Abstract
Germline mutations in LKB1 cause Peutz–Jeghers syndrome (PJS), an autosomal dominant disorder with a predisposition to gastrointestinal polyposis and cancer. Hyperactivation of mTOR-signaling has been associated with PJS. We previously reported that rapamycin treatment of LKB1+/− mice after the onset of polyposis reduced the polyp burden. Here we evaluated the preventive efficacy of rapamycin on Peutz–Jeghers polyposis. We found that rapamycin treatment of LKB1+/− mice initiated before the onset of polyposis in LKB1+/− mice led to a dramatic reduction in both polyp burden and polyp size and this reduction was associated with decreased phosphorylation levels of S6 and 4EBP1. Together, these findings support the use of rapamycin as an option for chemoprevention and treatment of PJS.
Keywords: Peutz–Jeghers syndrome, LKB1, Polyposis, Rapamycin, S6, 4EBP1
1. Introduction
Peutz–Jeghers syndrome (PJS) is a unique autosomal dominant disorder [1,2]. The causative gene LKB1, is located on chromosome 19p13.3 and encodes serine threonine kinase 11 [3–5]. Up to 80% of families with clinically defined PJS harbor germline mutations in the LKB1 gene [6–8]. Patients with PJS are characterized by cutaneous hypermelanocytic macules and hamartomatous polyp development in the gastrointestinal tract. In one study, polyps were detected in 88% of patients affected with PJS [9]. These hamartomas, although benign, may lead to significant clinical complications such as intestinal obstruction, abdominal pain, gastrointestinal bleeding, and anal extrusion of polyps. In the past, treatment of PJS was limited to surgical resection of clinically significant polyps and follow-up endoscopies [10,11], which may yield only partial improvement. In addition to the polyposis, PJS patients are also at increased risk for gastrointestinal and extraintestinal cancers [9,12–19]. For example, in a meta-analysis of 210 patients with PJS, the relative risk of cancer of the stomach, pancreas, lung and breast were found to be 213-, 132-, 17- and 15.2-fold higher than the general population, respectively; and the cumulative risk for developing cancer at any site is 93% [13]. Thus, there is an urgent need to identify effective preventive and chemotherapeutic agents for the treatment of PJS and PJS-associated cancers.
Mouse models provide useful tools for studying the mechanism of many diseases as well as potential therapeutic and preventive strategies. Previously, we and several other groups [20–24] reported that LKB1+/− knockout mice develop severe gastrointestinal polyposis, which model human PJS. Haploinsufficiency of LKB1 is responsible for the polyps seen in these LKB1+/− mice [20–24]. LKB1 is the major upstream kinase of AMPK [25–28] and directly phosphorylates Thr172 of AMPK. Upon phosphorylation, AMPK phosphorylates and activates TSC2, the gatekeeper for mTOR-signaling. Through AMPK/TSC2, LKB1 negatively regulates mTOR [mammalian target of rapamycin] signaling [29]. mTOR, a serine/threonine kinase, is a central regulator of cell growth and proliferation [30]. The mTOR-signaling pathway network is important for driving cell growth and proliferation, and hyperactivation of the mTOR-signaling pathway has been detected in many types of cancers. It has been reported that a reduced level of expression or loss of function of the LKB1 protein causes hyperactivity of mTOR-signaling in hamartomatous polyps from LKB1+/− mice and LKB1-deficent cells [29,31], as evidenced by the elevated phosphorylation levels of mTOR downstream targets such as the ribosomal protein S6 (S6) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4EBP1). More recently, Katajisto et al. demonstrated that rapamycin effectively blocked phospho-S6, indicating inactivation of the mTOR pathway, in LKB1lox/− myofibroblast-enriched cultures [32]. These observations suggested that mTOR inhibitors such as rapamycin and its analogues could be useful for Peutz–Jeghers polyposis treatment. In addition, in two small trials of tuberous sclerosis patients, treatment with rapamycin induced regression of the astrocytomas [33] and reduced facial angiofibroma [34].
