Abstract
Thrombocytopenia, fever, and acute renal failure are characteristic features of nephropathia epidemica, the predominant hantavirus infection in Europe. However, clinical presentation and blood cell counts may point to other disorders, such as a hematologic disease, particularly when impairment of renal function is not evident. This differential diagnosis often results in further extensive and unnecessary testing. We describe 3 patients with hantavirus infection with no renal failure, in whom a hematologic disorder was initially suspected. Serologic testing of hantavirus finally unraveled the mystery, and outcome of the patients was excellent. It is conceivable that similar cases often remain undiagnosed. Thus, testing for hantavirus should always be considered in cases of thrombocytopenia and fever of unknown origin, especially in areas endemic for the infection.
CRP = C-reactive protein; IUD = intrauterine device; NE = nephropathia epidemica; PUUV = Puumala virus
Thrombocytopenia is a common observation in hospitalized patients and is associated with a variety of diseases. Low platelet counts are most likely the result of decreased platelet production or increased platelet destruction. Rarely, pseudothrombocytopenia, distributional thrombocytopenia, or dilutional thrombocytopenia need to be considered. Clinical features such as petechiae, purpura, nose bleeding, or bruising may be the first sign of a low platelet count. Identifying the underlying cause is pivotal for appropriate diagnostic and therapeutic management strategies.1
Decreased platelet production may be seen in various circumstances: after viral infections or administration of drugs, in the course of a malignant hematologic disease, after chemotherapy or radiation of the bone marrow, as the result of direct alcohol toxicity or vitamin B12 and/or folic acid deficiency. Similarly, increased platelet destruction is seen in a number of immunologic conditions, like idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, systemic lupus erythematosus, and the antiphospholipid or HELLP (hemolytic anemia, elevated liver enzymes, low platelet count) syndrome. Drug-induced thrombocytopenia, in particular heparin-induced thrombocytopenia, always must be considered. Of note, various drugs and some viral infections may affect both platelet production and destruction. Sepsis is the prevailing cause of thrombocytopenia in the severely ill or intensive care unit patient.
In several pathologic conditions, thrombocytopenia and fever are often comorbid conditions,2 and low platelet counts may point to the severity of the underlying disorders. Typical viral diseases associated with thrombocytopenia are infections with cytomegalovirus, H5N1, and human immunodeficiency virus.3-5 Hantavirus infection is a less-known but important differential diagnosis in thrombocytopenia and fever. Although hantavirus infections are usually associated with impaired renal function, we describe 3 patients with the disease who presented without elevated serum creatinine levels.
CASE REPORTS
Case 1
A 30-year-old woman was admitted to a hospital elsewhere for persistent fever. She reported night sweats, weight loss, and fever (temperature, up to 40°C) despite taking oral antibiotics (clarithromycin) and paracetamol for 5 days. Blood tests showed bicytopenia (leukocytes, 2.7 × 109/L; platelets, 27 × 109/L) and elevated levels of lactate dehydrogenase (544 U/L), and C-reactive protein (CRP; 100 mg/L). Findings on electrocardiography, chest radiography, and echocardiography were normal. Abdominal ultrasonography revealed mild enlargement of the spleen. Gynecological examination revealed an intrauterine device (IUD) but was otherwise unremarkable. Treatment was begun with isotonic fluid and low-dose heparin intravenously, and broad antibiotic coverage with imipenem/cilastin therapy was provided for 3 days. The IUD, initially considered a possible focus, was removed; however, the fever persisted, and the patient was transferred to the University Hospital Tuebingen for further evaluation by an expert hematologist. The patient's medical history was notable for appendectomy and surgical repair of an inguinal hernia. She reported no coughing, diarrhea, dysuria, hematuria, or recent travel. Her partner had been treated with antibiotics for similar clinical symptoms 4 weeks previously. On admission to our hospital, the patient's blood pressure was 120/80 mm Hg, heart rate was 72 beats/min, and temperature was 39°C. No abnormalities were evident on physical examination. Blood tests (Table) using an autoanalyzer demonstrated the presence of atypical lymphocytes and plasma cells. Urinalysis revealed mild proteinuria and hematuria. Urine and blood cultures were negative as well as serologic testing for a variety of viruses, including human immunodeficiency virus, hepatitis B and C, Epstein Barr virus, cytomegalovirus, influenza, and parainfluenza. Within a few days, the patient's fever resolved and blood test results normalized (Table). Surprisingly, test results for Puumula virus (PUUV) serology obtained later were positive for IgG and IgM, pointing to a PUUV infection as the reason for the clinical symptoms.
