Abstract
INTRODUCTION
To report our initial experience of laparostomy and immediate intra-abdominal vacuum therapy in patients with severe peritonitis due to intra-abdominal catastrophes.
PATIENTS AND METHODS
Twenty-seven patients underwent emergency laparotomy and laparostomy formation with the application of immediate intra-abdominal TRAC–VAC® therapy (male:female ratio, 1:1.2; median age, 73 years; range, 34–84 years). Predicted mortality was assessed using the P-POSSUM score and compared with clinically observed outcomes.
RESULTS
Ten patients (37%) with a mean predicted P-POSSUM mortality of 72%, died of sepsis and multi-organ failure. Seventeen patients (mean P-POSSUM 48% expected mortality) survived to discharge. One patient with pancreatitis died from small bowel obstruction 1-year post discharge, two patients developed a small bowel fistula. One patient had an allergic reaction to the VAC dressing. Our patients, treated with laparostomy and TRAC VAC therapy, had a significantly improved observed survival when compared to P-POSSUM expected survival (P = 0.004).
CONCLUSIONS
Laparostomy with immediate intraperitoneal VAC therapy is a robust and effective system to manage patients with intra-abdominal catastrophes. There were significantly improved outcomes compared to the mortality predicted by P-POSSUM scores. Damage control surgery with laparostomy formation and intra-abdominal VAC therapy should be considered in patients with severe peritonitis.
Keywords: Laparostomy, Intraperitoneal VAC therapy, Damage control surgery, POSSUM
Introduction
Immediate closure of the abdominal wall following laparotomy may not be possible or desirable, particularly in patients with a severe abdominal catastrophe such as diffuse peritonitis, necrotising pancreatitis or trauma. Attempting to close the abdomen in such cases may result in potentially life-threatening intra-abdominal compartment syndrome. In such circumstances, it is preferable to leave the abdominal wall open initially as a laparostomy.
The use of laparostomy following damage-control surgery is well established,1–3 but the open abdomen is complex to manage and is itself associated with considerable morbidity and mortality rates in excess of 25%.4 Various measures for managing the open abdomen have been described such as the use of prosthetic mesh, the Bogota bag and Wittman's patch.1,5 Ideally, any temporary abdominal closure device should be able to contain the visceral contents, actively remove exudates, quantify third space losses, promote granulation tissue and aid subsequent abdominal closure. None of these temporary measures available to date satisfy all these requirements; therefore, a ‘gold standard’ technique for laparostomy management has remained elusive.4,6,7
Negative pressure therapy has become established in the management of ‘slow-to-heal’ superficial wounds. In addition, considerable evidence exists in the literature surrounding the role of using vacuum-assisted negative pressure dressings to achieve abdominal closure following trauma. However, relatively few prospective studies have been published evaluating the use of negative pressure therapy to achieve abdominal closure following surgery for intra-abdominal sepsis. A total of 144 patients, who had negative pressure dressings applied to aid in closure of abdominal wounds, were included in five studies.8–12 Abdominal closure was ultimately achieved in 52% of the cases (range, 31–75%). Fistula rates were 16% (range, 6–21%) and the overall mortality rate 30%.
Animal models have demonstrated an increase in cell division, proliferation and angiogenesis, factors crucial for effective healing, in wounds which are treated with vacuum therapy.13,14 The negative pressure enhances tissue perfusion, promotes local blood flow and tissue oxygenation, and stimulates the formation of granulation tissue. There is also evidence that the accumulation of chronic wound fluid inhibits cell proliferation,15 and that this inhibition is reduced when the wounds are exposed to negative pressure therapy, which actively removes such exudates.
We present our initial experience using a negative pressure laparostomy system, for patients who have suffered an intra-abdominal catastrophe and were at risk of developing abdominal compartment syndrome.
We have performed laparostomy with immediate application of intraperitoneal vacuum therapy and found it to be a robust technique that satisfies many of these requirements. TRAC–VAC® refers to the vacuum-assisted closure device, for the application of negative pressure therapy to laparostomy wounds (Kinetic Concepts Inc. (KCI) Medical, San Antonio, TX, USA).