Previously, we reported that 2 months of rapamycin treatment in LKB1+/− mice initiated at 9 months of age, which is after the onset of polyposis, effectively reduced the polyp burden by 47% [35]. In the current study, we explored the potential preventive effect of rapamycin on polyposis in LKB1+/− mice by initiating the treatment at 5 months of age, before the onset of polyposis. We found that early rapamycin treatment had a more pronounced effect on polyp burden reduction than such treatment initiated after the onset of polyposis. Together, our results suggest that rapamycin may be a useful therapeutic and preventive agent for Peutz–Jeghers polyposis.
2. Materials and methods
2.1. Animals
The animal study was performed in accordance with the guidelines for animal experiments of The University of Texas M.D. Anderson Cancer Center. Generation and geno-typing of heterozygous Lkb1 knockout mice were described previously [23]. All mice were in a C57BL/6J congenic background and housed in a conventional specific-pathogen-free facility. The mice had free access to food and water before and throughout the study.
2.2. Drug administration
Rapamycin (Wyeth Pharmaceuticals, St. Davids, PA, USA) solution was prepared and stored as described previously [35]. We selected 28 healthy mice at 5 months of age and randomly assigned them to treatment and control groups. The mice were then injected intraperitoneally with rapamycin (2 mg/kg) or vehicle alone (the solution used to dilute the rapamycin) 5 days per week for 6 months.
2.3. Polyp scoring
All mice were euthanized 1 h after the last intraperitoneal injection. The stomach and duodenum of the mice were dissected, coded, and scored. The polyp size was measured with a digital micrometer and the volume was calculated by using the formula: V = π/6 a3, where a is the diameter. The total polyp burden in each mouse was the sum of all polyps from that individual mouse.
2.4. Western blot analysis
Western blot analysis was performed by standard methods. Briefly, snap-frozen polyps dissected from Lkb1+/− mice were homogenized in NP-40 lysis buffer [150 mM NaCl, 1%NP-40, 50 mM Tris–HCl, 1.5 mM EDTA (pH 7.5)] containing protease inhibitors (1.6 μg/ml benzamidine HCl, 1 μg/ml phenanthroline, 1 μg/ml aprotonin, 1 μg/ml leupeptin, 1 lg/ml pepstatin A, 1 mM PMSF), and the total proteins were extracted. Equivalent amounts of protein were separated by SDS–PAGE and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with TBS-T [10 mM Tris-base, 100 mM NaCl, 0.1% Tween 20 (pH 7.5)] containing 5% nonfat dry milk at room temperature overnight and then blotted with relevant antibodies at room temperature for 1 h. HRP-conjugated secondary antibodies were detected by using the Enhanced Chemiluminescence kit (Amersham Biosciences, Piscataway, NJ, USA). Antibodies for S6, phospho-S6 (Ser235/236), phospho-4EBP1 (Thr70), and phospho-4EBP1 (Ser65) were obtained from Cell Signaling Technology (Beverly, MA). Actin and/or S6 were used as an internal control to visualize the total protein loading, and anti-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.5. Immunostaining for phosphorylated S6 (phospho-S6)
Tissues were fixed with 10% formalin solution and embedded in paraffin. Sections (4-μm thick) were deparaffinized in xylene and then rehydrated in decreasing concentrations of ethanol. Immunostaining for phospho-S6 expression was performed as described previously [35], using a 1:50 dilution of the anti-phospho-S6 antibody (S235/236) (Cell Signaling Technology).
2.6. Statistical analysis
We used Stata 10 statistical software (Stata, College Station, TX) for all analyses described here. Fisher's exact test was used to compare the frequency distribution of tumor sizes in the treated and control groups. Student's t-test was used to determine the statistical significance of the differences in polyp burden between the treatment and control groups. Spearman correlations were used to assess the correlation among polyp numbers in different size categories in the two groups. Data are presented as means ± standard error.