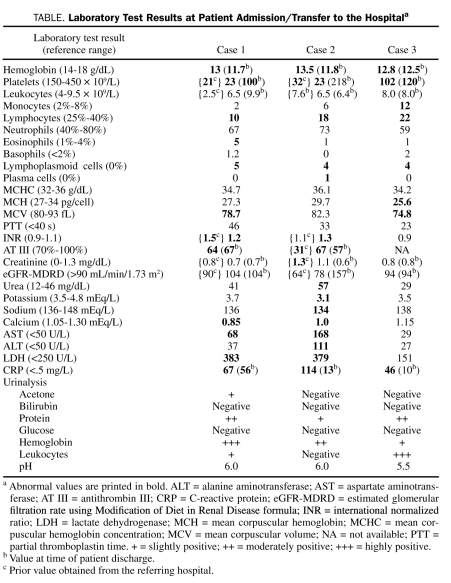
TABLE.
Laboratory Test Results at Patient Admission/Transfer to the Hospitala
Case 2
A 42-year-old man was admitted to a tertiary care hospital for continuous fever (temperature, up to 40°C), watery diarrhea, and cephalgia. The patient reported that he had traveled to Spain 2 weeks earlier and to Egypt 6 months previously. He smoked about 20 cigarettes per day (30-pack-years), did not use illicit drugs, and had no risky sexual contacts. Initial blood tests showed thrombocytopenia, reduced antithrombin III, and increased international normalized ratio levels. Clinically, no focus for infection could be detected. In addition, chest radiography, abdominal ultrasonoghrapy, and spinal tap analysis revealed normal findings, and endocarditis was ruled out by echocardiography. Empirical antibiotic therapy was initiated with metronidazole, ciprofloxacin, and ceftriaxone. Additional blood cell counts using an automated cell counter revealed a substantial number of nonclassifiable white blood cells; because an unspecified leukemia was suspected, the patient was transferred to our hospital for further evaluation by an expert hematologist. Blood test results on admission are shown in the Table. Extensive microbiological, serologic, and hematologic analyses were performed. Computed tomography revealed signs of pulmonary congestion and free abdominal fluid with an increased number of mesenterial and retroperitoneal lymph nodes (Figure, A and B). Differential blood cell count slides viewed by an expert hematologist were inconclusive; bone marrow aspiration showed a normal number of megakaryocytes and discrete hemophagocytosis (Figure, C). Because of persistent undulating fever, antibiotic treatment was changed to meropenem and levofloxacin. Subsequently, the patient's status improved, and blood values normalized. Serologic testing revealed that IgG and IgM were positive for PUUV.
FIGURE.
