Abstract
A novel linear multifunctional polyethylene glycol (PEG)-dexamethasone (Dex) conjugate (click PEG-Dex) was synthesized using facile Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition (a click reaction). Dex was conjugated to the click PEG via an acid-labile hydrazone bond to allow the drug release in pathophysiological environment. To evaluate click PEG’s potential as a versatile drug delivery platform, the click PEG-Dex conjugates were tested in an adjuvant-induced arthritis (AA) rat model. In vivo optical imaging data confirmed the arthrotropism of the conjugates in the arthritic rats. Long-term treatment study revealed that a single click PEG-Dex conjugate administration provided sustained (> 15 days) amelioration of ankle joint inflammation to the AA rats. Treatment with equivalent dose of dexamethasone phosphate sodium (free Dex) only provides temporal resolution of the arthritis, which recurred upon treatment withdrawal. Further histological and bone mineral density comparison between the ankle joints from both click PEG-Dex and free Dex treatment groups confirmed the superior anti-inflammatory and disease modifying effects of the novel polymer-drug conjugates.
Keywords: Click PEG, Dexamethasone, Polymer-drug Conjugate, Rheumatoid Arthritis, Arthrotropism
Introduction
The advance of biological sciences in the past few decades has revolutionized our understanding of etiologies of many human diseases from cellular and molecular levels. Though these developments have reinvigorated the drug discovery realm with great excitement, the number of new drugs approved by the US FDA is somewhat disappointing considering the significant public investment and anticipation. Part of the problem may stem from the current drug discovery paradigm, which almost exclusively focuses on the identification of new molecular target for disease intervention, with the drug concentrations in vivo being somewhat passively handled by the human body itself.
Proactive management of therapeutic agents’ concentration in vivo was first conceptualized by Paul Ehrlich as the magic bullet. It has evolved during the past century into the flourishing field of drug delivery and nanomedicine. Among the many delivery tools that have been developed, Ringsdorf’s design of polymeric drug conjugate was seminal,1 which was further potentiated by Maeda’s discovery of the enhanced permeability and retention (EPR) effects of macromolecules in the solid tumors.2 Since then, great achievements have been made the field of polymer therapeutics with nearly a dozen polymer-drug conjugates being evaluated clinically. Among the biocompatible polymers being used, N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers, poly(ethylene glycol) (PEG), and poly(glutamic acid) (PGA) are the top choices.3
As a nonimmunogenic and biocompatible water-soluble polymer, PEG not only gained particular success in protein conjugation, but also was widely used in low molecular weight (MW) drug conjugation.4 In addition to the marketed PEGylated protein drugs, several low MW drug-PEG conjugates have also entered clinical trials for cancer treatment (e.g. Pegamotecan, a camptothecin-PEG conjugate).5 Low camptothecin loading in Pegamotecan (1.7 wt. %), however, caused the company to eventually abort the product. Apparently this is caused by the fact that PEGs only have two chain terminal hydroxyl groups for chemical modification or conjugation regardless of their molecular weight. This intrinsic chemical limitation has significantly limited the development of small drug-PEG conjugates. Contrary to linear PEGs, the presence of side chain functionalities allows high drug loading capacities in HPMA copolymers and PGAs.6 Using multi-arm PEGs, the drug loading can be increased to 3 folds.7 In addition, several studies have also been made to develop linear multi-functional PEGs.8–11 Nevertheless, challenges still remain for these approaches due to the complex syntheses.
Recently, we have successfully developed a linear, multifunctional poly(ethylene glycol) (click PEG)12 by using the facile Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition (a click reaction).13–15 The copolymerization is modular, simple, and highly efficient. In this manuscript, we present the first synthesis of a click PEG theranostic and its successful application in the diagnosis and treatment of a rat model of inflammatory arthritis.
Experimental Section
Materials and methods
N,N-dioctadecyl-N’,N’-bis(2-hydroxyethyl)propanediamine (LA),16 2,2-bis(azidomethyl)propane-1,3-diol, acetylene-terminated PEG 2000 (acetylene PEG), acetylene-terminated monomethylether PEG 1900 (acetylene mPEG), and tris-(hydroxypropyltriazolylmethyl)amine (THPTA) were synthesized as described previously.12 Methyl 4-bromoacetate, sodium ascorbate, and copper sulfate were purchased from ACROS (Morris Plains, NJ, USA). Hydrazine hydrate was purchased from Fisher Scientific (Pittsburgh, PA). Dexamethasone (Dex) and dexamethasone phosphate were purchased from Hawkins, Inc. (Minneapolis, MN, USA). IRDye 800CW NHS ester was purchased from LI-COR Biosciences (Lincoln, NE, USA). Mycobacterium tuberculosis H37Ra (heat-killed, desiccated) was obtained from VWR International (West Chester, PA, USA). Paraffin oil (low viscosity, Bayol F) was obtained from Crescent Chemical Company, Inc. (Islandia, NY, USA). PEG and PEO calibration standards were purchased from Chrom Tech, Inc. (Apple Valley, MN, USA). Superdex 200 (HR 10/30) column, PD-10 columns and Sephadex LH-20 resins were obtained from GE HealthCare (Piscataway, NJ, USA). All solvents and other reagents if not specified were purchased from Fisher Scientific or ACROS and used without further purification.
1H and 13C NMR spectra were recorded on a 500 MHz NMR spectrometer (Varian, Palo Alto, CA) separately. The high-resolution mass spectra (HRMS) were obtained on a high-resolution 4800 MALDI TOF/TOF™ analyzer (Applied Biosystems/MDS SCIEX); α-Cyano-4-hydroxy-cinnamic acid was used as the matrix and xenon as the ionizing gas. The weight average molecular weight (Mw) and number average molecular weight (Mn) of copolymers were determined by size exclusion chromatography (SEC) using the ÄKTA FPLC system (GE HealthCare) equipped with UV and RI (KNAUER, Berlin, Germany) detectors. SEC measurements were performed on Superdex 200 (HR 10/30) with phosphate-buffered saline (pH = 7.3) as the eluent. PEG or PEO standards with narrow polydispersity were used as calibration standards. Copper residue in the polymer was determined on a Varian ICP-MS (Inductively Coupled Plasma – Mass Spectrometer, software version 2.1, Walnut Creek, CA). HPLC analyses were performed on an Agilent 1100 HPLC system (Agilent Technologies, Inc., Santa Clara, CA, USA) with a reverse phase C18 column (Agilent, ZORBAX 300SB-C18, 4.6×250 mm, 5 µm). In vivo near-infrared fluorescence (NIRF)-based optical imaging was accomplished on a XENOGEN IVIS® 200 Series Imaging System (Hopkinton, MA, USA). Bone mineral density (BMD) was measured with a pDEXA® Sabre™ X-ray bone densitometer (Norland Medical System, Inc., Fort Atkinson, WI, USA). All animal experiments were performed according to a protocol approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and adhered to Principles of Laboratory Animal Care (National Institutes of Health publication 85-23, revised in 1985).