Patients and Methods
Between March 2003 and August 2008, a prospective database, containing all patients undergoing laparostomy formation, was established. Patient demographics, presenting diagnosis, individualised P-POSSUM scores, intra-operative findings and surgical procedures undertaken were recorded. Details of subsequent interventions, complications and outcomes were documented.
All patients were resuscitated with intravenous fluids and had an in-dwelling urethral catheter, nasogastric tube and antibiotic therapy prior to surgery. The decision was made in the operating theatre by the senior surgeon in attendance that it would be preferable to perform a laparostomy rather than to attempt primary abdominal closure. The reasons for this were variable but included the following: (i) approximation of the fascia was impossible; (ii) damage control surgery with planned relaparotomy had been performed and a second early repeat laparotomy was planned for definitive treatment; or (iii) there was an attempted initial fascial closure in theatre but physiological parameters deteriorated suggesting the patient was at significant risk of abdominal compartment syndrome.
The P-POSSUM score, initially designed for risk adjusted audit, is used ubiquitously in general surgical practice in the UK not only for audit, but also for institutional and surgeon-specific performance and for aiding informed consent. Our observed outcomes were compared to expected outcomes as predicted by the P-POSSUM score, using standard statistical analysis. P-POSSUM scores are individualised and specific for a particular patient depending on individual patient factors. Therefore, each patient's observed P-POSSUM was compared with their individualised patient outcome.
Temporary abdominal closure was achieved with the use of the TRAC–VAC® system. The dressing system contains an encapsulated, non-adherent, polyurethane foam. The foam is enclosed in a fenestrated bilayer dressing that allows fluid and effluents to be sucked towards the central foam whilst maintaining a protective wrapping around the bowel. The dressing is placed over the exposed bowel and wrapped around it to contact with the visceral and parietal peritoneum. A second piece of foam was cut to match the size of the laparostomy and placed to contact the edges of the abdominal wall. This was followed by application of a semi-occlusive drape over the abdominal wound. A small, 2-cm hole was made in the drape and a suction pad applied. This was then connected to the suction device and set to a subatmospheric pressure of 125 mmHg using continuous therapy mode to maintain this pressure. If the patient had had a stoma fashioned, an occlusive dressing was placed over the stoma and a small window then cut in it to allow the application of a stoma bag on top. This avoided losing suction to the main wound. Laparostomy inspections were made in theatre every 24–48 h as required individually, and new dressings re-applied afterwards. At inspection, if the patients' physiological and pathological conditions were favourable, delayed primary closure of the abdominal wall was attempted. If sutured abdominal wall closure was ultimately not possible, often as a result of lateral contracture of the abdominal wall muscles, treatment with ‘intraperitoneal’ negative pressure therapy was continued until such time that the bowel was ‘fixed’ and free evisceration of the intestine would not occur. The wounds were then matured to heal by secondary intention, either using a wound manager stoma bag (e.g. DermaSure® wound management system [ADI Medical Ltd, Sunny Hollow, Handleton Common, Lane End, Bucks, UK]), or with further on lay negative pressure therapy without the intraperitoneal fenestrated dressing of the TRAC–VAC® system. In these cases, Mepitel dressings (Direct Medical Inc., Alabaster, AL, USA), a porous, semi-transparent, low adherent wound contact layer, consisting of a flexible polyamide net coated with soft silicone, were placed over the exposed abdominal contents and around the skin edges of the wounds before applying the foam dressing on top. The wounds were allowed to heal by secondary intention until completely epithelialised.
We chose to compare our observed outcomes against expected outcomes using the P-POSSUM scoring system. The P-POSSUM system, originally described by Copeland et al.16 and subsequently modified with the ‘Portsmouth predictor’, has been shown to be a valid and reliable model for the prediction of morbidity and mortality based on 12 physiological and 6 operative parameters.16,17
Results
Between March 2003 and August 2008, 27 patients with severe diffuse intra-abdominal sepsis underwent laparotomy and laparostomy formation with immediate application of negative pressure – vacuum assisted therapy. There were 13 men and 14 women, median age 73 years (range, 34–84 years). Mean ASA score was 3.75 (median, 3; range, 2–5).