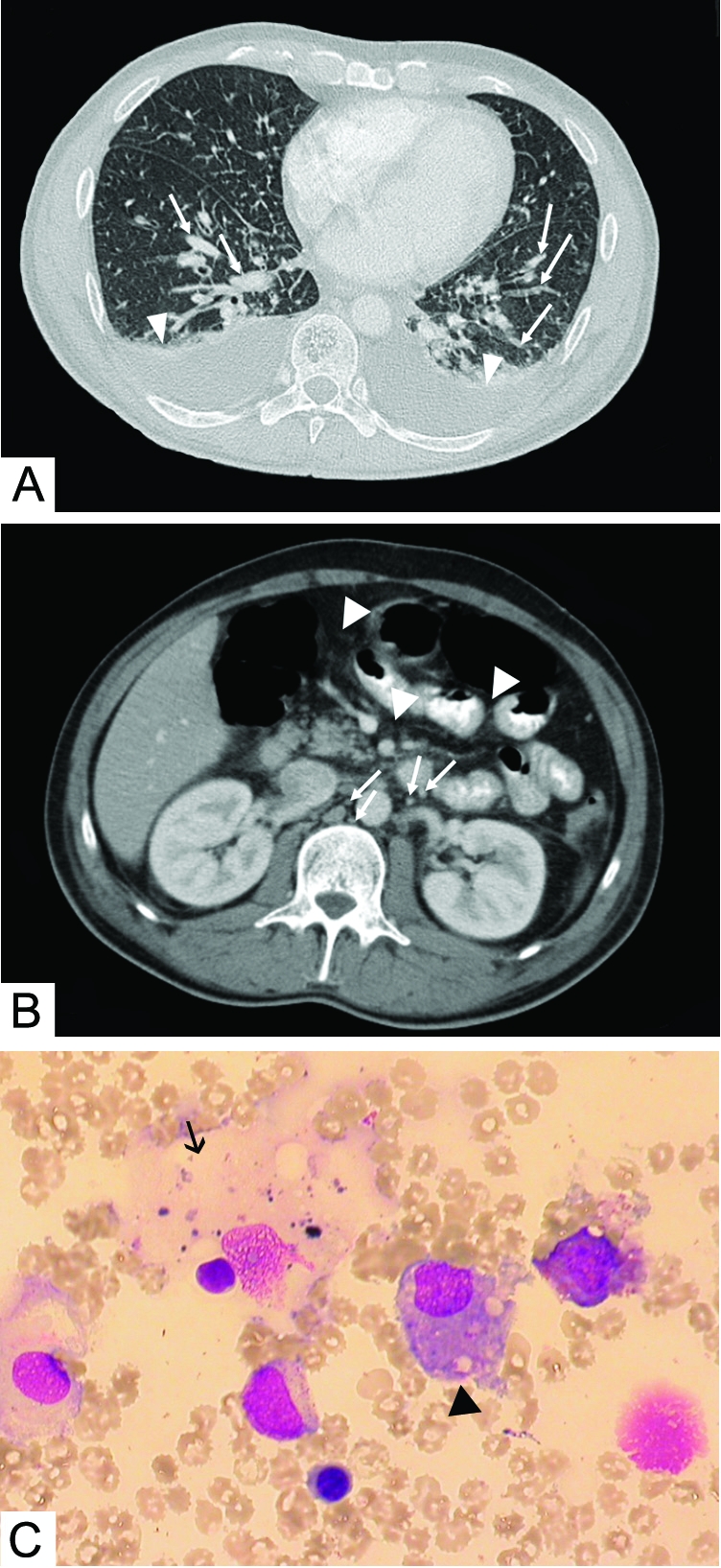
A, Chest computed tomographic scan, revealing signs of pulmonary congestion (arrows) and minor bilateral effusions (arrowheads). B, Abdominal computed tomographic scan, showing multiple enlarged lymph nodes (arrows) and intra-abdominal fluid (arrowheads). C, Representative bone marrow sample, showing normal megakaryocytes (arrow) and discrete hemophagocytosis (arrowhead).
Case 3
A 23-year-old woman presented to the emergency department with fever of about 1 week's duration, associated with chills and side pain. Treatment with ciprofloxacin for 3 days on an outpatient basis had not improved her symptoms. In addition, she experienced spontaneous petechiae on her legs and arms. Her medical history was remarkable for treatment of Hashimoto thyroiditis. Physical examination confirmed some petechial lesions on the extremities. Laboratory analysis revealed thrombocytopenia, slightly elevated CRP value, as well as leukocyturia and proteinuria (Table). Clinical symptoms resolved within 24 hours, and the patient was discharged. Serologic test results for PUUV IgM and IgG obtained a few days later were clearly positive.
