Abstract
Summary
FK 506 was given for immunosuppression in 14 liver recipients. The drug was used in the first 10 cases because the recipients under conventional immunosuppression had rejection, nephrotoxicity, or both. This salvage therapy was successful in 7 of the 10 attempts. 2 of the 10 patients in the original salvage group as well as 4 new patients underwent fresh orthotopic liver transplantation under FK 506 plus low-dose steroids from the outset. None of these 6 patients had rejection although 1 with preexisting cor pulmonale and coronary atherosclerosis died of a myocardial infarction. In addition, 2 of the 14 liver recipients were given cadaveric kidneys, either from the same donor or from a different donor, and a third was given a pancreas as well as a kidney from the liver donor. There were no rejections of the kidney and pancreas grafts, and serious side-effects were not encountered.
Introduction
Cyclosporin has been an essential factor in the expansion of transplantation services during the past decade.1 However, its nephrotoxicity and other limitations have stimulated a continuing search for alternative agents. We report here the first clinical trials in liver recipients of a new drug that is not related chemically to cyclosporin or to other standard immunosuppressants. FK 506, a macrolide produced by Streptomyces tsukubaensis, was discovered in 1984 in Japan during a search for new immunosuppressive and cancer chemotherapeutic agents. It was shown to be immunosuppressive in vitro by Kino et al2 and in rats by Ochiai et al3 and by numerous others (see ref 4) throughout 1987. A major divergence of opinion quickly surfaced about the potential clinical value of the agent. From our studies of heart, kidney, or liver transplantation in rats,5 dogs,6–8 and subhuman primates,8,9 we judged it worthy of a clinical trial. In contrast, workers at Cambridge University reported unacceptable toxicity including widespread arteritis.10 However, we8 and Ochiai et al11 have shown such vascular lesions to be present in non-immunosuppressed dogs after whole organ transplantation and in dogs treated with other agents including cyclosporin. Arteritis has not been a feature in baboons treated with FK 5069 or in formal toxicology studies.8 When examining its effects in man we did not feel justified in conducting potentially dangerous pharmacokinetic studies in normal volunteers, as is the usual practice in drug development. Instead the agent was given, in the first instance, to patients in desperate plight because their liver grafts were being rejected despite conventional immunosuppression.
Later, it was combined with low doses of steroids as primary antirejection treatment for high-risk recipients of livers, kidneys, and in one case a pancreas.
Methods
Liver Graft Rescue
The original hepatic diagnoses in these 10 patients were postnecrotic cirrhosis (6) and sclerosing cholangitis (4). Conventional treatment was deemed to have failed if there was acute or chronic graft rejection despite immunosuppression with cyclosporin and steroids with or without adjuvant antilymphocyte globulin (polyclonal or monoclonal) and/or azathioprine. In most patients, abnormal renal function had imposed limits on the amount of cyclosporin that could be given. Patient 3 had undergone a renal tranplantation in December, 1988, but this kidney showed end-stage chronic rejection on biopsy. The hepatic grafts that were undergoing rejection had been in place for 12 days to 6½ years. 4 of the 10 patients had already undergone retransplantation one to four times, and the starting point for the FK 506 study was based on the last graft. 9 of the 10 patients had abnormal tests of liver function and/or injury—jaundice in 7 (total bilirubin 37·6–359 μmol/l) and raised serum aminotransferases and canalicular enzymes (alkaline phosphatase and/or gamma-glutamyl transferase) in 9. Patients 5 and 10 were gravely ill with acute hepatic failure when FK 506 was started and they had already been listed with the national organ procurement network as candidates for emergency retransplantation. The diagnosis and characteristics of rejection were established by liver biopsy in all 10 patients; duct injury and cellular infiltration were prominent features, vascular injury and fibrosis being less common.
An additional patient who had undergone hepatic and renal transplantation in November, 1988, for polycystic liver and kidney disease was entered into the FK 506 rescue protocol on April 8, 1989, but was withdrawn a few days later when the diagnosis was revised to acute B virus hepatitis.
Fresh Liver Grafts
Patients 2 and 9, whose livers could not be salvaged with KF 506, received new grafts (their sixth and second liver, respectively) under FK 506 and low-dose steroids. This sixth liver replacement in patient 2 was performed under emergency conditions when occlusion of the arterial conduit to graft number 5 led to septic hepatic gangrene of the hilum, gram-negative bacteraemia, and septic shock. Patient 9 was jaundiced but in good condition at the time of retransplantation.