3. Results
3.1. Early rapamycin treatment strongly reduces PJ polyposis in Lkb1+/− mice
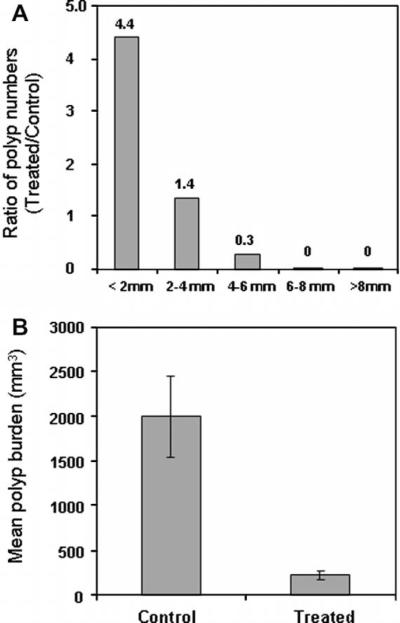
We observed polyps in all of the mice in both groups, with no significant difference in the number of polyps observed in each individual mouse between the treatment and control groups (data not shown). However, when we compared the numbers of polyp in each size category (diameter in mm) between the rapamycin-treated and control groups (summarized in Table 1), we observed a statistically significant difference in polyp size distribution (Fisher's exact test, P < 0.0001). Spearman correlation test (P < 0.00001) demonstrated the trend of polyp distribution in the rapamycin-treated vs. control group. There were more polyps in the smaller size categories and fewer polyps in larger size categories in the rapamycin-treated group, while in the control group there were fewer polyps in the smaller size categories and more polyps in larger size categories. This observation is in agreement with our previous report, in which rapamycin treatment of Lkb1+/− mice was initiated after the onset of polyposis [35], but with a higher level of statistical significance. The size distribution of polyps was further compared as a polyp ratio of treated/control. As shown in Fig. 1A, this ratio decreased dramatically as the polyp size increased, indicating a reduction of larger polyps following rapamycin treatment. It is important to point out that a majority of the polyps in the treated group fell into the <2 mm (48.4%, 31 of 64) and 2–4 mm (42.2%, 27 of 64) size categories, and no polyp was observed in the >6 mm categories, suggesting the presence of these small polyps in the rapamycin-treated mice was caused by inhibition of polyp progression.
Table 1.
Number, size, and burden of polyps observed in rapamycin-treated and control mice.
| Polyp size | <2 mm | 2–4 mm | 4–6 mm | 6–8 mm | >8 mm | Total |
|---|---|---|---|---|---|---|
| Number of polyps | ||||||
| Treated mice | 31 | 27 | 6 | 0 | 0 | 64 |
| Control mice | 7 | 20 | 22 | 16 | 7 | 72 |
| Tumor burden (mm3) | ||||||
| Treated mice | 437.5 | 1801.4 | 822.3 | 0.0 | 0.0 | 3061.3 |
| Control mice | 84.3 | 1281.3 | 6986.7 | 9959.6 | 9642.5 | 27954.3 |
Fisher's exact test: P < 0.0001; Spearman correlation test: P < 0.00001. Fourteen mice were analyzed for each group (control and treated).
Fig. 1.
Rapamycin treatment reduces polyp burden in Lkb1+/− mice. (A) Comparison of polyp size distribution in control and rapamycin-treated mice, shown as the treated/control ratio for the indicated size categories (diameter in mm). (B) The mean tumor burdens were 1996.7 ± 440 mm3 in control mice and 218.7 ± 52 mm3 in rapamycin-treated mice (14 mice in each group; P < 0.001, Student's t-test).
This reduction was even more pronounced when we analyzed the tumor burden of polyps in both groups (Table 1). The mean tumor burdens were 1996.7 ± 440 mm3 in the control mice and 218.7 ± 52 mm3 in the rapamycin-treated mice (14 mice in each group; P < 0.001, Student's t-test) (Fig. 1B), corresponding to an 89% decrease between the groups. This reduction was substantially greater than that seen in our previous study, in which we observed a 47% decrease in polyp burden when rapamycin treatment was initiated after the onset of polyposis (9–11-month trial) [35], suggesting that administration of rapamycin before the polyposis is more effective. Taken together, these data provide compelling evidence that early treatment with rapamycin, before the onset of polyposis, is an effective means of preventing and suppressing polyposis in this mouse model of PJS.
