Abstract
Techniques to detect and quantify DNA and RNA molecules in biological samples have played a central role in genomics research1–3. Over the past decade, several techniques have been developed to improve detection performance and reduce the cost of genetic analysis4–10. In particular, dramatic advances in label-free methods have been reported11–17. Yet, detection of DNA molecules at concentrations below femtomolar level requires amplified detection schemes1,8. Here we report a unique nanomechanical response of hybridized DNA and RNA molecules that serves as an intrinsic molecular label. Nanomechanical measurements on a microarray surface exhibit excellent background signal rejection that allows direct detection and counting of hybridized molecules. The digital response of the sensor provides a large dynamic range that is critical for gene expression profiling. We have measured differential expressions of miRNAs in tumor samples, which has been shown to help discriminate tissue origins of metastatic tumors18. 200 picograms of total RNA is found to be sufficient for this analysis. In addition, the limit of detection in pure samples is found to be 1 attomolar. These results suggest that nanomechanical readout of microarrays promises attomolar level sensitivity and large dynamic range for the analysis of gene expression, while eliminating biochemical manipulations, amplification, and labeling.
In a microarray experiment, single stranded probe DNA molecules with known sequences are immobilized on a surface at predefined locations. Hybridization of probe and target molecules is routinely measured by fluorescence, but it can also be detected by exploiting various nanoscale phenomena19 including nanomechanics. For example; changes in surface stress12 and added mass of target molecules13, electrical forces20, topographical changes in self assembled bar-coded nucleic acid probe tiles21, and hydration induced surface tension15 have been used for hybridization detection.
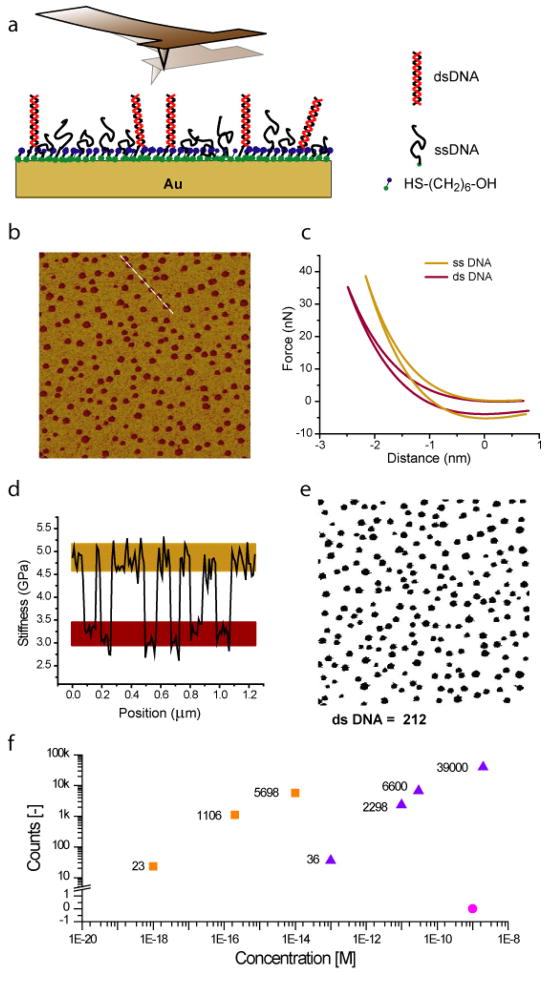
Our nanomechanical detection scheme relies on the changes in stiffness of DNA molecules immobilized on a surface before and after hybridization. We have used a recently developed atomic force microscope (AFM) technique22,23 to investigate the stiffness of single and double stranded DNA molecules, which is illustrated in Fig. 1a. The resulting stiffness maps, such as in Fig. 1b, show hybridized DNA molecules as dark brown spots. This is verified by a series of experiments with non-complementary targets and complementary targets with varying concentrations.
Figure 1.
Nanomechanical detection of DNA hybridization. (a) Schematic of the experiment. After hybridization, the surface of the array spot is scanned with the AFM to generate the stiffness map shown in (b). Scan size is 3 μm. Hybridized molecules are measured to be less stiff and appear as dark brown spots. (c) Stiffness at each pixel in (b) is calculated from force-distance curves. Effective stiffness values across the dashed line in (b) are plotted in (d). A computer generates a binary image (e) to count the hybridized molecules from stiffness values. The numbers of hybridized molecules found in 10 μm wide scan areas are plotted in (f) against varying target concentrations and for two probe immobilization areas (squares: 50 μm, triangles: 5 mm). Hybridization against a non-matching sequence is given by the pink circle.