In addition, four primary liver transplantations were done under FK 506 and low-dose steroids. The first patient (no 11) was a hepatitis B virus carrier with postnecrotic cirrhosis, juvenile diabetes mellitus, and renal failure. He had such severe autonomic neuropathy that he could no longer eat. The next 3 patients had postnecrotic cirrhosis and pulmonary hypertension (case 12), cirrhosis plus multifocal nodular hyperplasia (case 13), and postnecrotic cirrhosis (case 14). Patient 12 had cor pulmonale, pulmonary artery pressure 80/40 mm Hg, systemic blood pressure 120/60 mm Hg.
Fresh Kidney Transplantation
Liver recipients 1 and 3 had renal failure at the time of the switch from cyclosporin to FK 506. Twenty-seven and forty-seven days later, respectively, cadaver kidneys were transplanted under FK 506 and low-dose steroids. In addition patient 11, who was anuric, received a kidney from his liver and pancreas donor.
Pancreatico-duodeno-jejunal Transplantation
In patient 11 the whole pancreas was placed intraperitoneally and arterialised with a Carrell patch on the right common iliac artery. An iliac vein graft from the cadaver donor was used to extend the portal vein for an outflow anastomosis to the anterior surface of the recipient right common iliac vein.12 The duodenum was closed in two layers and a 35 cm segment of cadaveric jejunum was brought as a Brooke jejunostomy to the right lower quadrant for collection of pancreatic and exocrine secretions in an ileostomy bag.13 The pancreas graft, which was transplanted last of the three organs from the common donor, had a cold ischaemic period of 24 h compared with 12 h for the liver and 18 h for the kidney.
Immunosuppression and Monitoring for Liver Salvage
In the early cases we examined the effect of FK 506 on the pharmacokinetics of cyclosporin and observed increases in cyclosporin blood levels with associated nephrotoxicity. The results discouraged us from trying combined therapy.
All patients received an initial intravenous bolus of 0·15 mg/kg FK 506 over 1 h. Plasma concentrations, measured by the two-step monoclonal enzyme immunoassay technique of Tamura et al,14 were highest (9·0–20·0 ng/ml) at the end of the 1 h infusion and fell exponentially thereafter (to 0·7–3·8 ng/ml after 12 h). When intravenous therapy was continued, 0·075 mg/kg was given every 12 h until oral doses were started: at the lower intravenous doses, peaks ranged from 3·3 to 20 ng/l with 12 h troughs of 0·5 to 5·0 ng/ml.
Oral doses of FK 506 were 0·15 mg/kg every 12 h or 24 h. The absorption curves with oral administration showed only minor increases from trough to peak levels in stable patients, the maximum rise being observed after about 2 h. 12 h or 24 h plasma trough levels that were seemingly therapeutic ranged from 0·2 to as high as 6 or 7 ng/ml without a detectable association with toxicity. The oral doses were adjusted downward to provide trough levels of less than 3ng/ml.
Prednisone doses were left temporarily at their previous level if these were 20 mg/day or less, were reduced to 20 mg if they had been higher, and were subsequently reduced further.
Immunosuppression and Monitoring for Fresh Transplantations
A first intravenous dose of 0·15 mg/kg FK 506 was given after revascularisation of the graft, followed by 0·075 mg/kg twice a day; we proceeded to oral therapy in the same way as with graft salvage. 6 patients received 200 mg methylprednisolone at the time of operation, the dose being reduced in 40 mg decrements to 20 mg/day. In 2 others the previous maintenance dose of intravenous or oral methylpredinosolone was left unchanged at 20 mg/day.
Results
Tables I–III summarise patient details and results.
TABLE I.
LIVER RECIPIENTS GIVEN RESCUE TREATMENT: FOLLOW-UP TO SEPT 14, 1989
| Patient | Age/sex | Date of last liver graft | Date FK 506 started (1989) | Fate of liver graft | Serum creatinine (μmol/l) |
|
|---|---|---|---|---|---|---|
| Before | After | |||||
| 1 | 28/F | June 29, ’88★ | Feb 28 | Salvaged | 362 | 115 |
| 2 | 38/M | Jan 1, ’89★ | March 25 | Replaced July 2† | 195 | 195 |
| 3 | 30/F | Nov 18, ’87★ | April 4 | Salvaged | 477 | Dialysis |
| 4 | 42/M | April 30, ’89 | Feb 6 | Salvaged | 124 | 141 |
| 5 | 38/F | Feb 12, 82 | June 29 | Salvaged | 239 | 283 |
| 6 | 47/M | June 15, ’86 | Jan 7 | Salvaged | 248 | 239 |
| 7 | 18/F | July 18, ’86★ | July 8 | Salvaged | 141 | 203 |
| 8 | 63/F | May 8, ’89 | July 30 | Replacement attempted Sept 5; died† | 150 | 141 |
| 9 | 37/M | July 10, ’89 | Feb 8 | Replaced Aug28† | 88 | 106 |
| 10 | 45/F | July 29, ’89 | Aug 10 | Salvaged | 133 | 97 |
Third (case 1), fifth (case 2), and second (case 3 and 7) liver grafts.