The causative pathologies leading to laparostomy formation are shown in Table 1. Eight patients had perforated sigmoid diverticular disease (Hinchey grades 3 and 4) and one patient had a perforated colonic carcinoma. Three patients had the diseased sigmoid colon immediately resected without formation of an anastomosis or stoma: the ends of the colon proximal and distal to the resected segment being stapled off (damage-control surgery). One of these patients subsequently had a delayed primary anastomosis and closure of the abdominal wall. Two had an end colostomy fashioned (Hartmann's procedure) at their second-look laparotomy. The remaining six patients underwent a Hartmann's procedure and laparostomy at the initial laparotomy.
Table 1.
Intra-operative diagnosis and outcome
| Diagnosis | Number | Mortality |
|---|---|---|
| Perforated diverticular disease | 8 | 2 |
| Perforated sigmoid carcinoma | 1 | 0 |
| Bowel anastomotic leaks | 6 | 3 |
| Necrotising pancreatitis | 1 | 1 |
| Small bowel perforation | 6 | 1 |
| Abdominal compartment syndrome following emergency splenectomy | 1 | 1 |
| Perforated appendicitis | 1 | 0 |
| Biliary peritonitis following cholodochoduodenal anastomosis | 1 | 1 |
| Large pyosalpinx | 1 | 0 |
| Ischaemic colon secondary to C. difficile infection | 1 | 1 |
Six patients had laparostomy formation following surgery for an anastomotic leak. Five of these patients had previously undergone an anterior resection for rectal cancer, none of which had had defunctioning ileostomies. One patient suffered an anastomotic leak following reversal of a Hartmann's procedure. All this group underwent a laparotomy and extensive pelvic washout and all were defunctioned with an end colostomy.
One patient with necrotising pancreatitis (secondary to alcohol) and infected pancreatic necrosis, underwent a pancreatic necrosectomy, and laparostomy formation.
Six patients underwent surgery for small bowel perforation. Two of these were secondary to small bowel ischaemia. Two patients had multiple perforations in areas of acute Crohn's disease and one patient suffered an iatrogenic small bowel injury, presumably secondary to a unseen diathermy injury, following laparoscopic surgery for rectal carcinoma. One patient suffered an unrecognised iatrogenic bowel injury during a laparoscopic cholecystectomy. All the patients underwent abdominal washout and small bowel resection. Five patients had a primary small bowel anastomosis; one patient, with severe peritoneal contamination, had the diseased bowel resected and the bowel ends stapled off. A delayed primary anastomosis was performed at second-look laparotomy.
One patient developed signs of compartment syndrome following an emergency splenectomy for trauma and primary closure. At re-look laparotomy, the abdomen was washed out, a cholecystectomy was performed as acalulous cholecystitis was evident and the topical negative pressure laparostomy dressing was applied.
One patient suffered a deep wound dehiscence having previously undergone a laparotomy for perforated necrotic appendicitis with faecal peritonitis. One patient had biliary peritonitis and had suffered a leak from a cholodoco-duodenal anastomosis, which had been constructed during open surgery for cholangitis secondary to impacted distal common bile duct stones, in a patient with abnormal biliary anatomy who had undergone two failed endoscopic retrograde cholangiopancreatography procedures. One patient, had a massive pyosalphinx with diffuse peritonitis and underwent a salpingectomy and abdominal washout. One patient underwent a laparotomy, subtotal colectomy and laparostomy formation, for ischaemic pseudomembranous colitis secondary to proven Clostridium difficile infection. In this case, there was severe diffuse generalised peritonitis and retroperitoneal sepsis.
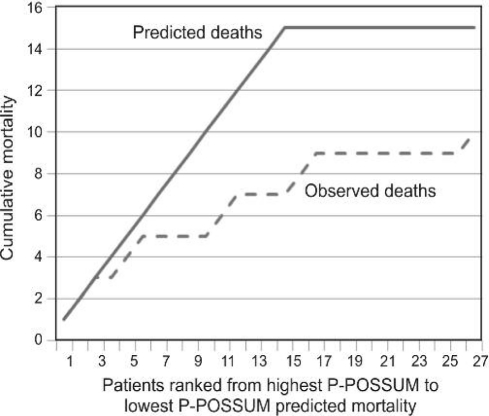
Of the 27 patients who underwent laparostomy with immediate application of negative pressure laparostomy, 10 patients (37%) with a mean P-POSSUM predicted mortality of 72% (median ASA 4) died on the same admission (Fig. 1). The remaining 17 patients survived to discharge (mean predicted P-POSSUM mortality rate 48%, median ASA 3), 16 patients are available for long-term follow-up. Of the 17 patients surviving to discharge, an average of four re-look laparotomies and ‘change of dressing’ were performed (Table 2).