When the sharp tip presses against the molecules on the surface, interaction forces increase at a rate proportional to local stiffness. The two force curves plotted in Fig. 1c show the surface is stiffer on the single stranded region. The responses of molecules against external forces are not only determined by their intrinsic mechanical properties, but also on their conformations on the surface. The higher stiffness observed on single stranded DNA is largely due to the influence of the gold substrate. Single stranded molecules either lay down on gold, or get squeezed under the influence of the tip without much resistance so that the tip is essentially feeling the stiffness of the substrate. This hypothesis is supported by the height measurements that show hybridized molecules to appear taller than single stranded regions (See supplementary information). Increased height of hybridized molecules also result in spot sizes larger than the size of DNA molecules due to tip convolution. The changes in the height of hybridized molecules have also been used to detect hybridization25,26; however these demonstrations required nano- to micromolar level target concentrations. In addition, height measurements provide limited discrimination against the roughness of the substrate surface; therefore atomically flat substrates had to be used. Measurement of a local material property like stiffness, however, provides the specificity necessary to discriminate single hybridized molecules on the surface.
Figure 1d shows calculated stiffness values over the dashed line in Fig. 1b. The stiffness values over single and double stranded molecules are well separated from each other. In addition, the values measured on different spots are sufficiently uniform to provide a clear signature for hybridized molecules. A computer can analyze the stiffness map with this signature and generate a binary image to count hybridized target molecules as seen Fig. 1e. RNA/DNA hybrids also exhibit the same mechanical signature as hybridized DNA (see supplementary information).
We have carried out hybridization experiments with varying target DNA concentrations and with two different areas of probe immobilization. The results plotted in Fig. 1f shows the dependency of surface density of hybridized molecules on target concentration and spot size; lower target concentrations and larger immobilization areas give less hybridized molecules at a given scan area. Concentration levels from 1 nM down to 1 aM are detectible. In addition, no hybridized molecules are found with the non-matching sequence. These numbers represent 3 to 8 orders of magnitude enhancements in the detection limit and detection range over the previously reported direct detection methods11–16,20,21.
We have further investigated the performance of the presented technique under the complex background of biological samples by analyzing total RNAs extracted from tumor tissues. Detection and quantification of RNA requires the use of large amounts of starting material or additional enzymatic steps involving reverse transcription and amplification. Furthermore, small regulatory RNA molecules like microRNAs27 are not directly compatible with conventional amplification schemes28. Therefore, the presented approach can simplify the analysis of microRNA content in biological samples to a great extent.
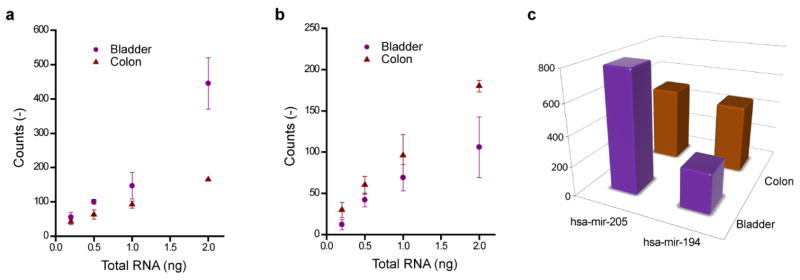
We have measured differential expressions of two microRNAs, hsa-mir-205 and hsa-mir-194, in colon and bladder tumors by analyzing total RNAs extracted from tissue samples. The extraction process includes disruption of cells and purification of RNAs. Material isolated this way is routinely used for gene expression profiling. Rosenfeld et al., have shown that microRNAs exhibit expression patterns that can be used to predict tissue origins of metastatic tumors and the particular microRNAs we investigate here discriminate tumors of gastrointestinal (colon) and nongastrointestinal-epithelial (bladder) origin18. The numbers of RNA/DNA hybrids are plotted in Fig. 2a for hsa-mir-205 and in Fig. 2b for hsa-mir-194. (Numerical values from each measurement are provided in the supplementary section). The chart in Fig. 2c compares average expression levels normalized to 10 picograms. The data shows that hsa-mir-205 is expressed more in bladder tumor sample and hsa-mir-194 is expressed more in colon tumor sample. The observed inversion in differential expression in these microRNAs is in agreement with the results of Rosenfeld et al. and we further verified this result by conventional microarray analysis of the tumor samples (NCode™ Human miRNA Microarray V3). The average numbers of microRNAs are also in broad agreement with the numbers derived from quantitative PCR experiments28. Note that the inversion in the differential expressions of the two microRNAs was detectible down to 0.2 ng of total RNA. This represents a dramatic reduction in the amount of total RNA compared to conventional microarray experiments, which typically require amounts in excess of 1 μg. These arrays are generally printed on glass slides and use spot sizes around 100 × 100 μm. Their unprinted areas are blocked to eliminate unspecific adsorption.