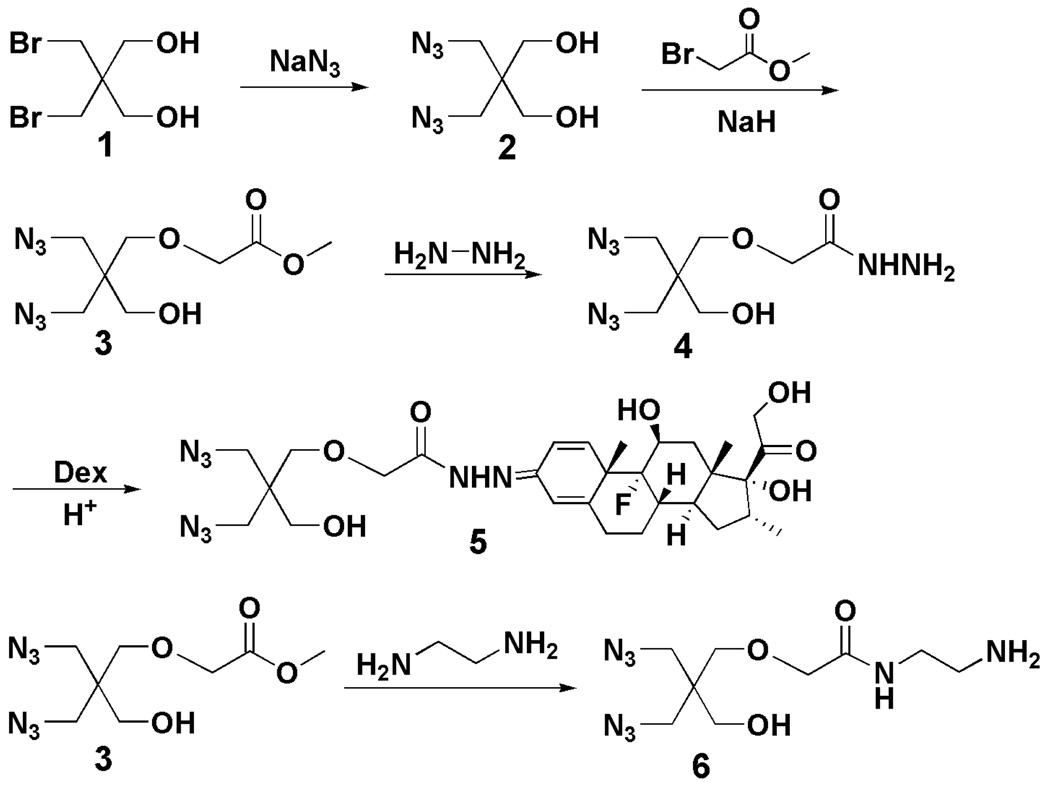
Synthesis of methyl 2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)-propoxy)acetate (3)
2,2-Bis(azidomethyl)propane-1,3-diol (2; 1.86 g, 10 mmol) was dissolved in 60 mL anhydrous THF. NaH (0.4 g, 10 mmol, 60% dispersion in mineral oil) was added slowly to the reaction solution and stirred at room temperature for 3 h. Methyl 4-bromoacetate (3 mL) in THF (10 mL) was then slowly added and stirred at room temperature overnight. Water was finally added slowly to quench the reaction. The products were extracted with EtOAc. The combined organic extracts were washed with brine, dried over MgSO4, filtered and concentrated to give oil. The oil was further purified by flash chromatography on a silica gel column using ethyl acetate/hexane (1:3.5, v/v) as eluent. Yield: 57 %. 1H NMR (d6-DMSO): δ = 4.87 (t, J = 4.9 Hz, 1 H), 4.13(s, 2 H), 3.66 (s, 3 H), 3.36 (s, 2 H), 3.35 s, 4 H), 3.30 (d, J = 4.9 Hz, 2 H); 13C NMR (d6-DMSO): δ = 170.5, 70.0, 67.9, 59.8, 51.5, 51.3, 45.0; HRMS calcd for C8H14N6O4 (M+) 258.1077, found at m/z 297.0538 potassium adduct (M+K+).
Synthesis of 2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)propoxy)-acetohydrazide (4)
Methyl 2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)-propoxy)acetate (3; 840 mg, 3.3 mmol) was dissolved in methanol (10 mL). Hydrazine hydrate (0.5 mL) in 5 mL of methanol was then added. The reaction solution was stirred overnight at 60 °C. After evaporation of the reaction solvent, the product was dried under vacuum. Yield: 87 %. 1H NMR (d6-DMSO): δ = 9.01 (s, 1 H), 4.94 (t, J = 4.9 Hz, 1 H), 4.29 (br, 2 H), 3.88 (s, 2 H), 3.39 (s, 2 H), 3.35 (s, 4 H), 3.32 (d, J = 4.9 Hz, 2 H); 13C NMR (d6-DMSO): δ = 167.7, 69.8, 69.7, 59.8, 51.1, 45.1; HRMS calcd for C7H14N8O3 (M+) 258.1189, found at m/z 281.0089 as sodium adduct (M+Na+).
Synthesis of acid-labile Dex-containing monomer (5)
Dexamethasone (784 mg, 2 mmol) and 2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)propoxy)-acetohydrazide (4; 568 mg, 2.2 mmol) were dissolved in THF (15 mL). Acetic acid (0.5 mL) was added into the reaction solution as catalyst. The reaction was stirred for 3 days at room temperature. After evaporation of the reaction solvent, the product was purified by silica gel flash column chromatography (chloroform/methanol = 10:1). Yield: 40 %. 1H NMR (d6-DMSO): δ = 10.83 (s, 0.5 H), 10.40 (s, 0.5 H), 6.78 (s, 0.5 H), 6.59 (s, 0.5 H), 6.42 (m, 1 H), 6.19 (m, 1 H), 5.13 (br, 1 H), 4.95 (s, 1 H), 4.90 (t, J = 5.4 Hz, 0.5 H), 4.87 (t, J = 4.9 Hz, 0.5 H), 4.69 (br, 1 H), 4.50 (dd, J1 = 19.0 Hz, J2 = 5.4 Hz, 1 H), 4.06 (m, 4 H), 3.40 (s, 4 H), 3.37 (s, 2 H), 3.34 (d, J = 4.9 Hz, 2 H), 2.94 (m, 1 H), 2.60 (m, 1 H), 2.27 (m, 2 H), 2.12 (m, 2 H), 1.72 (m, 1 H), 1.60 (q, J = 11.2 Hz, 1 H), 1.41 (s, 3 H), 1.24 (m, 2 H), 1.06 (m, 1 H), 0.85 (s, 3 H), 0.78 (d, 3 H, J = 7.3 Hz); 13C NMR (d6-DMSO): δ = 211.5, 173.9, 172.0 (d, syn and anti), 168.2 (d, syn and anti), 165.9 (d, syn and anti), 126.7 (d, syn and anti), 111.4 (d, syn and anti), 100.8 (d, JCF = 174 Hz), 90.4, 74.3 (d, syn and anti), 70.2 (d, JCF = 22 Hz), 69.7 (d, syn and anti), 66.5, 60.2 (d, syn and anti), 51.3 (d, syn and anti), 47.7, 47.4 (d, JCF = 22 Hz), 45.4 (d, syn and anti), 43.7, 36.1, 35.1, 34.0 (d, JCF = 20 Hz), 32.2, 31.0, 27.5, 24.4 (d, JCF = 6 Hz), 16.9, 15.6; HRMS calcd for C29H41FN8O7 (M+) 632.3082, found at m/z 633.2235 (M+H+).
Synthesis of N-(2-aminoethyl)-2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)propoxy) acetamide (6, amine-containing monomer)
Methyl 2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)-propoxy)acetate (3; 0.55 g, 2.1 mmol) was dissolved in ethylene diamine (10 mL, 150 mmol) under argon and allowed to stir at 55 °C overnight. TLC (1:2, v/v, ethyl acetate/hexane; silica TLC plate) verified that the reactant had been consumed. The solvent was then removed by rotary evaporation. Yield: 100%. 1H NMR (d6-DMSO): δ = 7.69 (br, 1H), 3.84 (s, 2H), 3.39 (s, 4H), 3.33 (s, 2H), 3.31 (s, 2H), 3.12-3.08 (m, 2H), 2.58 (t, J = 6.6 Hz, 2H); 13C-NMR (d6-DMSO): δ = 168.8, 70.2, 69.8, 59.8, 51.1, 45.1, 41.7, 41.3; HRMS calcd for C9H18N8O3 (M+) 286.1502, found at m/z 309.0206 as sodium adduct (M+Na+).