Graft replaced because of arterial thrombosis and septic hepatic gangrene (case 2) or because the small bileducts had already been destroyed (cases 8 and 9).
Liver Graft Rescue
Grafts salvaged
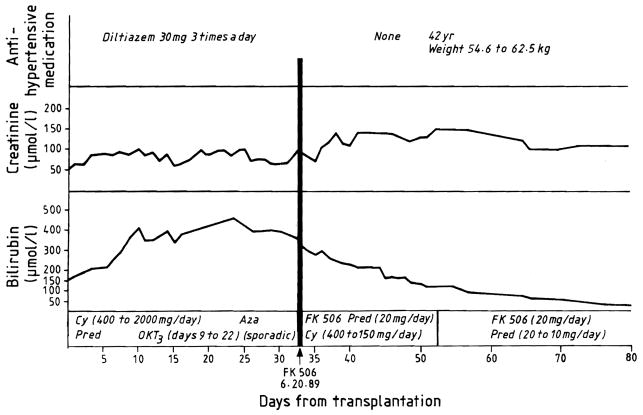
All 10 patients had a sustained improvement in at least some liver functions. 7 of the 10 liver grafts are still in place and providing good to normal function after FK 506 treatment for one to more than six months. Only 1 of these 7 patients (case 10) has jaundice; her bilirubin has fallen from 301·0 to 73·5 μmol/l. The improvements in liver function were very prompt (fig 1). Objective evidence of benefit was obvious by the time the first liver biopsy specimens were obtained after the start of therapy (fig 2). Table II records all biopsy data. The classic findings of liver rejection were ameliorated or eliminated. The ability to halt the duct injury was particularly striking in those patients whose duct injury was not already irreparable.
Fig 1. Use of FK for salvage in patient 4, whose liver biopsy appearances are shown in fig 2.
Cy = cyclosporin; aza = azathioprine; pred = prednisolone.
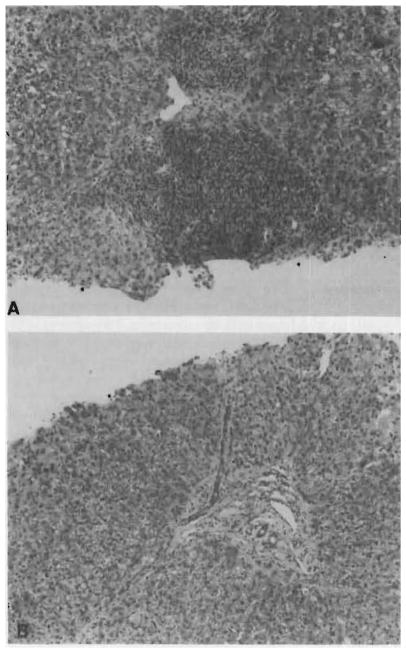
Fig 2. Liver biopsy specimens.
A, before FK 506. Note prominent portal inflammation. Liver damage persisted despite treatment with OKT3. B, three weeks after initiation of the FK 506, infiltrate has disappeared and repair of previous damage is evident.
TABLE II.