Figure 1.
Observed versus predicted cumulative mortality.
In five patients, the wound was deemed suitable for sutured closure at one of the second-look laparotomies. Four wounds were sutured using a mass closure technique and one was closed using interrupted deep tension sutures. Two of these patients had suffered small bowel perforations in areas of ischaemia, and had undergone a limited bowel resection and anastomosis. One patient had his abdominal wound closed 4 days following initial surgery; the second patient had his wound closed at 6 days. A further, young patient, who had been found to have suffered multiple small bowel perforations in an area of acute inflammatory bowel disease, underwent a limited right hemicolectomy and had his wound closed, during his first re-look laparotomy, 48 h following initial surgery. One of the patients, who had suffered an unrecognised iatrogenic small bowel injury during laparoscopic rectal resection, was closed with interrupted deep tension sutures, during his third re-look laparotomy, 6 days following initial surgery. A further patient, found to have a large pyosalpinx, underwent delayed primary closure of her abdominal wound during her first re-look procedure, following a further wash-out.
Patients in whom delayed primary closure of the abdominal wall was achieved were statistically younger (P = 0.03) but no difference in their presenting P-POSSUM scores was found. No patient with large bowel pathology achieved primary closure of the abdominal wound. There were no wound dehiscences following delayed primary abdominal wall closure in our series.
In the remaining 12 patients, the wound was left to granulate and heal by secondary intention. As the wounds healed and decreased in size, the dressings were changed for a ‘mini vac’ system, which in some instances, were managed in the community.
For this group, the average time to achieve ‘closure’ (discontinuation of mini VAC therapy) was 6 weeks. Although the wounds at this stage had significantly contracted, had healthy granulation tissue and were dry from secretions, complete epithelialisation had not always been achieved. The majority of these patients were further managed in the community and, as such, we do not have data pertaining to time of complete wound epithelialisation. No patients from our series required split skin grafting to achieve ultimate wound closure. Split skin grafts may have reduced the healing time in some cases, but was not used in any of the patients in this series.
Two patients (11%) developed enterocutaneous fistulas whilst on negative pressure therapy. One fistula was discovered during a second-look laparotomy. The defect in the small bowel was closed and the laparostomy wound subsequently healed by secondary intention. The patient survived and had no evidence of fistulation 6 months later. A second patient, who had undergone a small bowel resection and anastomosis, developed a low output fistula, which was managed conservatively and closed without requirement for further surgical intervention.
One patient developed a mild allergic reaction to the dressing drapes. This was not sufficient enough to warrant discontinuation of the negative pressure treatment.
Two patients were returned to theatre earlier than planned. This was because of lifting of the adhesive parts of the occlusive drapes and failure of the dressing to maintain constant negative pressure, causing small bowel evisceration. On both occasions, the bowel was inspected and was viable. It was returned to the abdominal cavity and a new dressing applied. Both patients recovered without further incident. Both these patients were obese and inability to maintain the negative pressure of the vacuum dressing was likely due to difficulties in maintaining the contact of the adhesive drapes to the abdominal wall skin, especially in the skin folds and in the groin creases.
In our series, there are no patients who have developed a ventral hernia that has required subsequent corrective intervention.
Applying the P-POSSUM scoring system to our patient series, we might have expected 15 deaths from our 27 patients (Fig. 1). However, we observed only 10 deaths. Four patients, out of a total of nine, with P-POSSUM scores of over 75% predicted mortality, survived to discharge (i.e. 44% observed survival in patients with P-POSSUM scores > 75%).