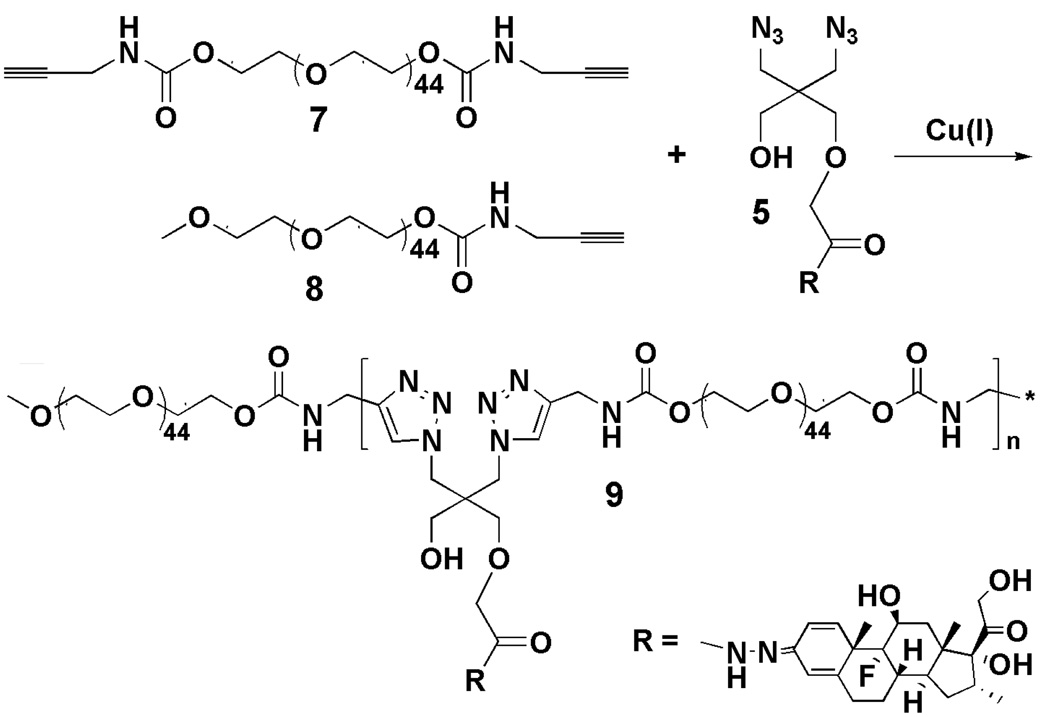
Synthesis of acid-labile click PEG-Dex conjugate (9)
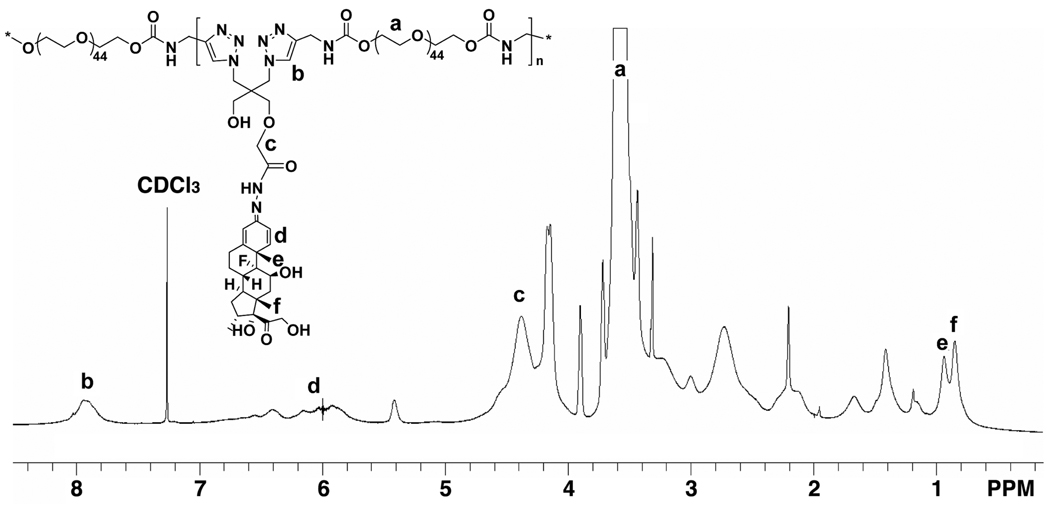
Acetylene terminated PEG 2000 (7; 200 mg, 92.5 µmol), Dex-containing monomer (5; 63.3 mg, 100 µmol), acetylene mPEG (8; chain terminator, 29.7 mg, 15 µmol), THPTA (8.64 mg, 20 µmol, stabilizing agent), and CuSO4·5H2O (2.5 mg, 10 µmol) were dissolved in water/methanol (2:4.5, v/v, 8 mL) with stirring. The solution was bubbled with argon to remove oxygen. Sodium ascorbate (20 mg, 100 µmol, in 1 mL H2O) was added into the reaction solution drop by drop with stirring to generate the Cu(I) catalyst by reducing the Cu(II). The reaction mixture was then allowed to stir at room temperature for 1 day. After evaporation of the solvent, the resulting polymer was dissolved in methanol and applied onto an LH-20 column to remove the unreacted low MW compounds. A large excess of EDTA disodium salt (> 10 times of Cu, molar ratio) was then added to the aqueous solution of the polymer, stirred for 1 hr at room temperature, and then dialyzed against water to remove the Cu catalyst. The MW cutoff size of the dialysis tubing is 12 kDa of globular protein. The polymer solution was lyophilized to obtain the final click PEG-Dex. Yield: 250 mg. The 1H NMR spectrum of click PEG-Dex is shown in Figure 1, with key structural elements assigned. Some peaks could not be assigned due to signal overlapping.
Figure 1.
1H NMR spectrum of click PEG-Dex. Key structural elements of click PEG-Dex are assigned.
To quantify the Dex loading in click PEG-Dex, it was hydrolyzed in 0.1 M HCl (2 mg/mL) overnight. The resulting solution was neutralized with NaOH (0.1 M) and analyzed by HPLC. Mobile phase, acetonitrile/water = 2/3; Detection, UV 240 nm; Flow rate, 1 mL/min; Injection volume: 10 µL. The analyses were performed in triplicate.
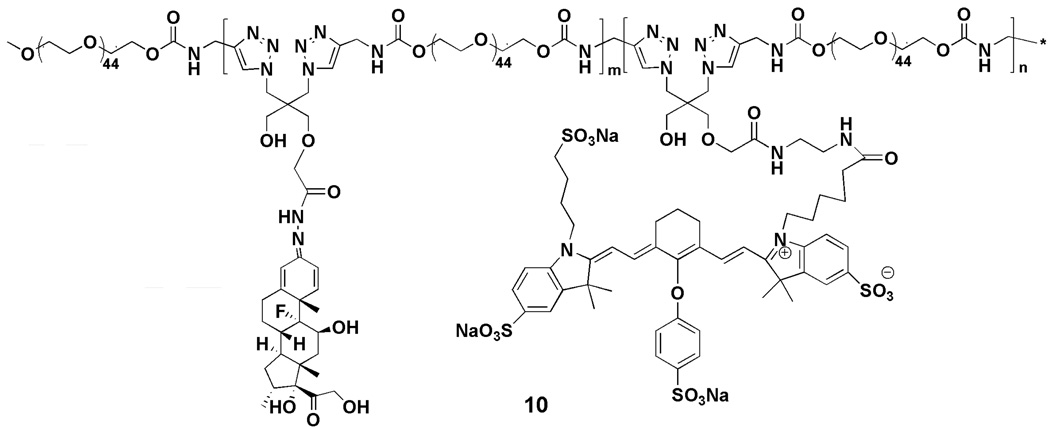
Synthesis of near infrared dye labeled click PEG-Dex conjugate (10)
Acetylene terminated PEG 2000 (7; 100 mg, 46.25 µmol), N-(2-Aminoethyl)-2-(3-azido-2-(azidomethyl)-2-(hydroxymethyl)propoxy)acetamide (6; 2.86 mg, 10 µmol), Dex-containing monomer (5; 25.32 mg, 40 µmol), and acetylene mPEG (8; 14.85 mg, 7.5 µmol) was polymerized using the method similar to that described in the synthesis of acid-labile click PEG-Dex conjugate. The resulting amine-containing click PEG-Dex (100 mg) was then labeled with 0.5 mg IRDye 800 CW NHS ester in DMF overnight at room temperature, and then purified by PD-10 column and lyophilized (Scheme 3). IRDye 800 CW content in click PEG was quantified by measurement of the UV absorption at 774 nm in water.
Scheme 3.
Structure of IRDye 800 CW-labeled click PEG-Dex.
Analysis of Cu residue in the polymer
The copper content in the copolymers after purification was determined by ICP-MS. Calibration standards (2 – 20 ng Cu/mL) were prepared by serial dilution of 1000 mg/L copper standard solution with 2% (v/v) nitric acid. Replicate copolymer samples were prepared by dissolving the conjugate in 50 µL trace metal grade nitric acid, then diluting with water (2 –10 mg of polymer/mL in 2% v/v nitric acid). The solutions were then assayed for copper by ICP-MS with no additional sample processing. Signals from both 63Cu and 65Cu isotopes were used to generate two independent calibration curves with 165Ho (10 ng/mL) as the internal standard (correlation coefficients = 0.9987). To evaluate matrix effects in the instrument, the method of standard additions was used on one sample.17 A weighed quantity of copolymer was dissolved in nitric acid, then diluted with distilled water (1:1). To aliquots of this solution, variable aliquots of copper standard were added (equivalent to 0, 2.5, 5, and 7.5 ug Cu/g-polymer); the resulting solution was diluted to 1 mL and assayed.