DISCUSSION
Hantaviruses are enveloped RNA viruses that cause 2 different human diseases: hemorrhagic fever with renal syndrome and human pulmonary syndrome.6 Although hantaviruses have attracted increasing attention as emerging pathogens in recent years, testing is not often included in routine diagnostic assessment, and they are rarely considered a possible pathogen in routine clinical practice. In Europe, Dobrova virus, Tula virus, and PUUV are the most important species. Rodents are the major reservoir for PUUV, but other free-living and domestic animals have been shown to be possible virus carriers7; transmission is mainly via the aerosol route.8 Puumula virus is reported throughout most of Europe, with parts of Sweden, Southern Germany, and Austria being endemic regions,9,10 and several outbreaks have been associated with an increase in the rodent population due to a milder climate or less snow during winter.11,12 The natural host of the virus is the bank vole (Myodes glareolus), and incubation time is from 2 to 4 weeks after exposure.13
Infections with PUUV cause hemorrhagic fever with renal syndrome,9 also called nephropathia epidemica (NE). Generally a mild disease, NE has a sudden onset and presents with headache, blurred vision, gastrointestinal symptoms, and back pain. The clinical course is usually benign; however, fatal cases have been reported.6,14 Up to 90% of affected patients have mild to severe thrombocytopenia and elevated lactate dehydrogenase levels, and most have a fever.14-16 Impaired renal function is the prominent feature that leads to the differential diagnosis of NE, whereas absence of an elevated serum creatinine level generally prompts the clinician to consider another disease. Pulmonary involvement may occur in NE, with radiologic findings making it difficult to distinguish NE from other diagnoses, such as pneumonia.17 Hantavirus infections affecting the respiratory system result in considerable morbidity and mortality, and a different treatment approach may be necessary. However, serious pulmonary involvement is rare in hantavirus infections associated with the prevailing subtypes of Puumula and Dobrava in Europe, although it is common in infections with various hantaviruses in the New World.7
The diagnosis of acute hantavirus infection is based on detection of virus-specific IgM.9 Management of NE consists of supportive measures and prevention of further damage; specific treatment is not available.18 However, in patients with severe thrombocytopenia, corticosteroids have been shown to be effective to some extent.19 In the current cases, NE was not seriously considered relevant, although all patients lived in an area endemic for PUUV infection. Serologic testing was performed out of curiosity and lack of other explanations in the first 2 patients, and experience from those cases led to further testing in the third patient. Although a thorough investigation may be helpful in establishing a diagnosis of PUUV infection, the transmission path often cannot be identified. In our patients, no prior exposure to rodents was evident. In addition, clinical presentation of patients 1 and 2 as well as normal serum creatinine levels initially resulted in the correct diagnosis being overlooked. In patient 1, transesophageal echocardiography was performed, and an IUD was removed to rule out a potential bacterial cause. In patient 2, suspicious findings on chest radiography as well as pulmonary congestion, intra-abdominal fluid, and an increased number of lymph nodes on chest and abdominal computed tomography led to equivocal interpretations. Additional tests, such as lumbar puncture and bone marrow aspiration, were performed. Both patients were transferred to our hospital with the tentative diagnosis of a malignant hematologic disease based on initial pancytopenia or atypical leukocytes, respectively. However, a differential blood cell count performed at our institution and reviewed by a hematologist did not support that diagnosis. Although the combination of fever, normal leukocyte count, slight lymphopenia, and moderately elevated CRP level clearly pointed to a viral cause, further diagnostic steps were performed to identify the disease and to rule out a more threatening disorder. In patient 3, because clinical symptoms and biochemical findings improved rapidly, despite marked thrombocytopenia and petechial lesions, further invasive tests were not performed. In all 3 patients, extensive biochemical analysis, including serologic testing for various viral infections, was initiated. Of note, antibiotic therapy was initiated in all 3 patients because of persistent fever, although a bacterial pathogen could not be identified. Elevated serum CRP levels were suggestive of an infection, consistent with a bacterial or viral disease. Braun et al16 recently demonstrated that NE is always associated with elevated CRP levels. However, it is unclear whether antibiotic therapy affected outcome in our patients.
