Abstract
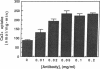
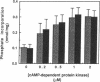
Studies in animal models have suggested that alterations affecting phospholamban-mediated stimulation of Ca2+ uptake by sarcoplasmic reticulum are involved in the pathophysiology of heart disease. A monoclonal antibody that binds to phospholamban and stimulates Ca2+ uptake was used to characterize phospholamban-mediated effects in human cardiac sarcoplasmic reticulum and to compare these effects in tissue from normal and failing hearts. Stimulation of Ca2+ uptake by anti-phospholamban monoclonal antibody simulated the effect of phosphorylation of phospholamban by cAMP-dependent protein kinase. Binding of anti-phospholamban antibody reduced the K0.5 of the Ca2(+)-transporting ATPase from 0.53 microM [( Ca2+]) to 0.29 microM [( Ca2+]), without affecting Vmax or nHill. At 0.2 microM Ca2+, stimulation was 1.93-fold in sarcoplasmic reticulum prepared from normal human left ventricular myocardium and 1.94-fold in sarcoplasmic reticulum prepared from the left ventricular myocardium of patients with heart failure resulting from idiopathic dilated cardiomyopathy. Stimulation of Ca2+ uptake in canine cardiac sarcoplasmic reticulum under identical conditions was 1.89-fold. Phospholamban-mediated stimulation of Ca2+ uptake in human cardiac sarcoplasmic reticulum is thus comparable in magnitude to that observed in other species and results from an increase in the apparent affinity of the Ca2(+)-transporting ATPase for Ca2+. The pathogenesis of heart failure in idiopathic dilated cardiomyopathy does not, however, appear to involve intrinsic alterations of this mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beekman R. E., van Hardeveld C., Simonides W. S. Effect of thyroid state on cytosolic free calcium in resting and electrically stimulated cardiac myocytes. Biochim Biophys Acta. 1988 Apr 2;969(1):18–27. doi: 10.1016/0167-4889(88)90083-3. [DOI] [PubMed] [Google Scholar]
- Beekman R. E., van Hardeveld C., Simonides W. S. On the mechanism of the reduction by thyroid hormone of beta-adrenergic relaxation rate stimulation in rat heart. Biochem J. 1989 Apr 1;259(1):229–236. doi: 10.1042/bj2590229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Grossman W., McLaurin L. P., Rolett E. L. Alterations in left ventricular relaxation and diastolic compliance in congestive cardiomyopathy. Cardiovasc Res. 1979 Sep;13(9):514–522. doi: 10.1093/cvr/13.9.514. [DOI] [PubMed] [Google Scholar]
- Guarnieri T., Filburn C. R., Beard E. S., Lakatta E. G. Enhanced contractile response and protein kinase activation to threshold levels of beta-adrenergic stimulation in hyperthyroid rat heart. J Clin Invest. 1980 Apr;65(4):861–868. doi: 10.1172/JCI109738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Inui M., Chamberlain B. K., Saito A., Fleischer S. The nature of the modulation of Ca2+ transport as studied by reconstitution of cardiac sarcoplasmic reticulum. J Biol Chem. 1986 Feb 5;261(4):1794–1800. [PubMed] [Google Scholar]
- Kirchberger M. A., Borchman D., Kasinathan C. Proteolytic activation of the canine cardiac sarcoplasmic reticulum calcium pump. Biochemistry. 1986 Sep 23;25(19):5484–5492. doi: 10.1021/bi00367a021. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGrew S. G., Wolleben C., Siegl P., Inui M., Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989 Feb 21;28(4):1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Bristow M. R., Krall J. Ca2+ uptake by cardiac sarcoplasmic reticulum from patients with idiopathic dilated cardiomyopathy. Circ Res. 1989 Oct;65(4):1141–1144. doi: 10.1161/01.res.65.4.1141. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Nishikawa M., Adelstein R. S. Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. Stimulation of cardiac sarcoplasmic reticulum calcium uptake. J Biol Chem. 1984 Jul 10;259(13):8029–8032. [PubMed] [Google Scholar]
- Nagai R., Zarain-Herzberg A., Brandl C. J., Fujii J., Tada M., MacLennan D. H., Alpert N. R., Periasamy M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Wang J. H. Stimulation of bovine cardiac sarcoplasmic reticulum Ca2+ pump and blocking of phospholamban phosphorylation and dephosphorylation by a phospholamban monoclonal antibody. J Biol Chem. 1986 May 25;261(15):7018–7023. [PubMed] [Google Scholar]
- Whitmer J. T., Kumar P., Solaro R. J. Calcium transport properties of cardiac sarcoplasmic reticulum from cardiomyopathic Syrian hamsters (BIO 53.58 and 14.6): evidence for a quantitative defect in dilated myopathic hearts not evident in hypertrophic hearts. Circ Res. 1988 Jan;62(1):81–85. doi: 10.1161/01.res.62.1.81. [DOI] [PubMed] [Google Scholar]
- Will H., Küttner I., Kemsies C., Vetter R., Schubert E. Comparative analysis of phospholamban phosphorylation in crude membranes of vertebrate hearts. Experientia. 1985 Aug 15;41(8):1052–1054. doi: 10.1007/BF01952139. [DOI] [PubMed] [Google Scholar]
- Xu Z. C., Kirchberger M. A. Modulation by polyelectrolytes of canine cardiac microsomal calcium uptake and the possible relationship to phospholamban. J Biol Chem. 1989 Oct 5;264(28):16644–16651. [PubMed] [Google Scholar]