We performed a randomisation test on the data by comparing the P-POSSUM scores with observed outcomes using a Bernouilli distribution test to simulate datasets of the expected survival for each of the 27 patients. Using this statistical technique, we generated 999 data sets; only 3 of these datasets had better survival rates than the observed data. By including the observed data set, only 4 sets predicted a survival rate of 10/27 or better. By performing a one-tailed test at P = 0.004, we can, therefore, conclude that the observed survival rate was significantly better than predicted by the P-POSSUM (P = 0.004).
Discussion
Primary abdominal wall closure following laparotomy is not always possible or advisable, especially in situations such as damage-limitation surgery, where it may precipitate cardiorespiratory, gastrointestinal and renal dysfunction secondary to abdominal compartment syndrome. The role of laparostomy in these circumstances is well established. Laparostomy management presents a formidable challenge and, in itself, is associated with significant morbidity and mortality in excess of 25%.18
Various methods for managing laparostomies have been described.6,19,20 Many of these are difficult to apply and somewhat cumbersome to manage. There is a requirement for a temporary closure device that is not only simple to apply, but also actively removes wound and visceral exudates, promotes tissue granulation, minimises complications, and provides mechanical containment of abdominal contents. We have found that intraperitoneal vacuum-assisted therapy largely fulfils these criteria. In our series of patients with a severe intra-abdominal catastrophe managed by laparostomy with immediate application of negative pressure therapy, there was a statistically significantly improved survivability when compared to expected outcomes as predicted by P-POSSUM scores.
Some authors, however, have found that the P-POSSUM scoring system has a tendency to over-predict morbidity and mortality, and that this over-prediction is particularly pronounced in the elderly, low-risk and elective surgical cases,21,22 which has resulted in various specialty specific modifications to the original scoring system (v-POSSUM, cr-POSSUM). However, a wealth of published studies have compared the available surgical scoring systems, particularly in the context of emergency and damage-control surgery, concluding that the P-POSSUM system is a robust scoring system which accurately predicts outcome.23,24
Although the precise mechanism by which negative pressure therapy works remains unclear, many authors believe that by actively removing excess interstitial fluid, the system improves local blood supply, bringing with it the oxygen and nutrients that are essential for effective tissue repair.17 Studies have shown that vacuum therapy is associated with a decrease in wound sepsis, possibly by lowering the bacterial load in the wound.14 By applying sub-atmospheric pressure, negative pressure therapy brings about the collapse of the foam placed between the wound edges. Banwell et al.18 suggest that this centripedial force promotes tissue granulation at the wound/foam interface.
In addition to promoting wound granulation, the negative pressure laparostomy system allows for the containment of the abdominal contents, whilst permitting drainage of purulent material and exudates between its interstices. It proved particularly popular in the ITU, where it was easier for nursing staff to look after than a traditional wound manager laparostomy bag, which we had routinely used previously.
Damage-control surgery was performed on three patients having suffered perforated diverticular disease and one patient who suffered a small bowel perforation with gross peritoneal contamination. The decision to perform damage-control surgery was taken jointly by the senior surgeon, anaesthetist and/or intensivist in attendance. The decisions were based on the haemodynamic status of the patient under anaesthetic (i.e. need for short operative time), hostile intraperitoneal conditions for primary anastomosis, severe peritoneal contamination rendering subsequent undrained intra-abdominal sepsis likely, prediction of significant risk of abdominal compartment syndrome by experienced clinicians (i.e. distended bowel, high ventilation pressures on attempted abdominal closure) and urgent requirement to reverse negative physiological responses to pathological and surgical trauma (i.e. coagulopathy, hypothermia, acidosis). We believe, particularly in patients with severe perforated diverticular disease, the added operative time to mobilise the descending colon and splenic flexure to fashion a stoma, which is unlikely to function for some time postoperatively, is sometimes not good use of this critical time. We feel that these patients benefit more from damage-control surgery and laparostomy formation followed by prompt admission to intensive care, institution of invasive monitoring and organ support, and correction of deranged physiology, blood clotting, etc. Return to theatre can be performed on an ‘elective’ basis following physiological optimisation, possibly by a specialist colorectal surgeon, for further peritoneal wash-out, end stoma formation. If the intra-abdominal conditions have improved significantly enough, primary anastomosis and delayed primary closure of the abdominal wall may be possible.
