Sir,—Bone-marrow-derived dendritic cells are powerful antigen-presenting cells, which are 100-times more effective than macrophages in activating T lymphocytes in mixed-lymphocyte culture1 and in intact animals.2 These cells are pivotal in the generation of the T-cell repertoire, including provision of the appropriate ligand for negative selection of potentially autoreactive T lymphocytes.3 We now describe the tissue distribution of donor dendritic accessory cells in fully xenogenic (F344 rat → B10 mouse) radiation bone-marrow chimeras that were permanently tolerant to donor-specific xenoantigens, yet fully reactive to third-party mouse and rat lymphoid cells.4,5
Laboratory animals were typed for chimerism by flow cytometry, and 3 per group were killed for a complete tissue survey at 1,2,3,4,6, and 8 weeks and at 8 months after reconstitution. Immunohistochemical staining for rat-derived dendritic cells (class II bright) was with the monoclonal antibody L-21-6 directed at the invariant chain of the rat class II molecule without cross-reactivity in mouse cells (provided by Dr Yuichi Iwaki, University of Pittsburgh).
In the first 2–3 weeks after reconstitution, the structure of the class-II-positive rat cells migrating into the tissue of the mice appeared more rounded than stellate and were thought to be an immature, less differentiated form of dendritic cell. By the end of one month and invariably thereafter, the rat dendritic cells were found in all of the mouse tissues examined: thymic cortex and medulla (figure, top), spleen, other lymphoid structures, liver, fat, peripheral nerve roots, brain (cerebellum, cerebrum), trachea, oesophagus, pancreas, lungs, bronchi, heart, and skin (figure, bottom). Their morphology, cell density, and distribution in the chimeras resembled that of normal rat tissues examined as controls.
Figure 1. Mouse thymus (top) and skin (bottom) six months after rat-to-mouse bone-marrow transplantation.
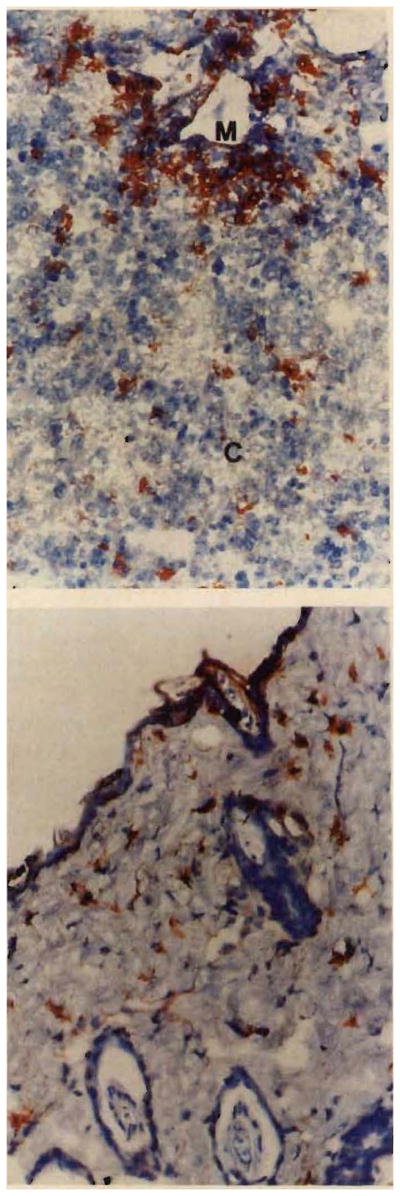
Top, staining by anti-MHC class II (L-21-6). Rust-coloured rat-derived cells are seen in both thymic cortex (C) and medulla (M) (× 300).
Bottom, L-21-6-positive-dendritic cells are shown in skin from the same animal (× 300).
The extent as well as the normal pattern of “homing” of the rat bone-marrow-derived dendritic cells in a xenogeneic mouse environment, suggests the presence of a highly conserved receptor-ligand mechanism. We are currently investigating the possible role of these ubiquitous cells in the induction and maintenance of specific transplantation tolerance across both allogeneic and xenogeneic histocompatibility barriers.
Acknowledgments
This work was partly supported by an American College of Surgeons Fellowship Award, 1990–92; Juvenile Diabetes Foundation Grant 1911433 and 1911421; NIH A130615; and Shannon Award DK43901 and NIH DK29961.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–97. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. III. Functional properties in vivo. J Exp Med. 1974;139:1431–45. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JL, Sharrow JO, Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990;171:935–40. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ildstad ST, Vacchio MS, Markus PM, Wren SM, Hodes RJ. Cross-species transplantation tolerance: rat bone-marrow-derived cells can contribute to the ligand for negative selection of mouse TCR-V6 in chimeras tolerant to xenogeneic antigens (mouse + rat-mouse) J Exp Med. 1992;175:147–55. doi: 10.1084/jem.175.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Y, Ricordi C, Tzakis A, Rilo HL, Carroll PB, Starzl TE. Long-term survival of donor-specific pancreatic islet xenografts in fully xenogeneic chimeras (WF-B10 mouse) Transplantation. 1992;53:277–83. doi: 10.1097/00007890-199202010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]