3.2. Rapamycin inhibition is associated with suppressing mTOR-signaling in Lkb1+/− polyps
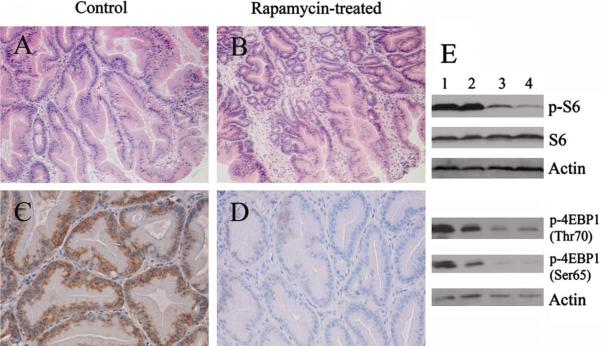
The tumor-suppressing effect of rapamycin was examined in the Lkb1+/− polyps. Both paraffin-embedded and fresh-frozen polyps were collected and further analyzed. As shown in Fig. 2A and B, we observed no significant differences in polyp morphology between the rapamycin-treated and control mice using H&E staining. To determine whether rapamycin treatment suppressed the polyposis by inhibition of its target mTOR, we examined the levels of phospho-S6 by immunohistochemical staining of polyps. Elevated phospho-S6 (Ser235/236) is a useful marker for increased mTOR kinase activity. We observed phospho-S6 (Ser235/236) staining in polyps from both the treated and control groups (Fig. 2C and D), indicating that activation of mTOR-signaling is associated with polyp development. However, the staining intensity of phospho-S6 observed in the polyps from the rapamycin-treated group was dramatically reduced compared that from the control group, suggesting that the anti-tumor effect of rapamycin is associated with suppression of mTOR-signaling in these Lkb1+/− polyps. This result was also confirmed by western blot analysis. As shown in Fig. 2E, there was a substantial decrease in phospho-S6 levels in polyps from rapamycin-treated mice compared to that from control mice. We also analyzed another downstream molecule of mTOR, 4EBP1 by western blotting. Hyperphosphorylation of 4EBP1 by mTOR on Ser65 and Thr70 disrupts the binding of 4EBP1 to eIF4E and results in activation of cap-dependent translation [36,37]. A decrease of phospho-4EBP1 levels reflects a decreased mTOR kinase activity. We observed a distinct decrease of phosphorylation levels of 4EBP1 at sites Ser65 and Thr70 in polyps from rapamycin-treated mice. The reductions in phosphorylation levels of S6 and 4EBP1 in Lkb1+/− polyps caused by rapamycin treatment are consistent with a direct effect of rapamycin on the mTOR-signaling pathway.
Fig. 2.
Rapamycin treatment decreases the phosphorylation level of S6 and 4EBP1 of Lkb1+/− polyps. (A and B) Representative H&E staining of the polyps from control (A) and rapamycin-treated (B) mice; original magnification, ×40. (C and D) Representative anti-phospho-S6 immunostaining of polyps sections from control (C) and rapamycin-treated (D) mice; original magnification ×200. (E) Polyp lysates from the control group (lanes 1 and 2) and the rapamycin-treated group (lanes 3 and 4) were subjected to western blotting with anti-phospho-S6 (Ser235/236) antibody (p-S6), anti-phospho-4EBP1 (Thr70) antibody [p-4EBP1 (Thr70)], and anti-phospho-4EBP1 (Ser65) antibody [p-4EBP1 (Ser65)], with anti-actin antibody (Actin) and /or anti-S6 antibody (S6) as a loading control.
4. Discussion
The current study was designed to evaluate the preventive efficacy of rapamycin on polyposis in an Lkb1+/− Peutz–Jeghers mouse model. We initiated the rapamycin treatment prior to the onset of polyposis and our results revealed a dramatic reduction of size and polyp burden after the rapamycin treatment.
Development of gastrointestinal hamartomatous polyps is one of the most significant clinical features of PJS. This polyposis occurs early in life, with over one-third of patients developing symptoms within the first decade of life and 60% before age 20 years [38]. In a 78-year follow-up study of the original PJS family [39], 16 affected people in that family had undergone 33 laparotomies due to bowel obstruction. In a recent study of 51 patients, the median patient age at first polypectomy was 13 years [7]. These figures emphasize the need for developing preventive and treatment options for patients with PJS.