The manufacturers recommend return to theatre for dressing changes every 48–72 h. In practice, we found that we had to perform re-look surgery slightly sooner in our patient series (24–48 h). Occasionally this was unplanned, due to failure of the laparostomy dressing resulting in a loss of negative pressure or, in one case, evisceration of abdominal contents. Other indications for early return to theatre (< 48 h) included: (i) on-going, high-volume drainage of grossly infected fluid; and (ii) deterioration of patients' condition thought to be due to further intra-abdominal pathology.
All dressing changes in our study were performed with sterile conditions in an operating theatre, under general anaesthesia. For the first dressing change, the majority of patients were still intubated on the intensive care unit, meaning transfer to theatre for dressing change was relatively straight forward. Following extubation, we elected to perform all further dressing changes under general anaesthesia until the ‘intraperitoneal’ component of the dressing could be avoided. Although we have no experience of changing ‘intraperitoneal’ laparostomy dressings ‘on the ward’ without anaesthesia or sedation, we feel this would be distressing for the patient and may hamper extensive abdominal lavage. More recently, we have begun to replace the intraperitoneal dressings for the Blue Sky Medical V1STA® (Smith & Nephew Ltd, UK) negative pressure dressing, a superficial vacuum therapy device, when delayed primary closure is deemed not possible, for maturation of the laparostomy wound. The V1STA dressing utilises a slightly lower negative pressure, and can be changed on the ward environment, negating the requirement for a return to theatre and further anaesthetics, in appropriate patients who have been fully counselled. We elect to use an intraperitoneal dressing initially,
We report two patients (11%) who developed a small bowel fistula related to the topical negative pressure dressing. Fistulas have been reported in up to 25% of patients who have had other types of laparostomy management.18,19 Fistulas are attributed to desiccation and erosion of the bowel wall, possibly as a result of adhesions with the underlying dressing.20
Rao et al.12 reported a 20% incidence of fistula development in their series of patients with open abdomens treated with negative pressure therapy; of these patients 66% eventually died. They recommended caution in the use of negative pressure dressings, particularly where a bowel anastomosis has been performed or enterotomies have been closed.12 In our experience, one patient, who had undergone a Hartmann's procedure, was noticed to have a small enterotomy in a portion of small bowel in close proximity to the dressings, during a re-look laparotomy. The enterotomy was closed and the patient recovered without evidence of fistulation. The second patient, who had suffered an iatrogenic small bowel injury and undergone a bowel resection and anastomosis, developed a low output enterocutaneous fistula, after the intraperitoneal component of the dressing had been discontinued and the wound was being managed with superficial negative pressure dressings. A fistulogram was performed and the distal bowel was catheterised by interventional radiology, to utilise the distal bowel limb for enteral nutrition. The fistula closed following discontinuation of feeding without the need for surgical intervention. The patient remains well at 18-month review.
The KCI negative pressure laparostomy system employs a polyurethane-coated, fenestrated dressing for placement over the omentum and exposed bowel. Many animal and human studies have demonstrated the benefit of polyurethane-coated intraperitoneal material in reducing adhesion formation and subsequent bowel erosion.25 Early development of multiple adhesions between the parietal peritoneum and the visceral peritoneum of the bowel wall may precipitate inadvertent enterotomies during dressing changes. We would expect, with the use of polyurethane-coated negative pressure dressing, to observe fewer adhesion-associated complications than have been reported with traditional laparostomy techniques, although would concur with Rao et al.,12 and advocate caution with the use of high negative pressures in patients with bowel anastomosis, particularly if insufficient omentum is available for complete visceral coverage.
In our series, one patient developed a mild skin reaction to the dressings. As we have previously reported,26 this is often not a major setback as the skin can be effectively protected by applying a silicone dressing beneath the laparostomy dressings.