Hantavirus infection was not initially considered as a cause because of the assumption that creatinine values always need to be elevated in NE. However, abnormal findings on urinalysis were suggestive of impaired renal function in each patient. Although renal involvement in NE is usually characterized by interstitial nephritis, glomerular dysfunction and morphological changes have been reported.15 In the current cases, it is conceivable that the glomerular filtration rate was more affected at some point in the disease course because discrepancies between the initial and last measurement of serum creatinine levels in patient 1 suggested renal dysfunction at one point in the disease course. Possibly, creatinine levels before admissioin were higher but were overlooked. In addition, reduced antithrombin III levels in patients 1 and 2 may have been a sign of renal involvement. Thus, despite the normal glomerular filtration rate at the time of presentation, it seems reasonable to use the term “nephropathia epidemica” to describe acute PUUV infection in our patients.
Thrombocytopenia associated with viral infections is often a result of the induction of antiplatelet antibodies, with subsequent accelerated platelet clearance and/or suppression of megakaryopoiesis.20 Heparin-induced thrombocytopenia and thrombotic thrombocytopenic purpura were unlikely as differential diagnoses because heparin had not been administered before patient presentation and because hemolysis was absent. Moreover, clinical features and laboratory test results did not suggest an immunologic disorder. Rather, slight lymphopenia and moderately elevated CRP levels pointed to a viral disease. Mechanisms by which hantaviruses cause thrombocytopenia are only partially understood. A common hypothesis for thrombocytopenia in NE includes destruction of platelets based on immunologic mechanisms because corticosteroids may be of benefit.19 Recently, Gavrilovskaya et al21 showed that hantaviruses might bind platelets and recruit them to the endothelial cell surface via a β3 integrin–dependent mechanism, thereby resulting in thrombocytopenia. Of interest, severe thrombocytopenia in PUUV-induced NE may predict a severe course of acute renal failure.22 However, outcome of NE with supportive therapy is generally excellent.15 Thus, it seems essential to discriminate PUUV infection from other potential life-threatening disorders shortly after patient presentation to prevent further extensive and unnecessary diagnostic measures. During the past few years, analytic systems have been developed that enable quick testing for hantavirus infection without the need for sophisticated laboratory equipment. Available point-of-care tests for hantavirus species, with a specificity from 97% to 99% and a sensitivity from 97% to 100%, may be helpful and cost-effective (~$30 per test) if used in selected patients who have persistent fever, hematologic abnormalities, and/or renal failure.13,23,24 However, serologic testing is needed to confirm the diagnosis. Because seroprevalence of hantavirus has been reported to reach 5% in some regions,10 PUUV infection is probably often undiagnosed and symptoms are attributed to other viral diseases. Thus, testing for hantavirus should always be considered in patients with thrombopenic fever of unknown origin, especially those who live in areas endemic for the infection.
CONCLUSION
Hantavirus infection is an important differential diagnosis in thrombocytopenic purpura, even if signs of acute renal failure are absent. However, abnormal findings on urinalysis may be the first clue for this usually benign disease. Because clinical presentation can mimic other potentially life-threatening disorders associated with thrombocytopenia, early point-of-care and serologic testing for hantavirus infection may help to establish the correct diagnosis and prevent further unnecessary and possibly harmful tests.