A recent pilot study suggested that celecoxib, a COX-2 inhibitor, could be beneficial in the chemoprevention of PJS polyposis [40]. Although COX-2 is upregulated in some polyps and in most cancers from patients with PJS [24,41–44], it is not currently known to be directly influenced by LKB1. Recently, the finding that LKB1 negatively regulates mTOR through AMPK/TSC2 positions hyperactivation of mTOR as the nexus of PJS and suggested that mTOR inhibitors could be useful in the treatment of PJS [29]. On the basis of these findings, we previously conducted a study on rapamycin treatment of Lkb1+/− mice after the onset of polyposis [35] to mimic the clinical situation in which PJS patients present with a significant polyp burden. This post-onset treatment of the Lkb1+/− mice led to a 47% reduction of total polyp burden as well as fewer large (>8 mm) polyps (one in the treated group vs. seven in the control group) in this mouse model [35]. These findings suggested that rapamycin might be effective in future clinical trials on PJS patients and might allow these patients to avoid surgical intervention.
The study presented here evaluates the preventive efficacy of rapamycin on the development of polyps in Lkb1+/− mice. We initiated the rapamycin treatment prior to the onset of polyposis (5 months of age) and observed an even more dramatic effect on polyposis after 6 months of treatment than in our previous study [35] described above. The total polyp burden was 89% reduction in the current study, with no polyps >6 mm in diameter observed in the treated mice, suggesting that administration of rapamycin before polyposis is more effective. Also, the immunostaining and/or western blotting results demonstrated dramatic decrease of phosphorylation levels of S6 and 4EBP1 in the Lkb1+/− polyps after the rapamycin treatment, which reflected an association between suppression of polyposis and downregulation of mTOR-signaling. Together, our data presented here and previously [35] suggest that rapamycin or its analogues may be useful as preventive and therapeutic agents for Peutz–Jeghers polyposis. Considering that polyposis in PJS patients typically begins to cause significant clinical complications in the second decade of life, the study presented here suggests a chemopreventive strategy of administrating mTOR inhibitors to PJS patients earlier in the life rather than waiting until symptoms develop.
PJS patients are also at higher risk for developing various types of cancer. For example, the cumulative risks for breast, lung, and pancreatic cancers are 54%, 15%, and 36%, respectively [13]. These figures also address the importance of developing effective chemoprevention strategies for PJS patients. mTOR inhibitors have been actively used in clinical trials and have shown great promise in the treatment of breast cancer and non-small cell lung cancer [45–47]. Recently, the observation of hyperactivation of the mTOR-signaling pathway in pancreatic cancer [48,49] suggested that mTOR inhibitors may be useful in the treatment of patients with this disease. Considering the finding that LKB1 negatively regulates mTOR through AMPK/TSC2 [29], all these observations suggest that mTOR inhibitors may suppress PJS-associated cancers in addition to Peutz–Jeghers polyposis. It will be interesting to investigate whether administration of rapamycin early in life prevents the formation of PJS-associated cancers such as breast, lung, and pancreatic cancers.
In conclusion, we found that targeted therapy with the mTOR inhibitor rapamycin dramatically suppressed hamartomatous polyposis in this PJS mouse model. Our findings provide validation for the utilization of mTOR inhibitors as an option for PJS treatment and will have important implications for the management of PJS. The challenge that remains is to translate these finding safely and effectively into clinically relevant improvement in the care of patients with PJS.
Acknowledgements
This study was supported by National Institutes of Health (NIH) R03 Grant (CA123603), NIH CRED Grant (P30 ES007784), NIH SPORE Grant (P50CA70907), and NIH CCSG (CA16672).
Footnotes
Conflicts of interest statement None declared.