In vitro Dex release from click PEG-Dex conjugate
Click PEG-Dex (3 mg/mL) was dissolved in pH 5.0 acetate buffer (0.01 M with 0.15 M NaCl), pH 6.0 phosphate buffer (0.01 M with 0.15 M NaCl), or pH 7.4 phosphate buffer (0.01 M with 0.15 M NaCl) and incubated at 37°C. At selected time intervals, the release medium (0.3 mL) was withdrawn and the release of Dex was determined by HPLC after neutralization with NaOH (0.1 M). The analysis of each sample was performed in triplicate.
In vivo NIRF imaging of click PEG-Dex’ distribution in AA rats
To induce arthritis, heat killed Mycobacterium tuberculosis H37Ra (1 mg) and LA (5 mg) were mixed in paraffin oil (100 µL), sonicated and injected subcutaneously into the tail base of male Lewis rats (175 to 200 g, Charles River Laboratories, Wilmington, MA, USA).18 The in vivo NIRF imaging was performed on day 15th post induction of arthritis. All adjuvant-induced arthritis (AA) rats were anesthetized with 2% isoflurane throughout all procedures. After acquisition of a background image, the rats were given a bolus injection of the IRDye 800CW labeled click PEG-Dex conjugate (100 nmol of IRDye 800CW equivalence per kilogram body weight) via tail vein. Rats were imaged at 2h, 8h, 1d, 3d, 7d post injection.
The imaging was accomplished using a 200 Series XENOGEN IVIS Imaging System. A laser source produced light at wavelengths of 785nm for illumination of the complete animal in a light-proof box inside the device. The emitted fluorescence signals were detected with an intensified charge-coupled device camera. Image acquisition and analysis were performed with the Living Imaging® 2.0 software.
Treatment of AA rats with click PEG-Dex conjugate
Rats with established arthritis were selected and randomly assigned into four groups, click PEG-Dex, free Dex, click PEG, and saline, with 6 AA rats per group. A healthy control group was also included. On day 15th post arthritis induction, click PEG-Dex (74 mg/kg, [Dex]click PEG-Dex = 135 mg/g) and click PEG (64 mg/kg) were given intravenously to two groups of AIA rats, respectively. An equivalent dose of free Dex (10 mg/kg of free Dex in the form of water-soluble dexamethasone sodium phosphate) was divided into four aliquots (2.5 mg/kg of free Dex) and was administered (i.p.) to the third group of AA rats daily on days 15–18. Saline was given similarly to the third group of AA rats. The clinical measurements of ankle joints were performed daily. On day 29th post arthritis induction, all animals were euthanized. The hind limbs were dissected at the knee joint. The BMDs of the region from distal tibia to the phalanges of the left foot were measured by peripheral dual energy x-ray absorptiometry (pDEXA).
Clinical measurements
The hind paws of all animals were observed and measured for articular index (AI) score and ankle diameter (medial to lateral) from day 8th to 29th post arthritis induction. The same worker (LDQ) always observed and measured the rats using a digital caliper (World Precision Instruments, Inc. Saraspta, FL, USA), while the 2nd worker verified the results. The criteria for AI score were as the following: no signs of swelling or erythema (score=0), slight swelling and/or erythema (+1), low-to-moderate edema and signs involving the tarsals (+2), pronounced edema with limited use of the joint and signs extending to the metatarsals (+3), and excessive edema with joint rigidity and severe signs involving the entire hind paw (+4).
Histological analysis
The hind limbs of all of the rats were isolated and fixed in buffered formalin (10 %) for 3 days, decalcified with 12.5% (v/v) HCl for 2 days, embedded in paraffin, cut into 5 µm thickness sections, and stained with hematoxylin and eosin (H&E) for histological evaluation. The histological score of the joints were graded according to a modified grading system with the following parameters: synovial cell lining hyperplasia (0–2); villous hyperplasia (0–2); mononuclear cell infiltration (0–3); polymorphnuclear leukocytes infiltration in periarticular soft tissue (0–3); cellular infiltration and bone erosion at the distal tibia (0–2); cellular infiltration and bone erosion at astragalus (0–1) and cellular infiltration of cartilage (0–1).19 It was evaluated and summarized by an examiner (JT), who was blind to the treatment group arrangement.
Statistic analysis
One-way analysis of variance (ANOVA) was performed followed by a post hoc test (Tukey-Kramer) for multiple comparisons using Instant Biostatistics (Version 3.0, GraphPad Software, La Jolla, CA, USA). A p value of less than 0.05 was considered statistically significant.
Results
Syntheses of acid-labile Dex-containing click PEG-Dex conjugates
To release Dex from click PEG-Dex conjugate when exposed to acidic conditions associated with inflammatory tissues,20–22 2,2-bis(azidomethyl)propane-1,3-diol was first synthesized according to the method reported previously 12 and further monocarboxylated with methyl 2-bromoacetate. The carboxylate was then converted to hydrazide by reacting with excess hydrazine. As the final step, the acid-labile hydrazone bond was formed by coupling Dex with the hydrazide product under acidic condition (Scheme 1). These reactions are straightforward and the structures of each intermediates and the final product were all confirmed by 1H NMR and 13C NMR.
Scheme 1.
Syntheses of diazide monomers for click copolymerization.
Using click copolymerization (Scheme 2), the click PEG-Dex was synthesized by simply mixing Dex-containing monomer, acetylene PEG, acetylene mPEG (chain terminator), THPTA (stabilizing agent), and the in-situ produced Cu(I) catalyst in the reaction solvent. Amine-containing monomer was added for the synthesis of IRDye 800CW-containing click PEG-Dex conjugate. The resulting conjugates were purified on an LH-20 column. Due to the poor solubility of the Dex monomer in water, copolymerization was performed in an optimized solvent system (methanol/water). The Cu residue in click PEG-Dex was removed by EDTA chelation and subsequent dialysis. The ICP-MS analysis showed that more than 99.95% of the catalyst was removed through this procedure. The final Cu content was 4.7 ± 0.5 µg of Cu/g of click PEG-Dex. No free Dex monomer or Dex was detected in the click PEG-Dex by HPLC after purification. The weight average molecular weight (Mw) of click PEG-Dex is 17 kDa with a polydispersity index (PDI) of 3.0. The Dex content in the click PEG-Dex was determined as 135 mg dexamethasone per gram of click PEG-Dex, which is close to 100 % conversion of the Dex monomer into click PEG-Dex. For the synthesis of IRDye 800CW-containing click PEG-Dex, the NH2-containing click PEG-Dex was directly reacted with IRDye 800CW NHS ester. The final IRDye 800CW content is 3.4 µmol/g of polymer.
Scheme 2.
Synthesis of click PEG-Dex.
In vitro Dex release from click PEG-Dex
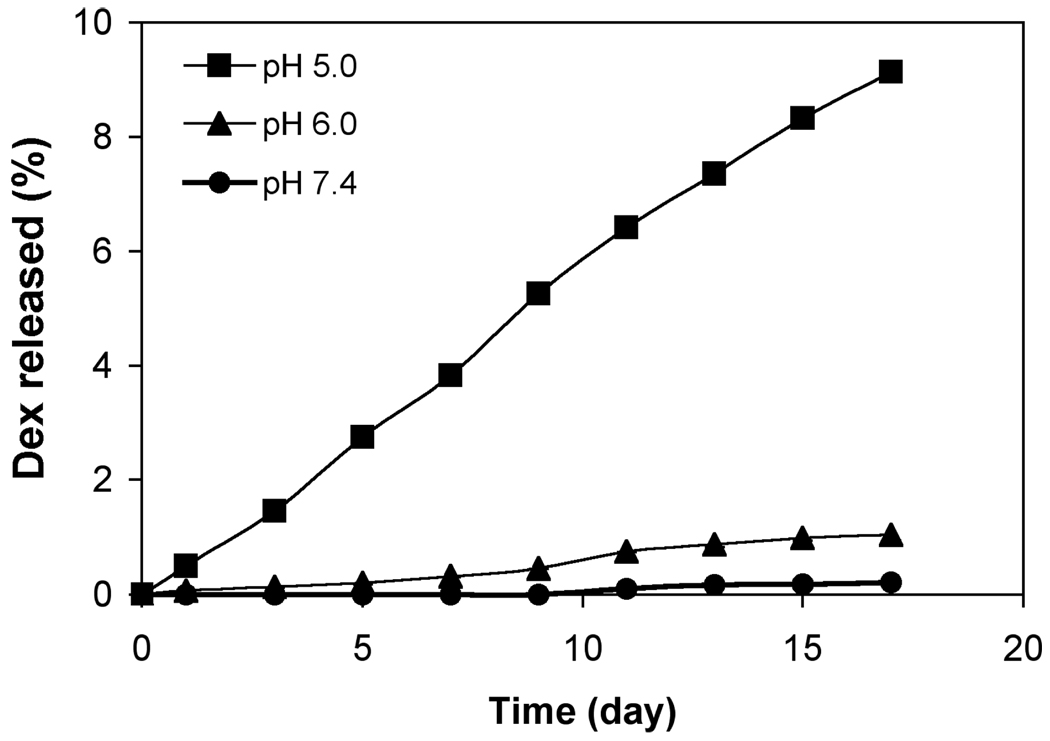
As shown in Figure 2, the in vitro Dex release profiles of click PEG-Dex is linear at pH 5.0. No Dex was released at pH 7.4 even after 17 days, within the detection limit of the HPLC system used (Agilent 1100). Less than 1 % of Dex was released at pH 6.0 within the time course of study. At pH 5.0, the release of Dex from click PEG-Dex was much faster compared to that of pH 6.0. By 17 days, a total of 9 % of Dex was released at pH 5.0, or an average release of 0.5 % per day.