LIVER HISTOLOGY BEFORE AND AFTER IN SALVAGE GROUP
| Patient | Day | Portal infiltrate | Duct damage | Duct loss | Fibrosis | Function |
|---|---|---|---|---|---|---|
| 1 | Pre-FK | 1+ | 1+ | 0 | 0 | Good |
| 15 | 0 | 0 | 0 | 0 | ||
| 69 | 0 | 0 | 0 | 0 | ||
| 2 | Pre-FK | 1+ | 1+ | 0 | 0 | Graft replaced |
| 12 | + | 0 | 0 | 0 | ||
| 34 | 0 | 0 | 0 | 0 | ||
| 94 | 0 | 0 | 0 | 0 | ||
| 3 | Pre-FK | 2+ | 1+ | 0 | 0 | Good |
| 14 | 0 | 0 | 0 | 0 | ||
| 31 | 0 | 0 | 0 | 0 | ||
| 141★ | 1+ | + | 0 | 0 | ||
| 4 | Pre-FK | 3+ | 2+ | 0 | 2+ | Good |
| 14 | + | + | 0 | 2 | ||
| 51 | 0 | 0 | + | + | ||
| 5 | Pre-FK | 1+ | 3+ | 0 | 0 | Good |
| 12 | + | 2+ | 0 | + | ||
| 19 | 0 | 2+ | 0 | + | ||
| 66 | 0 | 2+ | + | + | ||
| 6 | Pre-FK | 1+ | + | + | 0 | Good |
| 18 | 0 | + | 0 | 0 | ||
| 32 | 0 | 0 | 0 | 0 | ||
| 7 | Pre-FK | 2 | 2+ | + | + | Good |
| 16 | 1 | 1+ | + | + | ||
| 8 | Pre-FK | 2+ | 2+ | + | + | Graft replaced |
| 7 | 1 | 2+ | 1+ | + | ||
| 37 | + | 2+ | 2+ | + | ||
| 9 | Pre-FK | 3+ | 2+ | 0 | + | Graft replaced |
| 20 | 1 | 3+ | 2+ | 1 | ||
| 27 | + | 3+ | 3+ | 1 | ||
| 10 | Pre-FK | 2+ | 2+ | 0 | + | Improved; bilirubin now 73·5 μmol/l |
| 13 | + | + | 0 | 1+ | ||
| 27 | 0 | 0 | 0 | 1+ | ||
Patient placed back on cyclosporin after 120 days because of shortage of intravenous FK 506. This last biopsy was 21 days later. Patient has persistent low-grade lobular hepatitis.
Graft losses
Patient 9 had severe rejection with prominent duct injury and patient 8 had no identifiable ducts in her graft before beginning treatment. Although there was diminution of other histopathological evidence of rejection (table II), the ducts were not restored nor was jaundice relieved in those 2 patients. Retransplantation was successful in patient 9. Patient 8 died on the operating table during attempted retransplantation. Patient 1 had improvement of liver function and histopathological findings for almost three months until development of regional hepatic gangrene. The liver was replaced. The conduit used to rearterialise his fifth graft proved to have a tight stricture which was thought to have completely obstructed the blood flow.
Renal function
The previously placed renal homograft in patient 3 went on to complete failure. Renal function in the other 9 patients remained almost unchanged (table I). Several patients with preexisting hyperkalaemia remained hyperkalaemic and were successfully treated with fludrocortisone acetate. Patient 1, in whom hyperkalaemia was regarded as life-threatening, was treated by renal transplantation. 7 of the patients had arterial hypertension requiring medication before FK 506 was started: in 5 all antihypertensive drugs could be stopped, in 1 medication was reduced from two drugs to one, and in the 7th the regimen was unchanged.
Fresh Grafts
Livers
The two livers inserted at retransplantation (patients 2 and 9) functioned well from the outset with no clinical evidence of rejection in the 11 and 2½ weeks of follow-up. Postoperative biopsy showed no evidence of rejection. Patient 1 already had poor renal function and this did not become worse, and patient 9 had normal renal function throughout (tables I and III). The four primary liver grafts (patients 11–14) also performed flawlessly (fig 3). Biopsy specimens after 1 and 2 weeks showed no evidence of rejection. The recipients had no evidence of nephrotoxicity (table III). Patient 12, who had severe pulmonary hypertension, recovered smoothly until day 14 when she complained of abdominal pain and tenderness. Laparotomy showed no abnormality but she had a fatal myocardial infarction under anaesthesia. At necropsy there was extensive atherosclerosis of the aorta, and the left anterior descending coronary artery was occluded by plaque. There were typical fmdings of cor pulmonale including biventricular hypertrophy. The liver graft was normal except for a light mononuclear infiltrate in some of the portal areas, with scattered microabscesses and microgranulomas.
TABLE III.