References
- [1].Peutz JLA. Very remarkable case of familial polyposis of mucous membrane of intestinal tract and nasopharynx accompanied by peculiar pigmentations of skin and mucous membrane, Nederlands Maandschr. Geneesk. 1921;10:134–146. [Google Scholar]
- [2].Jeghers H, McKusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N. Engl. J. Med. 1949;241:1031–1036. doi: 10.1056/NEJM194912292412601. [DOI] [PubMed] [Google Scholar]
- [3].Hemminki A, Tomlinson I, Markie D, Jarvinen H, Sistonen P, Bjorkqvist AM, Knuutila S, Salovaara R, Bodmer W, Shibata D, de la CA, Aaltonen LA. Localization of a susceptibility locus for Peutz–Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat. Genet. 1997;15:87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- [4].Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la CA, Aaltonen LA. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- [5].Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M. Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- [6].Schumacher V, Vogel T, Leube B, Driemel C, Goecke T, Moslein G, Royer-Pokora B. STK11 genotyping and cancer risk in Peutz–Jeghers syndrome. J. Med. Genet. 2005;42:428–435. doi: 10.1136/jmg.2004.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amos CI, Keitheri-Cheteri MB, Sabripour M, Wei C, McGarrity TJ, Seldin MF, Nations L, Lynch PM, Fidder HH, Friedman E, Frazier ML. Genotype–phenotype correlations in Peutz–Jeghers syndrome. J. Med. Genet. 2004;41:327–333. doi: 10.1136/jmg.2003.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Volikos E, Robinson J, Aittomaki K, Mecklin JP, Jarvinen H, Westerman AM, deRooji FW, Vogel T, Moeslein G, Launonen V, Tomlinson IP, Silver AR, Aaltonen LA. LKB1 exonic and whole gene deletions are a common cause of Peutz–Jeghers syndrome. J. Med. Genet. 2006;43:e18. doi: 10.1136/jmg.2005.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Utsunomiya J, Gocho H, Miyanaga T, Hamaguchi E, Kashimure A. Peutz–Jeghers syndrome: its natural course and management, Johns. Hopkins. Med. J. 1975;136:71–82. [PubMed] [Google Scholar]
- [10].Giardiello FM, Trimbath JD. Peutz–Jeghers syndrome and management recommendations. Clin. Gastroenterol. Hepatol. 2006;4:408–415. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- [11].McGrath DR, Spigelman AD. Preventive measures in Peutz–Jeghers syndrome. Fam. Cancer. 2001;1:121–125. doi: 10.1023/a:1013896813918. [DOI] [PubMed] [Google Scholar]
- [12].Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD. Increased risk of cancer in the Peutz–Jeghers syndrome. N. Engl. J. Med. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- [13].Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- [14].Spigelman AD, Murday V, Phillips RK. Cancer and the Peutz–Jeghers syndrome. Gut. 1989;30:1588–1590. doi: 10.1136/gut.30.11.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hizawa K, Iida M, Matsumoto T, Kohrogi N, Kinoshita H, Yao T, Fujishima M. Cancer in Peutz–Jeghers syndrome. Cancer. 1993;72:2777–2781. doi: 10.1002/1097-0142(19931101)72:9<2777::aid-cncr2820720940>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [16].Boardman LA, Thibodeau SN, Schaid DJ, Lindor NM, McDonnell SK, Burgart LJ, Ahlquist DA, Podratz KC, Pittelkow M, Hartmann LC. Increased risk for cancer in patients with the Peutz–Jeghers syndrome. Ann. Intern. Med. 1998;128:896–899. doi: 10.7326/0003-4819-128-11-199806010-00004. [DOI] [PubMed] [Google Scholar]
- [17].Foley TR, McGarrity TJ, Abt AB. Peutz–Jeghers syndrome: a clinicopathologic survey of the “Harrisburg family” with a 49-year follow-up. Gastroenterology. 1988;95:1535–1540. doi: 10.1016/s0016-5085(88)80074-x. [DOI] [PubMed] [Google Scholar]