REFERENCES
- 1.Levi M, Lowenberg EC. Thrombocytopenia in critically ill patients. Semin Thromb Hemost. 2008;34(5):417-424 [DOI] [PubMed] [Google Scholar]
- 2.Wilson JJ, Neame PB, Kelton JG. Infection-induced thrombocytopenia. Semin Thromb Hemost. 1982;8(3):217-233 [DOI] [PubMed] [Google Scholar]
- 3.Wiwanitkit V. Hemostatic disorders in bird flu infection. Blood Coagul Fibrinolysis. 2008;19(1):5-6 [DOI] [PubMed] [Google Scholar]
- 4.Torre D, Pugliese A. Platelets and HIV-1 infection: old and new aspects. Curr HIV Res. 2008;6(5):411-418 [DOI] [PubMed] [Google Scholar]
- 5.Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muranyi W, Bahr U, Zeier M, van der Woude FJ. Hantavirus infection. J Am Soc Nephrol. 2005;16(12):3669-3679 [DOI] [PubMed] [Google Scholar]
- 7.Zeier M, Handermann M, Bahr U, et al. New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention–a review. Virus Genes. 2005;30(2):157-180 [DOI] [PubMed] [Google Scholar]
- 8.Escutenaire S, Pastoret PP. Hantavirus infections. Rev Sci Tech. 2000;19(1):64-78 [DOI] [PubMed] [Google Scholar]
- 9.Vapalahti O, Mustonen J, Lundkvist A, et al. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3(10):653-661 [DOI] [PubMed] [Google Scholar]
- 10.Ulrich R, Meisel H, Schutt M, et al. Prevalence of hantavirus infections in Germany [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004;47(7):661-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettersson L, Boman J, Juto P, Evander M, Ahlm C. Outbreak of Puumala virus infection, Sweden. Emerg Infect Dis. 2008;14(5):808-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann J, Meisel H, Klempa B, et al. Hantavirus outbreak, Germany, 2007. Emerg Infect Dis. 2008;14(5):850-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hujakka H, Koistinen V, Eerikainen P, et al. New immunochromatographic rapid test for diagnosis of acute Puumala virus infection. J Clin Microbiol. 2001;39(6):2146-2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand J Infect Dis. 2000;32(2):125-132 [DOI] [PubMed] [Google Scholar]
- 15.van Ypersele de Strihou C, Mery JP. Hantavirus-related acute interstitial nephritis in western Europe: expansion of a world-wide zoonosis. Q J Med. 1989;73(270):941-950 [PubMed] [Google Scholar]
- 16.Braun N, Haap M, Overkamp D, et al. Characterization and outcome following Puumala virus infection: a retrospective analysis of 75 cases. Nephrol Dial Transplant. doi: 10.1093/ndt/gfq118. [published online ahead of print March 17, 2010] doi:10.1093/ndt/gfq118. [DOI] [PubMed] [Google Scholar]
- 17.Paakkala A, Mustonen J. Radiological findings and their clinical correlations in nephropathia epidemica. Acta Radiol. 2007;48(3):345-350 [DOI] [PubMed] [Google Scholar]
- 18.Stock I. Hantavirus infections [in German]. Med Monatsschr Pharm. 2008;31(4):127-136 [PubMed] [Google Scholar]
- 19.Dunst R, Mettang T, Kuhlmann U. Severe thrombocytopenia and response to corticosteroids in a case of nephropathia epidemica. Am J Kidney Dis. 1998;31(1):116-120 [DOI] [PubMed] [Google Scholar]
- 20.Rand ML, Wright JF. Virus-associated idiopathic thrombocytopenic purpura. Transfus Sci. 1998;19(3):253-259 [DOI] [PubMed] [Google Scholar]
- 21.Gavrilovskaya IN, Gorbunova EE, Mackow ER. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J Virol. 2010;84(9):4832-4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasche FM, Uhel B, Kruger DH, et al. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg Infect Dis. 2004;10(8):1420-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hujakka H, Koistinen V, Eerikainen P, et al. Comparison of a new immunochromatographic rapid test with a commercial EIA for the detection of Puumala virus specific IgM antibodies. J Clin Virol. 2001;23(1-2):79-85 [DOI] [PubMed] [Google Scholar]
- 24.Hujakka H, Koistinen V, Kuronen I, et al. Diagnostic rapid tests for acute hantavirus infections: specific tests for Hantaan, Dobrava and Puumala viruses versus a hantavirus combination test. J Virol Methods. 2003;108(1):117-122 [DOI] [PubMed] [Google Scholar]