Figure 2.
Direct detection and quantification of miRNA expression in cancer tissues. Total RNAs isolated from bladder and colon tumor samples are analyzed for hsa-mir-205 and hsa-mir-194 content. The average numbers of target molecules found at varying amounts of total RNA are plotted for (a) hsa-mir-205 and (b) hsa-mir-194 (10 μm scan, 200 μm immobilization). Standard deviations are calculated whenever two or more measurements are available from experiments carried out in triplicates and they are given as error bars. (c) Comparison of expression levels in each sample estimated by normalizing the data to 10 pg, averaging, and scaling to entire area of immobilization.
An important advantage of the presented nanomechanical detection technique is its compatibility with multiplexed analysis in microarray platforms. Multiplexed analysis demands generation of stiffness maps and counting hybridized molecules for each array spot. Our instrument is not optimized for scan speed, yet it provides the throughput, reliability, and robustness for multiplexed analysis. As a demonstration, figure 3 shows a 25 μm wide stiffness map of hybridized molecules at 6 nm pixel size that is generated in 2 hours. In principle, a scan area of this size is sufficient to read out an array with a thousand spots by sampling 800 nm wide regions in each array spot. This level of multiplexing is sufficient for whole genome microRNA expression profiling and for many clinical applications. Throughput can be further enhanced by combining our high spatial resolution readout scheme with “DNA nanoarrays” formed by dip-pen lithography29 and Supramolecular NanoStamping30. Multiplexed analysis can also create more complex situations due to probes with secondary structures, mismatches, potential unusual base pairing, and free termini. We have analyzed several probe sequences that are representative of these cases and validated the utility of our stiffness based hybridization detection principle under these situations (See supplementary information).
Figure 3.
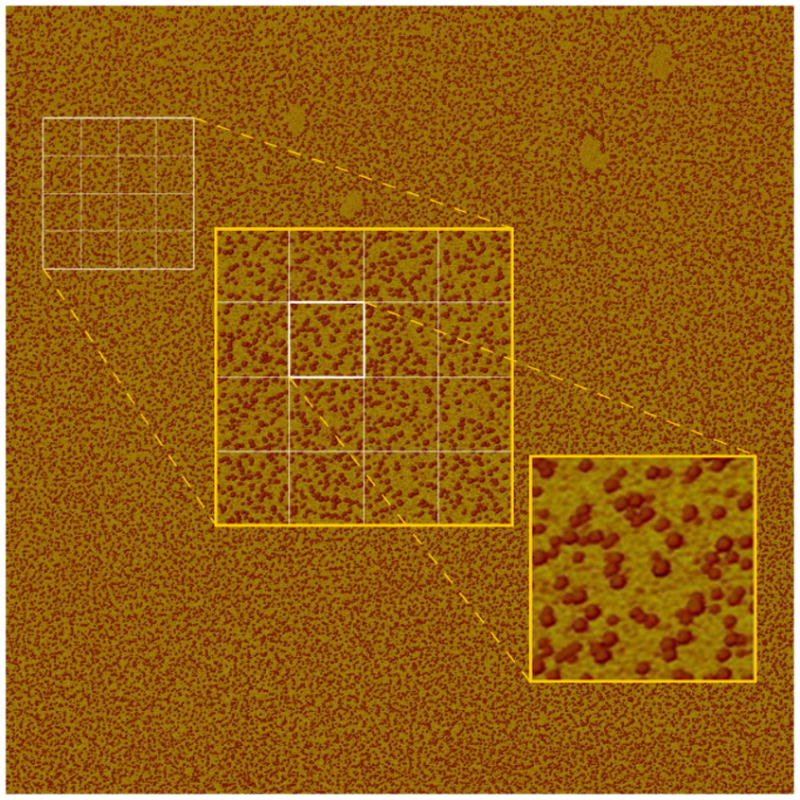
High throughput and robust nanomechanical readout for multiplexed detection. 25 × 25 μm stiffness map at 6 nm pixel resolution shows individual hybridized molecules. Scan time is 2hrs. Target DNA concentration is 32 pM and immobilization area is 5 × 5 mm. The number of dsDNA is ~ 41250. The rectangular grid illustrates the possibility to share the scan area among different microarray spots. Insets are showing two levels of zoom into the stiffness data (not a rescan). The original image is given separately in supplementary information.