FRESH TRANSPLANTATIONS
| Patient | Date started (1989) | Date of fresh transplantation | Organ transplanted | Graft function (Sept 10) | Total bilirubin (μmol/l) | Creatinine (μmol/l) |
|---|---|---|---|---|---|---|
| 1 | Feb 28 | March 27 | Kidney | Normal | 5·1 | 106 |
| 2 | March 25 | July 2 | 6th liver | Normal | 10·2 | 195 |
| 3 | April 4 | May 21 | 2nd kidney | Removed June 20 | 12·0 | — |
| 9 | Aug 2 | Aug 28 | 2nd liver | Normal | 44·5 | 106 |
| 11 | Aug 17 | Aug 17 | Liver | Normal | 20·5 | 97 |
| . . | Kidney | Normal | ||||
| . . | Pancreas | Normal | ||||
| 12 | Aug 18 | Aug 18 | Liver | Died Sept 1★ | 18·8 | 106 |
| 13 | Aug 24 | Aug 24 | Liver | Normal | 6·8 | 61 |
| 14 | Sept 3 | Sept 3 | Liver | Normal | 32·4 | 124 |
Myocardial infarction.
Fig 3. Use of FK 506 with steroids in a diabetic recipient of liver, kidney, and pancreas.
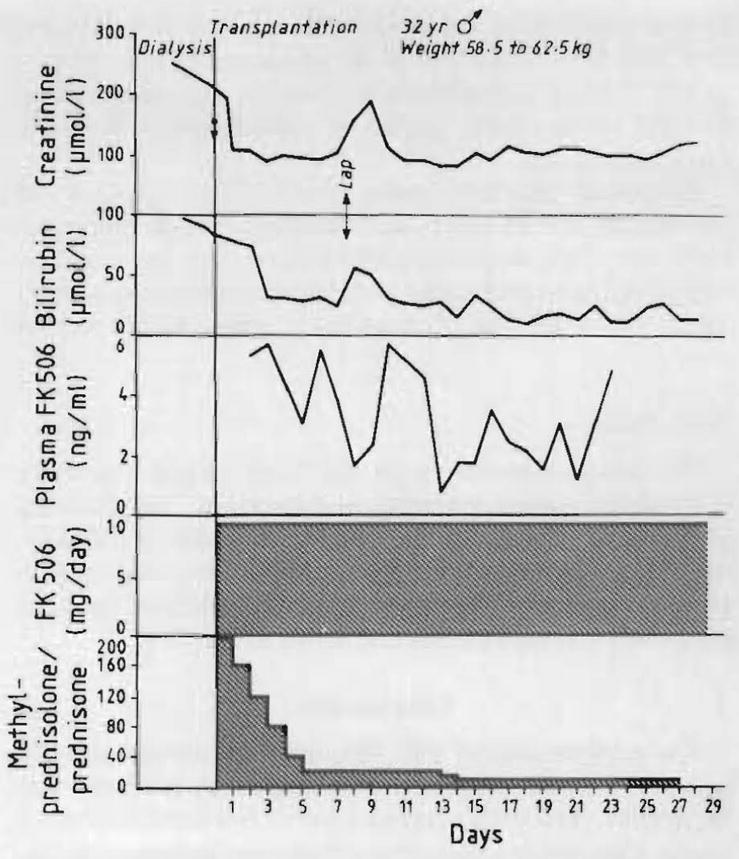
Lap = laparotomy for a liver laceration and severe haemorrhage caused by too low placement of a chest catheter.
Kidneys
The cadaveric renal transplants in liver patients 1 and 11 have functioned well for five and a half months and one month, respectively. Current serum creatinines are 106 and 97 μmol/l (table III). These patients are normotensive without antihypertensive therapy. The cadaveric kidney transplanted to liver patient 3 passed through a 2-week period of acute tubular necrosis before diuresis began. 30 days postoperatively, disruption of the end-to-side renal arterial anastomosis to the external iliac artery (because of a candida infection) necessitated graft nephrectomy and ligation of the iliac artery. The removed renal graft showed acute fibrin thrombi in some of the polar arterioles. These were attributed to seeding from the suture line infection. There was no evidence of cellular rejection or drug toxicity. Under immunosuppression with cyclosporin, azathioprine, and prednisone, the recipient of the other kidney from the same donor likewise had disruption of the renal arterial suture line on the 11 th postoperative day and died. In our programme this complication arises in about 1 in 1500 kidney transplantations, and in this instance was thought to have been caused by contamination from the shared donor.
Pancreatico-duodeno-jejunal graft
The patient is insulin-free one month postoperatively. Biopsy specimens were taken from the jejunostomy bud on postoperative days twelve and twenty-one and were histopathologically normal except for small foci of granulation tissue in the lamina propria.