Figure 2.
Cumulative in vitro Dex release profiles of click PEG-Dex at pH = 5.0, 6.0 and 7.4. Each sample was measured 3 times. The mean values and standard deviation were calculated with Microsoft Excel.
In vivo NIRF imaging of click PEG in the AIA rats
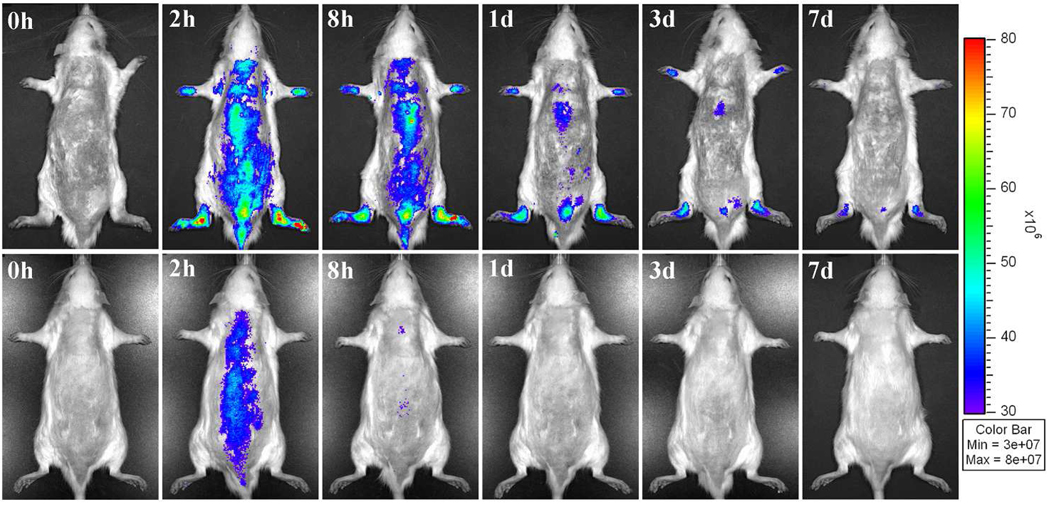
Prior to the injection of IRDye 800CW-labeled click PEG-Dex, no background signal was detectable in either healthy or arthritic rats (Figure 3). After the injection, visible NIRF signal was detected in the hind limbs of the AA rats. Imaging at 2 h post injection showed peak fluorescence signals in the arthritic ankle joints. The signal intensity was generally retained with a slow decline thereafter. At 7 days post injection, the fluorescence signals were still clearly detectable. In contrast, the healthy rats only showed a weak fluorescence signal in the hind limbs after the injection of IRDye 800CW-labeled click PEG-Dex and the signal declined rapidly. At 8 h post injection, the only signal detectable was located close to the heart. By 24 h post injection, no signal was detected.
Figure 3.
In vivo NIRF imaging of rats at different time points post injection of IRDye CW 800-labeled click PEG-Dex. Top panels, arthritic rats; bottom panels, healthy controls.
Clinical evaluations of different treatments on AIA rats
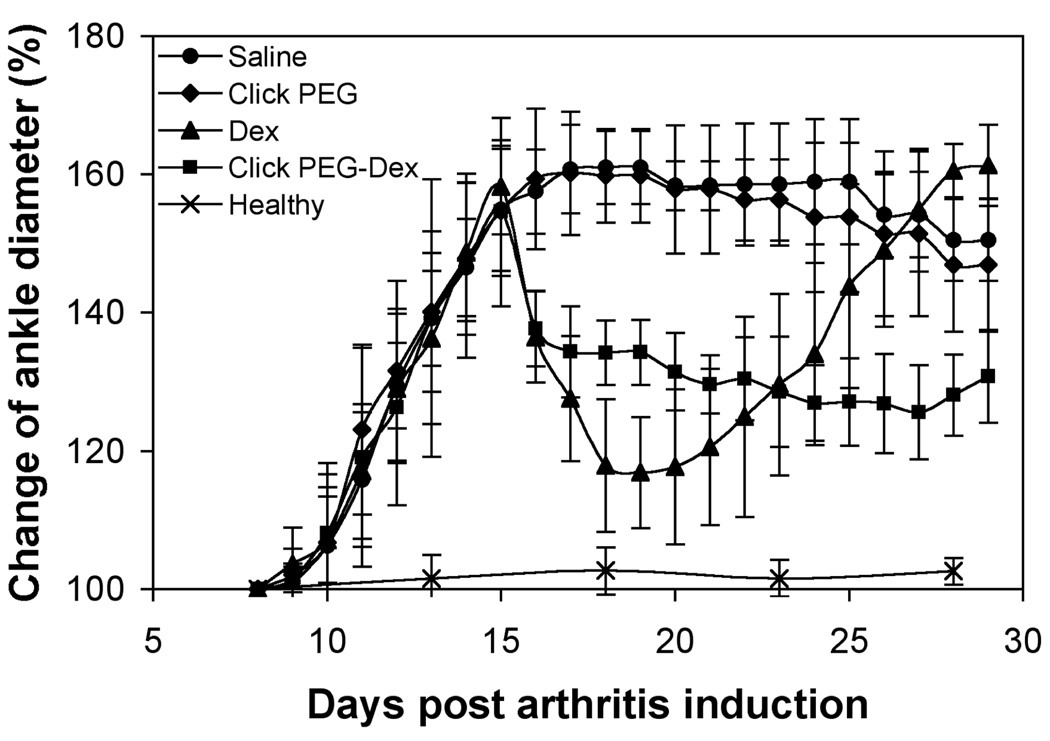
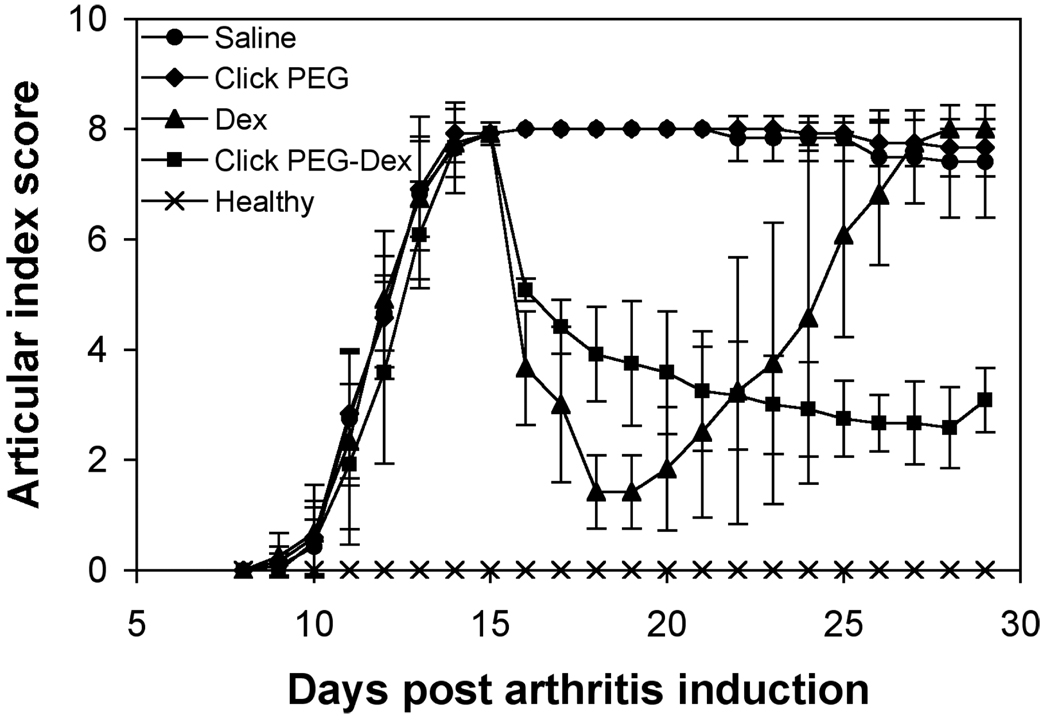
The adjuvant-induced arthritis onset started with mild swelling and increased ankle diameter on day 9th post induction (Figures 4 & 5). The ankle joint swelling started to plateau on day 14. As can be seen in Figures 4 & 5, saline and click PEG administration did not result in any reduction of clinical arthritis parameters, while treatments with both free Dex and click PEG-Dex resulted in immediate inflammation resolution. Ankle diameter and articular index (AI) scores for free Dex and click PEG-Dex groups were greatly reduced on day 16; the animals were more mobile and active. However, for the free Dex treatment group, inflammation flare was observed with a rapid decrease in mobility upon cessation of the free Dex treatment on day 19, and the ankle diameter and AI scores were at similar levels as those in the saline group at day 26th post arthritis induction. On the contrary, single click PEG-Dex treatment effectively ameliorated the ankle joint inflammation until day 28th post arthritis induction. Though presented as a well-sustained treatment, click PEG-Dex-induced inflammation resolution was relatively mild when compared to the short-lived free Dex treatment.