In summary, we observed that the nanomechanical signatures of hybridized DNA and RNA molecules provide an excellent background rejection that leads to digital readout with attomolar level detection limit in micro and nanoarray platforms, while eliminating external labeling and enzymatic manipulations of the target molecules. Furthermore, the analysis of microRNA contents in two tumor tissues illustrates the potential of this technique in medical applications. This opens the prospects of working with extremely small amounts of genetic material in combination with substantial simplifications and cost reduction in genetic analysis.
Methods Summary
Sample Preparation
The probe chips are prepared by immobilizing single-stranded and thiolated DNA molecules on a gold coated silicon substrate. Immobilization areas are defined lithographically using a standard lift-off procedure. After passivating the surface with mercaptohexanol24, the chips are immersed in a solution containing target DNA or total RNA (no labels or any other chemical modifications) for overnight hybridization, washed with a buffer solution, and dried under a stream of nitrogen. Each sample is visually inspected and the samples that appear contaminated or scratched are not included in the AFM analysis.
AFM analysis
Samples are imaged in tapping-mode AFM under ambient conditions. T-shaped cantilevers are used to generate time-varying tip-sample forces. Effective elastic modulus is derived from these waveforms using our previously described mathematical procedure22. This procedure provides quantitative values on samples with flat surfaces. In the case of the tip interacting with DNA molecules, the calculated values provide a measure of stiffness, but they do not correspond to true elastic modulus values.
Methods
Substrate preparation
5 × 5 mm gold coated silicon chips were prepared by sputter coating (Au = 20 nm and Ti = 10 nm, ‘AJA sputtering system’, CNS, Harvard University) on Si wafer (3 inch, silicon quest international). Diamond scribe was used for cutting wafers into 5 × 5 mm chips. Substrates with 50 × 50 and 200 × 200 μm gold spots were defined by photolithography followed by standard lift-off procedure.
Immobilization of ssDNA on gold surface
Gold chips were pretreated with UV-ozone procleaner (Bioforce Nanosciences) for ~ 30 min and washed with nanopure water (deionized and filtered with inorganic membrane filter with 20 nm pores). Thiolated ssDNA (single stranded DNA, concentration 1μM) were immobilized on the freshly prepared gold surface through thiol-gold covalent bonding. High salt buffer was used for immobilization of ssDNA (1M NaCl, 50 mM phosphate buffer pH 7.2). Immobilization was done at room temperature under constant mixing, incubation time ~ 15 hrs. The immobilized ssDNA chip surfaces were passivated by washing with water and subsequent incubation with mercaptohexanol (MCH, 1mM in nanopure water) for ~1 hr.
Synthetic DNA samples
All oligos were purchased from MWG Biotech Inc. (Huntsville, AL 35805 USA) or from Integrated DNA Technologies (Coralville, IO 52241 USA) used within a month. Thiolated probes were reduced according to manufacturers’ suggested protocols.
DNA Hybridization
Prior to hybridization, ssDNA immobilized chips were thoroughly washed with nanopure water. DNA/DNA hybridization reactions were carried out at room temperature under constant mixing in 1x SSC buffer (150 mM NaCl, 15 mM sodium citrate, pH 7.0). Volume of the hybridizing solution was 200 uL for 5 × 5 mm gold chips and 1 mL for 50 μm gold spots. Finally, hybridized chips were washed with low salt buffer (25 mM NaCl, 10 mM Tris HCl pH 7.4) and gently dried by weak stream of nitrogen.