In our series, five of the surviving patients (29%) underwent delayed primary wound closure during a second-look laparotomy. We believe that this is possible, and indeed desirable. Kaplan et al.27 reported that, if the abdomen is not primarily closed within 7–10 days, adhesion formation and fascia retraction can render this impossible. Conversely, some authors have found that, when employing negative pressure laparostomy systems, delayed primary closure, even up to 49 days' postoperatively, has been successful.21
However, if delayed primary closure is not possible, the negative pressure effect of the laparostomy system opposes the action of the oblique muscles (otherwise acting unopposed in other laparostomy techniques) and draws the fascia together, resulting in a lower incidence of ventral herniation than has been found in other methods of laparostomy management.6
By quantifying third space losses, the negative pressure system significantly aids the clinician with fluid replacement in demanding and frequently unstable patients. Barringer et al.28 reported two cases of hypovolaemia directly related to the use of a vacuum system. The group concluded that judicious management of fluid balance during negative pressure therapy is required.
Conclusions
Laparostomy with immediate application of negative pressure vacuum therapy is a robust and simple technique that not only promotes tissue repair but is associated with fewer complications than seen with some other laparostomy techniques. Our series has demonstrated a statistically significant trend towards a reduction in morbidity and mortality than might otherwise be expected in patients with such intra-abdominal catastrophes. Although there are a number of possible confounding factors, we believe that the use of a laparostomy dressing, by avoiding the considerable mortality associated with of abdominal compartment syndrome, was partly responsible for the improve outcomes observed in our series.
We have found the system to be straight forward to nurse and associated with minimal patient discomfort. In addition, the laparostomy dressings can easily be converted to ‘mini-VAC therapy’ (KCI), to allow on-going wound care in the community. We believe that laparostomy with negative pressure therapy should be used more frequently in the setting of damage-control surgery.
References
- 1.Hadeed JG, Staman GW, Sariol HS, Kumar S, Ross SE. Delayed primary closure in damage control laparotomy: the value of the Wittmann patch. Am Surg. 2007;73:10–2. [PubMed] [Google Scholar]
- 2.Chorbadjian M, Bown M, Graham C, Sayers R. Laparostomy healing by secondary intention after ruptured abdominal aortic aneurysm repair. J Tissue Viability. 2004;14:24–7. doi: 10.1016/s0965-206x(04)41003-1. [DOI] [PubMed] [Google Scholar]
- 3.Finlay IG, Edwards TJ, Lambert AW. Damage control laparotomy. Br J Surg. 2004;91:83–5. doi: 10.1002/bjs.4434. [DOI] [PubMed] [Google Scholar]
- 4.de Laet IE, Malbrain M. Current insights in intra-abdominal hypertension and abdominal compartment syndrome. Med Intensiva. 2007;31:88–99. doi: 10.1016/s0210-5691(07)74781-2. [DOI] [PubMed] [Google Scholar]
- 5.Brox-Jimenez A, Ruiz-luque V, Torres-Arros C. Experience with the Bogata bag technique for temporary abdominal closure. Cir Esp. 2007;83:150–4. doi: 10.1016/s0009-739x(07)71690-1. [DOI] [PubMed] [Google Scholar]
- 6.Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: A 7 year experience with 112 patients. J Trauma. 2000;48:201–7. doi: 10.1097/00005373-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Morris JA, Jr, Eddy VA, Blinman TA, Rutherford EJ, Sharp KW. The staged celiotomy for trauma. Issues in unpacking and reconstruction. Ann Surg. 1993;217:567–84. doi: 10.1097/00000658-199305010-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durmishi Y, Gervaz P, Buhler L, Bucher P, Zufferey G, et al. Vacuum-assisted abdominal closure: its role in the treatment of complex abdominal and perineal wounds. Experience in 48 patients. J Chir (Paris) 2007;144:209–13. doi: 10.1016/s0021-7697(07)89516-0. [DOI] [PubMed] [Google Scholar]