Figure 4.
The right ankle joint diameter of the five animal groups (click PEG-Dex, Dex, click PEG, saline, and healthy; 6 rats/group) during the entire experiment.
Figure 5.
The articular index (AI) scores of the five animal groups (click PEG-Dex, Dex, click PEG, saline, and healthy; 6 rats/group) during the entire experiment.
BMD assessment
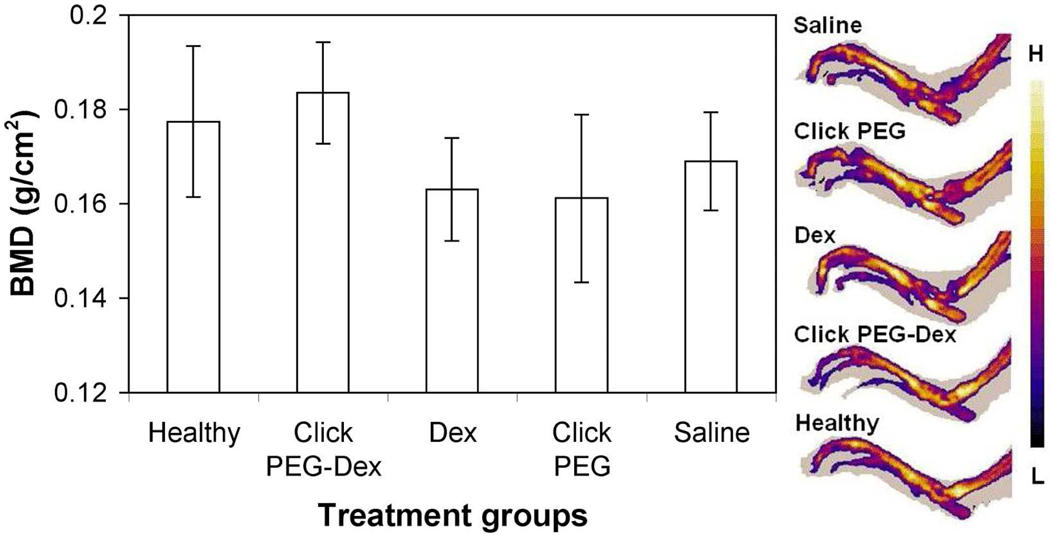
The hind limb bone mineral density (BMD) (from distal tibia to the phalanges) was analyzed with pDEXA. As shown in Figure 6, the saline-treated, click PEG-treated, and free Dex-treated groups were found to have similar level of BMD (BMD ≈ 0.16), which is lower than the BMD values shared by the healthy control and click PEG-Dex treatment groups (BMD ≈ 0.18). The pDEXA images suggested that the major bone destruction of arthritic joints was at the distal tibia.
Figure 6.
The endpoint bone mineral density (BMD) and representative pDEXA images of the right ankle joints of the five animal groups (click PEG-Dex, Dex, click PEG, saline and healthy). One-way ANOVA analysis, p < 0.01.
Histological evaluation of ankle joints
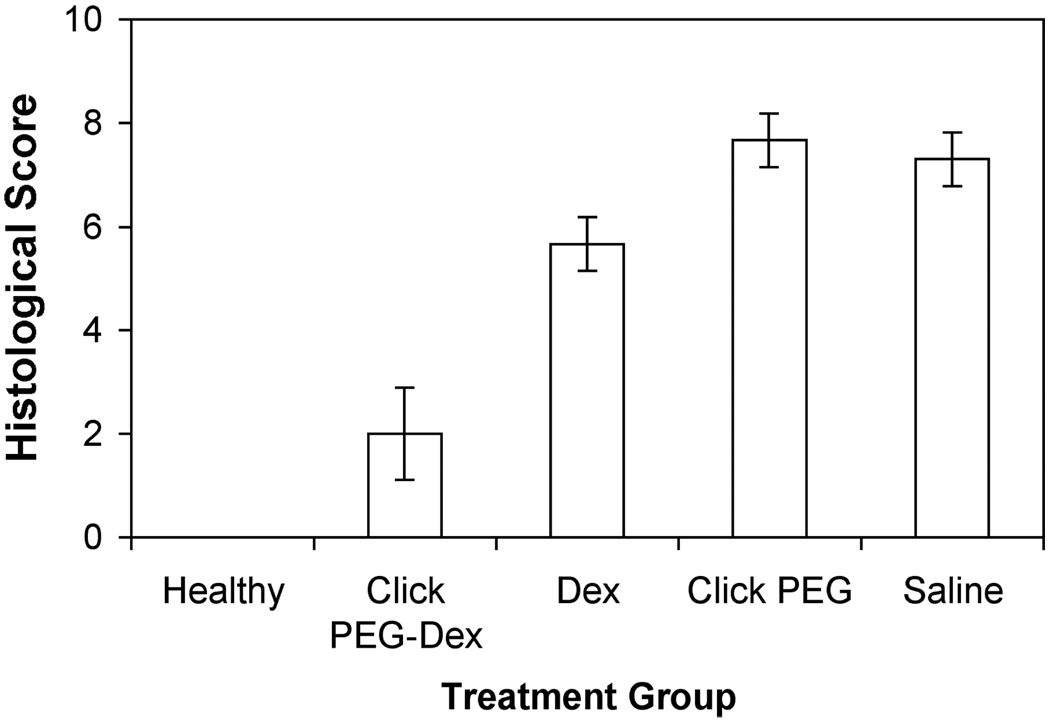
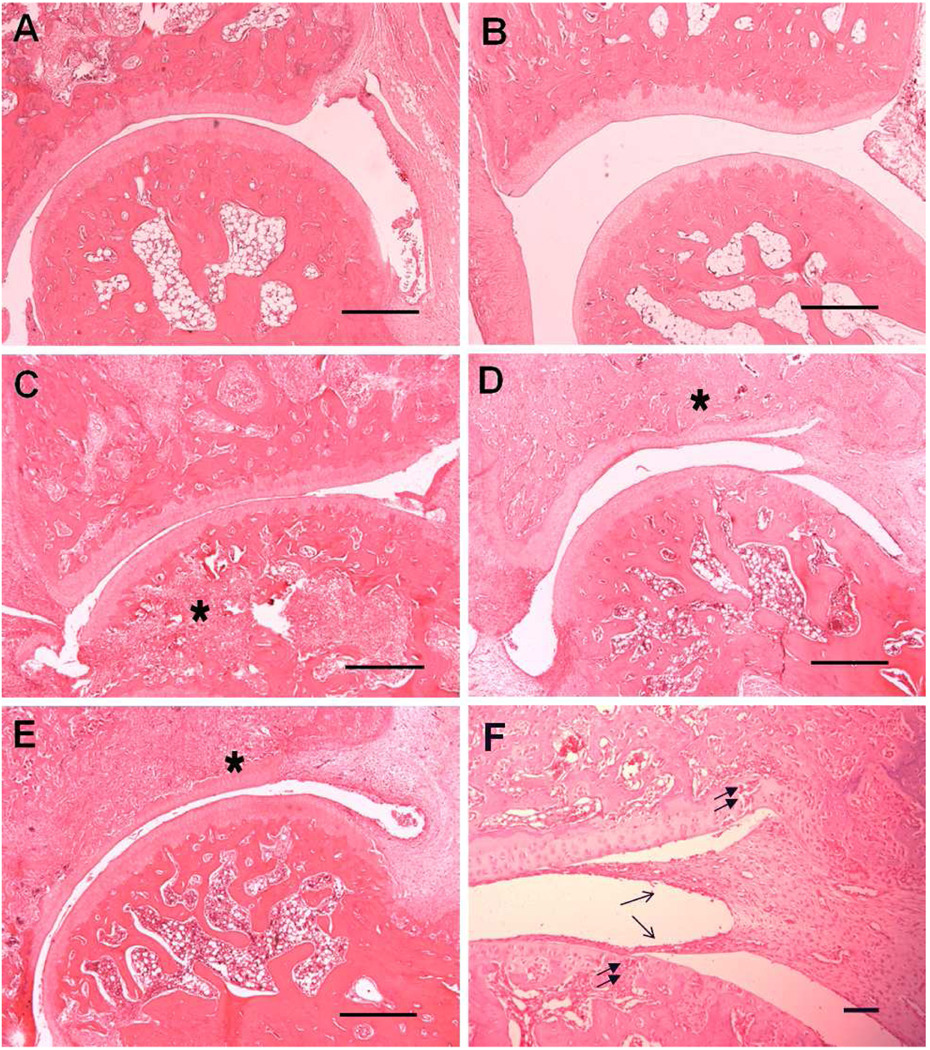
To confirm the therapeutic benefits of click PEG-Dex treatment of AA rats, the hind limbs of different treatment groups were processed and evaluated histologically (Figures 7 and 8). The saline-treated and click PEG-treated AA rats exhibited massive inflammatory cell infiltration with extensive pannus formation, severe cartilage destruction and irregular joint space, with each parameter of histological grading showed the highest scores among all of the treatment groups (> 7.0). The free Dex-treated group showed moderate synovial cell lining and villous hyperplasia. Inflammatory cell infiltration into periarticular soft tissue was observed. Bone destruction was present with total histological score of 6.0 for this group. AA rats treated with click PEG-Dex demonstrated the best anti-inflammatory effect with profound bone and cartilage protection (histological score 2.0). Inflammatory cell infiltration, pannus formation, and bone and cartilage destruction were absent in most cases, which is similar to the healthy control group. Mild synovial and villous hyperplasia was observed in this group, suggesting the presence of minor inflammation.
