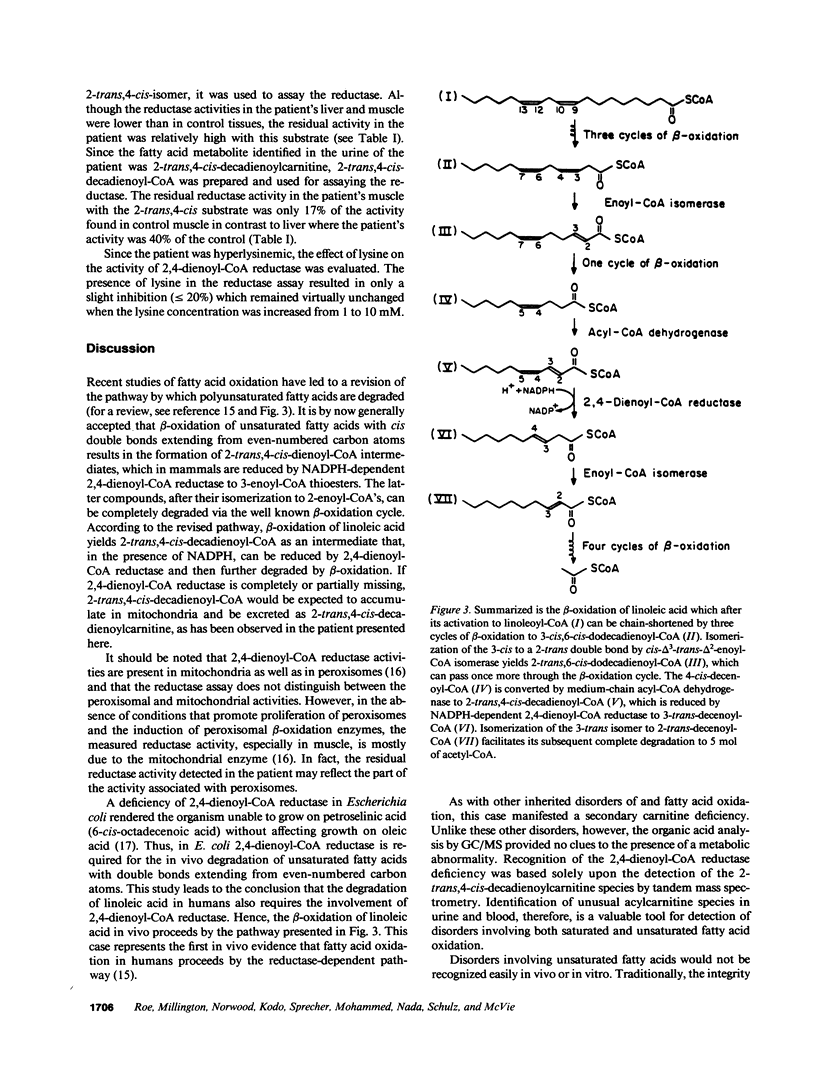
Abstract
Several inherited disorders of fatty acid beta-oxidation have been described that relate mainly to saturated precursors. This study is the first report of an enzyme defect related only to unsaturated fatty acid oxidation and provides the first in vivo evidence that fat oxidation in humans proceeds by the reductase-dependent pathway. The patient was a black female, presenting in the neonatal period with persistent hypotonia. Biochemical studies revealed hyperlysinemia, hypocarnitinemia, normal organic acid profile, and an unusual acylcarnitine species in both urine and blood. The new metabolite was positively identified by mass spectrometry as 2-trans,4-cis-decadienoylcarnitine, derived from incomplete oxidation of linoleic acid. In spite of dietary therapy, the patient died of respiratory acidosis at four months of age. Samples of liver and muscle from the autopsy were assayed for 2,4-dienoyl-coenzyme A reductase activity. Using the substrate 2-trans,4-cis-decadienoylcoenzyme A, the reductase activity was 40% of the control value in liver and only 17% of that found in normal muscle. It is suggested that unsaturated substrates should be used for in vitro testing to cover the full range of potential beta-oxidation defects and that acylcarnitine species identification be used for in vivo detection of this disorder.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brass E. P., Hoppel C. L. Carnitine metabolism in the fasting rat. J Biol Chem. 1978 Apr 25;253(8):2688–2693. [PubMed] [Google Scholar]
- Dommes V., Baumgart C., Kunau W. H. Degradation of unsaturated fatty acids in peroxisomes. Existence of a 2,4-dienoyl-CoA reductase pathway. J Biol Chem. 1981 Aug 25;256(16):8259–8262. [PubMed] [Google Scholar]
- Dommes V., Kunau W. H. 2,4-Dienoyl coenzyme A reductases from bovine liver and Escherichia coli. Comparison of properties. J Biol Chem. 1984 Feb 10;259(3):1781–1788. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millington D. S., Norwood D. L., Kodo N., Roe C. R., Inoue F. Application of fast atom bombardment with tandem mass spectrometry and liquid chromatography/mass spectrometry to the analysis of acylcarnitines in human urine, blood, and tissue. Anal Biochem. 1989 Aug 1;180(2):331–339. doi: 10.1016/0003-2697(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Roe C. R., Millington D. S., Maltby D. A., Bohan T. P., Kahler S. G., Chalmers R. A. Diagnostic and therapeutic implications of medium-chain acylcarnitines in the medium-chain acyl-coA dehydrogenase deficiency. Pediatr Res. 1985 May;19(5):459–466. doi: 10.1203/00006450-198505000-00011. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Cuebas D., Schulz H. 3-Hydroxyacyl-CoA epimerases of rat liver peroxisomes and Escherichia coli function as auxiliary enzymes in the beta-oxidation of polyunsaturated fatty acids. J Biol Chem. 1986 Sep 15;261(26):12238–12243. [PubMed] [Google Scholar]