- 9.Wondberg D, Larusson HJ, Metzger U, Platz A, Zingg U. Treatment of the open abdomen with the commercially available vacuum-assisted closure system in patients with abdominal sepsis: low primary closure rate. World J Surg. 2008;32:2724–9. doi: 10.1007/s00268-008-9762-y. [DOI] [PubMed] [Google Scholar]
- 10.Perez DG, Loprinzi CL, Barton DL, Pockaj BA, Sloan J, et al. Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg. 2007;204:586–92. doi: 10.1016/j.jamcollsurg.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO, 3rd, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma. 2008;65:337–42. doi: 10.1097/TA.0b013e31817fa451. [DOI] [PubMed] [Google Scholar]
- 12.Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum-assisted closure of abdominal wounds: a word of caution. Colorectal Dis. 2007;9:266–8. doi: 10.1111/j.1463-1318.2006.01154.x. [DOI] [PubMed] [Google Scholar]
- 13.Ingber D. In search of cellular control: signal transduction in context. J Cell Biochem Suppl. 1998;30/31:232–7. [PubMed] [Google Scholar]
- 14.Chen SZ, Li J, Li XY. Effects of vacuum-assisted closure: an experimental study. Asian J Surg. 2005;28:211–7. doi: 10.1016/S1015-9584(09)60346-8. [DOI] [PubMed] [Google Scholar]
- 15.Bucalo B, Eaglestein W, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen. 1993;1:181–6. doi: 10.1046/j.1524-475X.1993.10308.x. [DOI] [PubMed] [Google Scholar]
- 16.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 17.Midwinter MJ, Tytherleigh M, Ashley S. Estimation of mortality and morbidity risk in vascular surgery using POSSUM and the Portsmouth predictor equation. Br J Surg. 1999;86:471–4. doi: 10.1046/j.1365-2168.1999.01112.x. [DOI] [PubMed] [Google Scholar]
- 18.Banwell PE, Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care. 2003;12:22–8. doi: 10.12968/jowc.2003.12.1.26451. [DOI] [PubMed] [Google Scholar]
- 19.Aprahamian C, Wittman DH, Bergstein JM. Temporary abdominal closure (TAC) for planned relaparotomy in trauma. J Trauma. 1990;30:719–23. doi: 10.1097/00005373-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Navaria PH, Bunting M, Omoshoro-Jones J, Nicol AJ, Kahn D. Temporary closure of open abdominal wounds by the modified sandwich-vacuum pack technique. Br J Surg. 2003;90:718–22. doi: 10.1002/bjs.4101. [DOI] [PubMed] [Google Scholar]
- 21.Isbister WH, Al-Sanea N. POSSUM: a re-evaluation in patients undergoing surgery for rectal cancer. The Physiological and Operative Severity Score for Enumeration of Mortality and Morbidity. Aust NZ J Surg. 2002;72:421–5. doi: 10.1046/j.1445-2197.2002.02436.x. [DOI] [PubMed] [Google Scholar]
- 22.Tang TY, Walsh SR, Prytherch DR, Wijewardena C, Gaunt ME, et al. POSSUM models in open abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 2007;34:499–504. doi: 10.1016/j.ejvs.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Stawicki SP, Brooks A, Bilski T, Scaff D, Gupta R, et al. The concept of damage control: extending the paradigm to emergency general surgery. Injury. 2008;39:93–101. doi: 10.1016/j.injury.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. POSSUM is an optimal system for predicting mortality due to colorectal perforation. Hepatogastroenterology. 2008;55:430–3. [PubMed] [Google Scholar]
- 25.Ansaloni L, Catena F, Coccolini F, Fini M, Gazzotti F, et al. Peritoneal adhesions to prosthetic materials: an experimental comparative study of treated and untreated polypropylene meshes placed in the abdominal cavity. J Laparoendosc Adv Surg Tech A. 2009;19:369–74. doi: 10.1089/lap.2008.0366. [DOI] [PubMed] [Google Scholar]
- 26.Quah HM, Maw A, Young T, Hay DJ. Vacuum-assisted closure in the management of the open abdomen : a report of a case and initial experiences. J Tissue Viability. 2004;14:59–62. doi: 10.1016/s0965-206x(04)42003-8. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan M, Banwell P, Orgill D, Ivatury R, Demetriades D, et al. Guidelines for the management of the open abdomen. Supplement to Wounds: A Compendium of Clinical Research and Practice. 2005 [Google Scholar]
- 28.Barringer CB, Gorse SJ, Burge TS. The VAC dressing – a cautionary tale. Br J Plast Surg. 2004;57:482. doi: 10.1016/j.bjps.2004.01.005. [DOI] [PubMed] [Google Scholar]