Total RNA Hybridization
Total RNAs extracted from human bladder and colon tumors were purchased from Stratagene and kept at −70 °C until used. Samples were diluted with hybridization buffer (3x SSPE with Tween 20, pH 7.4 (Teknova)) to their final total RNA concentrations in Fig. 2. Hybridizations were carried out on a shaker for ~ 16 hrs at 42 °C. All microcentrifuge tubes, tweezers and gold coated silicon chips were cleaned with RNaseZap (Ambion) before immobilization of DNA molecules, and washed extensively with DNase, RNase free water (MP Biomedicals).
AFM measurement conditions
Tapping-mode AFM was used for the measurements under ambient conditions. Torsional harmonic cantilevers (HarmoniX Probes, Veeco Instruments) were used (resonance frequency f ~ 60 KHz) to generate time-varying tip-sample forces. Driving amplitude was adjusted so that peak tapping forces during the scan are approximately 30–50 nN (peak tapping forces are different on single and double stranded molecules). Set point amplitude is set to ~ 60 nm.
Calculation of elastic modulus
The numerical procedure to reconstruct tip-sample force waveform from the raw deflection signals involve averaging vibration waveforms over several consecutive oscillation cycles to reduce noise, correcting the effect of the torsional frequency response, and eliminating the cross talk from large vertical signals, and fitting the tip-sample force distance curves to a contact mechanics model. Details of these steps and data acquisition hardware are described in Ref. 22. Calculations are carried out in Labview. Calibration of the time varying force waveform (Volts to Newtons conversion) is performed according to Sahin, O. (Rev. Sci. Instrum. 78, 103707 (2007)). Effective elastic moduli of single and double stranded DNA in Fig. 1 are calculated for a tip radius of 7 nm. Subsequent images are calibrated by using the single stranded background in Fig.1 as a reference.
Supplementary Material
Acknowledgments
This research is supported by the Rowland Junior Fellows Program. H. H. J. P. was supported in part by NIH HG000205. We thank Ronald W. Davis for helpful discussions.
Footnotes
Author Contributions S.H. performed the experiments, prepared gold substrates and developed experimental protocols, H.H.J.P. and O.S. contributed to the experiments, SH and H.H.J.P designed and performed surface chemistry, O.S. designed the cantilevers and wrote the stiffness calculation program, H. H. J. P. and O.S. designed the biological assay, O.S. directed the research and wrote the paper, all authors discussed the results and commented on the manuscript.
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare competing financial interests. A patent application has been filed by Stanford University. Correspondence and requests for materials should be addressed to O. S. (sahin@rowland.harvard.edu)
Supplementary information is linked to the online version of the paper at www.nature.com/nature
References
- 1.Higuchi R, Dollinger G, Walsh PS, Griffith R. Simultaneous amplification and detection of specific DNA sequences. Nature Biotech. 1992;10:413–417. doi: 10.1038/nbt0492-413. [DOI] [PubMed] [Google Scholar]
- 2.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405:827–836. doi: 10.1038/35015701. [DOI] [PubMed] [Google Scholar]
- 4.Taton TA, Mirkin CA, Letsinger RL. Scanometric DNA array detection with nanoparticle probes. Science. 2000;289:1757–1760. doi: 10.1126/science.289.5485.1757. [DOI] [PubMed] [Google Scholar]
- 5.Fritz J, Cooper EB, Gaudet S, Sorger PK, Manalis SR. Electronic detection of DNA by its intrinsic molecular charge. Proc Natl Acad Sci USA. 2002;99:14142–14146. doi: 10.1073/pnas.232276699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao YC, Jin R, Mirkin CA. Nanoparticles with Raman Spectroscopic Fingerprints for DNA and RNA Detection. Science. 2002;297:1536–1540. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, Taton TA, Mirkin CA. Array-based electrical detection of DNA with nanoparticle probes. Science. 2002;295:1503–1506. doi: 10.1126/science.1067003. [DOI] [PubMed] [Google Scholar]
- 8.Nam JM, Stoeva SI, Mirkin CA. Bio-Bar-Code-Based DNA Detection with PCR-like Sensitivity. J Am Chem Soc. 2004;126:5932–5933. doi: 10.1021/ja049384+. [DOI] [PubMed] [Google Scholar]