Side-effects
The first intravenous doses of FK 506 caused headaches in 4 patients, nausea, vomiting, or anorexia in 7, and flushing or burning of the feet in 3 (table IV). The oral preparation was well tolerated, causing transient nausea or vomiting in only 2 patients. There were no correlation between symptoms and plasma concentrations of FK 506.
TABLE IV.
COMPLAINTS DURING TREATMENT WITH FK506
Transient.
Discussion
The performance of FK 506, both for salvage and for primary immunosuppression in high-risk patients, was impressive. The drug was remarkably free from unwanted effects. One disadvantage of our study was the impossibility of ruling out nephrotoxicity, but the weight of evidence is that the drug is not nephrotoxic in therapeutic doses and that it does not cause hypertension. With other agents such as azathioprine and cyclosporin, a dose ceiling is imposed by specific toxicity (bone marrow depression and nephrotoxicity, respectively). A comparable toxicological “litmus paper” to guide dose standardisation has not been identified with FK 506 and the therapeutic window may well be wide. With oral therapy, the only complaints or findings were nausea and vomiting and these were transient.
In many of the first investigations with FK 506, the possibility was entertained of combining small doses with agents such as cyclosporin, with which it is synergistic in vitro15,16 and in animals.5,6,8,11 Our clinical experience so far has not encouraged such combination therapy. The FK 506 may have increased the toxicity of cyclosporin, possibly by raising its blood concentration. FK 506 was so potent and free of side-effects that the simplest expedient was to use it alone.
Acknowledgments
This work was supported by research grants from the Veterans Administration and project grant no DK 29961 from the National Institutes of Health.
References
- 1.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;ii:1033–36. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 2.Kino T, Hatanaka H, Miyata S, et al. FK506, a novel immunosuppressant isolated from streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 1987;40:1256–65. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 3.Ochiai T, Nakajima K, Nagata M, et al. Effect of a new immunosuppressive agent, FK506, on heterotopic allotransplantation in the rat. Transplant Proc. 1987;19:1284–86. [PubMed] [Google Scholar]
- 4.Starzl TE, Makowka L, Todo S, editors. Transplant Proc. suppl 6. 19 . 1987. FK-506: a potential breakthrough in immunosuppression; pp. 3–104. [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Todo S, Lee PH, et al. Heterotopic heart transplantation in the rat receiving FK-506 alone or with cyclosporine. Transplant Proc. 1987;19:71–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Todo S, Dernetris AJ, Ueda Y, et al. Canine kidney transplantation with FK -506 alone or in combination with cyclosporine and steroids. Transplant Proc. 1987;19 (suppl 6):57–61. [PMC free article] [PubMed] [Google Scholar]
- 7.Todo S, Podesta L, ChapChap P, et al. Orthotopic liver transplantation in dogs receiving FK-506. Transplant Proc. 1987;19 (suppl 6):64–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Ueda Y, Demetris JA, et al. Immunosuppression of canine, monkey, and baboon allografts by FK 506 with special reference to synergism with other drugs, and to tolerance induction. Surgery. 1988;104:239–49. [PMC free article] [PubMed] [Google Scholar]
- 9.Todo S, Demetris A, Ueda Y, et al. Renal transplantation in baboons under FK 506. Surgery. 1989;106:444–51. [PubMed] [Google Scholar]
- 10.Thiru S, Collier DStJ, Calne R. Pathologic studies in canine and baboon renal allograft recipients immunosuppressed with FK506. Transplant Proc. 1987;19(suppl 6):98–99. [PubMed] [Google Scholar]
- 11.Ochiai T, Sakamoto K, Gunji Y, et al. Effects of combination treatment with FK506 and cyclosporine on survival time and vascular changes in renal-allograft-recipient dogs. Transplantation. 1989;48:193–97. doi: 10.1097/00007890-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Tzakis A. Pancreatico-duodenal transplantation with enteric exocrine drainage. In: Groth CG, editor. Pancreatic transplantation. Philadelphia: WB Saunders; 1988. pp. 113–30. [Google Scholar]
- 13.Starzl TE, Iwatsuki S, Shaw BW, et al. Pancreaticoduodenal transplantation in humans. Surg Gynecol Obstet. 1984;159:265–72. [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Kobayashi M, Hasimoto K, et al. A highly sensitive method to assay FK506 levels in plasma. Transplant Proc. 1987;19 (suppl 6):23–29. [PubMed] [Google Scholar]