Figure 7.
Histological evaluation of the ankle joint sections from the five animal groups (click PEG-Dex, Dex, click PEG, saline and healthy). One-way ANOVA analysis, p < 0.0001.
Figure 8.
Photomicrographs of representative histological sections of the ankle joint from four treatment groups and a healthy control group. A. Click PEG-Dex; B. Healthy; C. Free Dex; D. Saline; E. Click PEG. F. Saline, at higher magnification. Finger-like pannus formation (single arrow), bone destruction (asterisk) and cartilage damage (double arrow) are clearly evident in free Dex, click PEG and saline groups. Bar=0.5 mm in A~E, bar = 0.05mm in F.
Discussion
To address the limited functionalities of PEG (an otherwise excellent biocompatible synthetic polymer for drug delivery and nanomedicine), we have developed a novel linear multifunctional PEG (click PEG) by connecting acetylene-modified short PEGs with functionalized low MW diazide using facile Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition (a click reaction).12 We envisioned that the new delivery platform may have the following unique features when compared to the more established systems, such as PGA and HPMA copolymers. (1) No vinyl monomers need to be synthesized, which would greatly simplify the conjugation of many “difficult” drugs to the polymeric carrier; (2) The intramolecular aggregation or micellization can be minimized as each drug molecule is separated by well-defined short PEG segments of the similar length; (3) Different drugs, imaging probes and targeting moieties can be conveniently included (under mild condition) on the same polymer chain to allow true combination therapies and theranostic developments; (4) Biodegradability of the copolymer conjugates can be easily developed by modification of the two PEG termini with biodegradable structures (e.g. peptides, -S-S-, ester, etc.) prior to capping with acetylene; (5) Facile incorporation of short polymer segments of different physicochemical properties [e.g. poly(acrylic acid), poly(N-isopropylacrylamide), etc.] to click PEG could yield functional polymer conjugates with sensitivities to environmental pH, temperature, light, etc.; (6) The drug loading can be freely adjusted by using short PEG with different length; (7) Thread-on of modified alpha-cyclodextrin may further enhance the functionality of the click PEG. To exploit these advantages, a proof-of-concept in vivo study must be done to validate the potential of click PEG as a polymeric drug carrier. For this purpose, we conjugated a potent anti-inflammatory drug dexamethsone (Dex) to click PEG and evaluated its diagnostic and therapeutic potential in the adjuvant-induced arthritis (AA) rat model, which we have accumulated a lot of experience using HPMA copolymer conjugates.23–25
From the synthesis aspect, click PEG-Dex conjugate in the present study is relatively easier to obtain when compared to the synthesis of HPMA copolymer-Dex conjugate (P-Dex).25 As a technical challenge, Dex-containing vinyl monomer was hard to synthesis. The reactions have to be closely monitored with adequate amount free radical inhibitor to prevent premature polymerization of the monomer or its intermediates. For click PEG-Dex, however, the synthesis of Dex-containing diazide is rather robust and autopolymerization of the monomer is impossible. Click PEG-Dex was synthesized by simply mixing Dex-containing monomer, PEG monomer, and other reactants together and reacting at room temperature using Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition. The result shows that the conversion of Dex monomer into click PEG is nearly 100 % corresponding to 135 mg Dex in 1 gram of click PEG-Dex. Different with conventional linear PEG and multi-arm PEG (1.7 wt.% and 3.7 wt.% separately), the drug loading in our click PEG is very high (more than 10 wt.%). If needed, the drug loading can be further increased by copolymerizing Dex monomer with the acetylene PEG of shorter length. For drug or imaging agent’s conjugation to the polymeric delivery systems, polymer analogous reaction is a facile method being used frequently. To understand if it is also applicable to the newly developed click PEG conjugate, we incorporated IRDye 800CW into click PEG-Dex using this method. The reaction proceeded smoothly, with high imaging probe conversion ratio (81%). Due to the polycondensation reaction nature of the click PEG synthesis, the PDI of all the resulting polymeric conjugates have been rather wide. With optimization of the solvent system, the incorporation of stabilizing agent for Cu(I) and the use of a chain terminator (mPEG), we managed to keep the PDI of our conjugates at about 3. Ongoing experiments are also being conducted to evaluate if copolymerization of PEG diazide and low MW functional diacetylene monomer would avoid the “self-catalyzing” effect26 and further improve the PDI. Theoretically, however, the PDI of click PEG conjugates will remain higher than 2 due to the polycondensation mechanism. Such polymer conjugate may be acceptable in clinical evaluation as a cyclodextrin-PEG conjugate with PDI higher than 2 has been in phase II clinical trail for cancer treatment.10,27 Ultrafiltration, dialysis and column fractionation are practical methods that could be used to efficiently narrow down the PDI value to 1.5–1.6 of HPMA copolymer conjugates that have been evaluated in clinical trials.28
Compared to other polymeric delivery systems, a unique feature associated with click PEG development is the use of copper as catalyst. Although copper is a trace element required for physiological function, repeat administration of copper-contaminated therapeutic agents in vivo could lead to serious toxic consequences.29 To overcome this limitation, we used LH-20 column fractionation and EDTA disodium salt-assisted dialysis to remove the Cu catalyst. After lyophilization, the ICP-MS analysis of the final product showed that these procedures are sufficient to remove 99.95% of the Cu catalyst added to the reaction. Due to the concern of Cu toxicity and potential impact of variable Cu catalyst potency during the polymerization process, copper-free click reactions will be considered in the future development of click PEG delivery system.30,31
From the biological activity aspect, click PEG system works similarly to the HPMA copolymer conjugates.23–25 As shown in Figure 3, click PEG-Dex labeled with IRDye 800CW demonstrated strong arthrotropism in the AA model we tested, which agrees with our previous finding of the joint-targeting capability of DOTA/Gd3+-labeled HPMA copolymer using MRI imaging technique.23 Different from the MRI study, however, the click PEG was loaded with Dex. Therefore, the anti-inflammatory effect of activated Dex on the redistribution and clearance of the copolymer conjugate from the ankle joints was included in this present imaging study. It is very interesting to observe the sustained but gradual decline of click PEG-Dex’s signal (IRDye 800CW-labled) in the ankle joints, which coincided with the joint size reduction and may serve as a clinical evidence of the continuous inflammation resolution due to click PEG-Dex treatment (Figures 3–5). This data strongly implicate the utility of click PEG-IRDye 800CW conjugate as a powerful theranostic tool for clinical diagnosis and therapy outcome evaluation. It is important to note that while optical imaging has been largely restricted to preclinical applications in animals due to its tissue penetration limitation, clinical diagnosis and therapy evaluation for rheumatoid arthritic joints with optical imaging may be an exception due to the unique anatomical tissue access the joints provide.
