1987 Feb;31(2):197–201. doi: 10.1128/aac.31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. G., Smith J. S., Meier J. L. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest. 1986 Jun;54(6):609–615. [PubMed] [Google Scholar]
- McCarthy S. A., Kruisbeek A. M., Uppenkamp I. K., Sharrow S. O., Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988 Nov 3;336(6194):76–79. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- McLeod E., Caldwell J. L., Kaltreider H. B. Pulmonary immune responses of inbred mice. Appearance of antibody-forming cells in C57BL/6 mice after intrapulmonary or systemic immunization with sheep erythrocytes. Am Rev Respir Dis. 1978 Sep;118(3):561–571. doi: 10.1164/arrd.1978.118.3.561. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Eisen H., Joskowicz M., Pereira P., Coutinho A. Suppression of polyclonal antibody production in Trypanosoma cruzi-infected mice by treatment with anti-L3T4 antibodies. J Immunol. 1987 Jul 15;139(2):545–550. [PubMed] [Google Scholar]
- Ognibene F. P., Masur H., Rogers P., Travis W. D., Suffredini A. F., Feuerstein I., Gill V. J., Baird B. F., Carrasquillo J. A., Parrillo J. E. Nonspecific interstitial pneumonitis without evidence of Pneumocystis carinii in asymptomatic patients infected with human immunodeficiency virus (HIV). Ann Intern Med. 1988 Dec 1;109(11):874–879. doi: 10.7326/0003-4819-109-11-874. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L. Effects of bacterial pneumonitis on development of pneumocystosis in rats. Am Rev Respir Dis. 1982 Jun;125(6):723–726. doi: 10.1164/arrd.1982.125.6.723. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L. Phospholipid profile of Pneumocystis carinii and its interaction with alveolar type II epithelial cells. Infect Immun. 1987 Mar;55(3):736–741. doi: 10.1128/iai.55.3.736-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Sidman C. L. Genetically determined murine models of immunodeficiency. Annu Rev Immunol. 1987;5:367–403. doi: 10.1146/annurev.iy.05.040187.002055. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Von Behren L. A., Pesanti E. L. Uptake and degradation of Pneumocystis carinii by macrophages in vitro. Am Rev Respir Dis. 1978 Dec;118(6):1051–1059. doi: 10.1164/arrd.1978.118.6.1051. [DOI] [PubMed] [Google Scholar]
- Walzer P. D. Attachment of microbes to host cells: relevance of Pneumocystis carinii. Lab Invest. 1986 Jun;54(6):589–592. [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Perl D. P., Krogstad D. J., Rawson P. G., Schultz M. G. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974 Jan;80(1):83–93. doi: 10.7326/0003-4819-80-1-83. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Schnelle V., Armstrong D., Rosen P. P. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science. 1977 Jul 8;197(4299):177–179. doi: 10.1126/science.301657. [DOI] [PubMed] [Google Scholar]
- Wharton J. M., Coleman D. L., Wofsy C. B., Luce J. M., Blumenfeld W., Hadley W. K., Ingram-Drake L., Volberding P. A., Hopewell P. C. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trial. Ann Intern Med. 1986 Jul;105(1):37–44. doi: 10.7326/0003-4819-105-1-37. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Chiang N. Y., Greenspan J. S., Ermak T. H. Treatment of murine lupus with monoclonal antibody to L3T4. I. Effects on the distribution and function of lymphocyte subsets and on the histopathology of autoimmune disease. J Autoimmun. 1988 Oct;1(5):415–431. doi: 10.1016/0896-8411(88)90065-0. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]