- 9.Fang SP, Lee HJ, Wark AW, Corn RM. Attomole microarray detection of MicroRNAs by nanoparticle-amplified SPR imaging measurements of surface polyadenylation reactions. J Am Chem Soc. 2006;128:14044–14046. doi: 10.1021/ja065223p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiss GK, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nature Biotech. 2008;26:317 – 325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 11.Hahm J-i, Lieber CM. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2004;4:51–54. [Google Scholar]
- 12.Fritz J, et al. Translating biomolecular recognition into nanomechanics. Science. 2000;288:316–318. doi: 10.1126/science.288.5464.316. [DOI] [PubMed] [Google Scholar]
- 13.Ilic B, et al. Enumeration of DNA molecules bound to a nanomechanical oscillator. Nano Lett. 2005;5:925–929. doi: 10.1021/nl050456k. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, et al. Rapid and label-free nanomechanical detection of biomarker transcripts in human RNA. Nature Nanotech. 2006;1:214–220. doi: 10.1038/nnano.2006.134. [DOI] [PubMed] [Google Scholar]
- 15.Mertens J, et al. Label-free detection of DNA hybridization based on hydration-induced tension in nucleic acid films. Nature Nanotech. 2008;3:301. doi: 10.1038/nnano.2008.91. [DOI] [PubMed] [Google Scholar]
- 16.Clack NG, Salaita K, Groves JT. Electrostatic readout of DNA microarrays with charged microspheres. Nature Biotech. 2008;26:825–830. doi: 10.1038/nbt1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burg TP, et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature. 2007;446:1066–1069. doi: 10.1038/nature05741. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld N, et al. MicroRNAs accurately identify cancer tissue origin. Nature Biotech. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 19.Cheng MMC, et al. Nanotechnologies for biomolecular detection and medical diagnostics. Curr Opin Chem Biol. 2006;10:11–19. doi: 10.1016/j.cbpa.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Sinensky AK, Belcher AM. Label-free and high-resolution protein/DNA nanoarray analysis using Kelvin probe force microscopy. Nature Nanotech. 2007;2:653 –659. doi: 10.1038/nnano.2007.293. [DOI] [PubMed] [Google Scholar]
- 21.Ke YG, Lindsay S, Chang Y, Liu Y, Yan H. Self-assembled water-soluble nucleic acid probe tiles for label-free RNA hybridization assays. Science. 2008;319:180–183. doi: 10.1126/science.1150082. [DOI] [PubMed] [Google Scholar]
- 22.Sahin O, Su C, Magonov S, Quate CF, Solgaard O. An atomic force microscope tip designed to measure time-varying nanomechanical forces. Nature Nanotech. 2007;2:507. doi: 10.1038/nnano.2007.226. [DOI] [PubMed] [Google Scholar]
- 23.Sahin O, Erina N. High-resolution and large dynamic range nanomechanical mapping in tapping-mode atomic force microscopy. Nanotechnology. 2008;445:445717. doi: 10.1088/0957-4484/19/44/445717. [DOI] [PubMed] [Google Scholar]
- 24.Herne TM, Tarlov MJ. Characterization of DNA probes immobilized on gold surfaces. J Am Chem Soc. 1997;119:8916 –8920. [Google Scholar]
- 25.Zhou DJ, Sinniah K, Abell C, Rayment T. Label-free detection of DNA hybridization at the nanoscale: A highly sensitive and selective approach using atomic-force microscopy. Angew Chem Int Edit. 2003;42:4934–4937. doi: 10.1002/anie.200352178. [DOI] [PubMed] [Google Scholar]
- 26.Mirmomtaz E, et al. Quantitative Study of the Effect of Coverage on the Hybridization Efficiency of Surface-Bound DNA Nanostructures. Nano Lett. 2008;8:4134–4139. doi: 10.1021/nl802722k. [DOI] [PubMed] [Google Scholar]
- 27.He L, Hannon GJ. Micrornas: Small RNAs with a big role in gene regulation. Nature Rev Genetics. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 28.Chen CF, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demers LM, et al. Direct patterning of modified oligonucleotides on metals and insulators by dip-pen nanolithography. Science. 2002;296:1836–1838. doi: 10.1126/science.1071480. [DOI] [PubMed] [Google Scholar]
- 30.Yu AA, et al. Supramolecular nanostamping: Using DNA as movable type. Nano Lett. 2005;5:1061–1064. doi: 10.1021/nl050495w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.