For Dex activation study, click PEG-Dex demonstrated accelerated hydrolysis and release of Dex at pH 5.0 and remained intact at 6.0 and physiological pH. The release rate of Dex from click PEG-Dex (0.5 %/day), however, is relatively slow when compared to the Dex release from HPMA copolymer-Dex conjugate (P-Dex, ~ 1 %/day).24 In both conjugates, Dex was linked to the polymer backbone via the same acid-labile hydrazone bond. It is not clear if the difference in the chemical structure of the polymer backbone is the direct cause of the slower release of Dex from click PEG-Dex or it is due to the different proximate chemical environments of the Dex-containing monomers. Additional experiment are being performed to investigate if introduction of electron withdrawal structures (e.g. aromatic rings) next to the hydroazone would allow further manipulation of the Dex release rate from click PEG-Dex.
When compared to P-Dex, click PEG-Dex seems to possess a relatively mild anti-inflammatory potency than P-Dex. While a similar dose of P-Dex could completely restore ankle size and AI scores of the AA rats within 3 day,24,25 the amelioration of joint inflammation by click PEG-Dex is partial. This may be attributed to the aforementioned delayed activation of click PEG-Dex under acidic pH when compared to the activation rate of P-Dex (1 %/day at pH 5.0). It is also noted that the cell internalization rates of PEG and HPMA copolymer are different, with HPMA copolymer being internalized faster than PEG.32 Therefore, assuming the observed joint-retention of the polymeric drug delivery systems in arthritis joint is due to internalization of the polymers by activated synoviocytes, a slower cell-internalization may translate into a lower conjugate deposition in the arthritic joints, which provide another explanation of click PEG-Dex’ mild anti-inflammation potency.
Albeit less potent than P-Dex in reducing clinical inflammation, click PEG-Dex provides similar bone and cartilage protection to the ankle joints when compared to that of P-Dex.24,25 As evident in Figures 6–8, the BMD of AA rats treated with click PEG-Dex is similar to the healthy, and is significantly better than the free Dex and saline control groups (p < 0.01). The advantage of click PEG-Dex is even more pronounced in the histological evaluation. The histology score of the click PEG-Dex group is much lower than free Dex group and appears similar to the healthy controls.
Conclusion
To summarize, we have successfully provided the first set of in vivo evidence for click PEG as a novel polymeric theranostic platform in the present study. When loaded with potent anti-inflammatory drug dexamethasone (Dex), a single injection of the conjugate led to sustained amelioration of joint inflammation in the AA rats tested. Compared to the more established polymeric drug delivery systems, click PEG has distinct advantages in synthesis, structural design and multifunctional loading capacity. But its activation rate at acidic pH is slower, which may contribute to its mild potency when compared to HPMA copolymer-dexamethasone conjugate (P-Dex). Additional study is underway to further improve the Dex activation rate and the control of PDI of the polymer-drug conjugate. In the meantime, conventional methods such as column fractionation are sufficient to provide a linear multifunctional polymeric carrier with well-defined chemical structure and narrow polydispersity.
Acknowledgment
This work was supported in part by an NIH grant R01 AR053325 (DW). Purchase of the ICP-MS at the University of Nebraska at Omaha was supported by NSF grant 0411164 (FCL and others).
References
- 1.Ringsdorf H. J. Polym. Sci. Symp. 1975;51:135–153. [Google Scholar]
- 2.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 3.Maeda H. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 4.Pasut G, Veronese FM. Adv. Drug Delivery Rev. 2009;61:1177–1188. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK, Rizzo J, Ochoa L, Takimoto CH, Forouzesh B, Schwartz G, Hammond LA, Patnaik A, Kwiatek J, Goetz A, Denis L, McGuire J, Tolcher AW. J. Clin. Oncol. 2003;21:148–157. doi: 10.1200/JCO.2003.03.143. [DOI] [PubMed] [Google Scholar]
- 6.Langer CJ. Clin. Lung Cancer. 2004;6 Suppl 2:S85–S88. doi: 10.3816/clc.2004.s.020. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Rubio B, Sapra P, Wu D, Reddy P, Sai P, Martinez A, Gao Y, Lozanguiez Y, Longley C, Greenberger LM, Horak ID. Bioconjug. Chem. 2008;19:849–859. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 8.Nathan A, Zalipsky S, Ertel SI, Agathos SN, Yarmush ML, Kohn J. Bioconjug. Chem. 1993;4:54–62. doi: 10.1021/bc00019a008. [DOI] [PubMed] [Google Scholar]
- 9.Pechar M, Ulbrich K, Subr V, Seymour LW, Schacht EH. Bioconjug. Chem. 2000;11:131–139. doi: 10.1021/bc990092l. [DOI] [PubMed] [Google Scholar]
- 10.Cheng J, Khin KT, Jensen GS, Liu A, Davis ME. Bioconjug. Chem. 2003;14:1007–1017. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Chen MH, Parmar VS, Samuelson LA, Kumar J, Nicolosi R, Yoganathan S, Watterson AC. J. Am. Chem. Soc. 2004;126:10640–10644. doi: 10.1021/ja039651w. [DOI] [PubMed] [Google Scholar]
- 12.Liu XM, Thakur A, Wang D. Biomacromolecules. 2007;8:2653–2658. doi: 10.1021/bm070430i. [DOI] [PubMed] [Google Scholar]
- 13.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Kolb HC, Sharpless KB. Drug Discovery Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 15.Hein CD, Liu XM, Wang D. Pharm. Res. 2008;25:2216–2230. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronin TH, Faubl H, Hoffman WW, Korst JJ, inventors. 4,034,040. US patent. 1977
- 17.Bader M. J. Chem. Educ. 1980;57:703–706. [Google Scholar]
- 18.Chang YH, Pearson CM, Abe C. Arthritis Rheum. 1980;23:62–71. doi: 10.1002/art.1780230111. [DOI] [PubMed] [Google Scholar]
- 19.van Dijke CF, Peterfy CG, Brasch RC, Lang P, Roberts TP, Shames D, Kneeland JB, Lu Y, Mann JS, Kapila SD, Genant HK. Magn. Reson. Imaging. 1999;17:237–245. doi: 10.1016/s0730-725x(98)00167-2. [DOI] [PubMed] [Google Scholar]
- 20.Levick JR. J Rheumatol. 1990;17:579–582. [PubMed] [Google Scholar]
- 21.Andersson SE, Lexmuller K, Johansson A, Ekstrom GM. J. Rheumatol. 1999;26:2018–2024. [PubMed] [Google Scholar]
- 22.Farr M, Garvey K, Bold AM, Kendall MJ, Bacon PA. Clin. Exp. Rheumatol. 1985;3:99–104. [PubMed] [Google Scholar]
- 23.Wang D, Miller SC, Sima M, Parker D, Buswell H, Goodrich KC, Kopečková P, Kopeček J. Pharm. Res. 2004;21:1741–1749. doi: 10.1023/b:pham.0000045232.18134.e9. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR. Arthritis Res. Ther. 2007;9:R2. doi: 10.1186/ar2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, Wang D. Pharm. Res. 2008;25:2910–2919. doi: 10.1007/s11095-008-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodionov VO, Fokin VV, Finn MG. Angew. Chem. Int. Ed. Engl. 2005;44:2210–2215. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
- 27.Davis ME. Adv. Drug Delivery Rev. 2009;61:1189–1192. doi: 10.1016/j.addr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Duncan R. Adv. Drug Delivery Rev. 2009;61:1131–1148. doi: 10.1016/j.addr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Guo Z. Curr. Med. Chem. 2006;13:525–537. doi: 10.2174/092986706776055742. [DOI] [PubMed] [Google Scholar]
- 30.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc. Natl. Acad. Sci. U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc. Natl. Acad. Sci. U S A. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Pechar M, Li W, Kopečková P, Brömme D, Kopeček J. Biochemistry. 2002;41:8849–8859. doi: 10.1021/bi0257080. [DOI] [PubMed] [Google Scholar]