- [18].Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Moslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS. Frequency and spectrum of cancers in the Peutz–Jeghers syndrome. Clin. Cancer Res. 2006;12:3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- [19].Lim W, Hearle N, Shah B, Murday V, Hodgson SV, Lucassen A, Eccles D, Talbot I, Neale K, Lim AG, O'Donohue J, Donaldson A, Macdonald RC, Young ID, Robinson MH, Lee PW, Stoodley BJ, Tomlinson I, Alderson D, Holbrook AG, Vyas S, Swarbrick ET, Lewis AA, Phillips RK, Houlston RS. Further observations on LKB1/STK11 status and cancer risk in Peutz–Jeghers syndrome. Br. J. Cancer. 2003;89:308–313. doi: 10.1038/sj.bjc.6601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jishage K, Nezu J, Kawase Y, Iwata T, Watanabe M, Miyoshi A, Ose A, Habu K, Kake T, Kamada N, Ueda O, Kinoshita M, Jenne DE, Shimane M, Suzuki H. Role of Lkb1, the causative gene of Peutz–Jegher's syndrome. In embryogenesis and polyposis. Proc. Natl. Acad. Sci. USA. 2002;99:8903–8908. doi: 10.1073/pnas.122254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miyoshi H, Nakau M, Ishikawa TO, Seldin MF, Oshima M, Taketo MM. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res. 2002;62:2261–2266. [PubMed] [Google Scholar]
- [22].Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, Depinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- [23].Wei C, Amos CI, Stephens LC, Campos I, Deng JM, Behringer RR, Rashid A, Frazier ML. Mutation of Lkb1 and p53 genes exert a cooperative effect on tumorigenesis. Cancer Res. 2005;65:11297–11303. doi: 10.1158/0008-5472.CAN-05-0716. [DOI] [PubMed] [Google Scholar]
- [24].Rossi DJ, Ylikorkala A, Korsisaari N, Salovaara R, Luukko K, Launonen V, Henkemeyer M, Ristimaki A, Aaltonen LA, Makela TP. Induction of cyclooxygenase-2 in a mouse model of Peutz–Jeghers polyposis. Proc. Natl. Acad. Sci. USA. 2002;99:12327–12332. doi: 10.1073/pnas.192301399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, Depinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- [29].Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, Depinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- [30].Tee AR, Blenis J. mTOR, translational control and human disease. Semin. Cell Dev. Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- [31].Corradetti MN, Inoki K, Bardeesy N, Depinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz–Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Katajisto P, Vaahtomeri K, Ekman N, Ventela E, Ristimaki A, Bardeesy N, Feil R, Depinho RA, Makela TP. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat. Genet. 2008;40:455–459. doi: 10.1038/ng.98. [DOI] [PubMed] [Google Scholar]
- [33].Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- [34].Hofbauer GF, Marcollo-Pini A, Corsenca A, Kistler AD, French LE, Wuthrich RP, Serra AL. The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. Br. J. Dermatol. 2008 doi: 10.1111/j.1365-2133.2008.08677.x. [DOI] [PubMed] [Google Scholar]
- [35].Wei C, Amos CI, Zhang N, Wang X, Rashid A, Walker CL, Behringer RR, Frazier ML. Suppression of Peutz–Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin. Cancer Res. 2008;14:1167–1171. doi: 10.1158/1078-0432.CCR-07-4007. [DOI] [PubMed] [Google Scholar]
- [36].Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr., Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- [37].Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tovar JA, Eizaguirre I, Albert A, Jimenez J. Peutz–Jeghers syndrome in children: report of two cases and review of the literature. J. Pediatr. Surg. 1983;18:1–6. doi: 10.1016/s0022-3468(83)80262-0. [DOI] [PubMed] [Google Scholar]
- [39].Westerman AM, Entius MM, de Baar E, Boor PP, Koole R, van Velthuysen ML, Offerhaus GJ, Lindhout D, de Rooij FW, Wilson JH. Peutz–Jeghers syndrome: 78-year follow-up of the original family. Lancet. 1999;353:1211–1215. doi: 10.1016/s0140-6736(98)08018-0. [DOI] [PubMed] [Google Scholar]
- [40].Udd L, Katajisto P, Rossi DJ, Lepisto A, Lahesmaa AM, Ylikorkala A, Jarvinen HJ, Ristimaki AP, Makela TP. Suppression of Peutz–Jeghers polyposis by inhibition of cyclooxygenase-2. Gastroenterology. 2004;127:1030–1037. doi: 10.1053/j.gastro.2004.07.059. [DOI] [PubMed] [Google Scholar]
- [41].McGarrity TJ, Peiffer LP, Amos CI, Frazier ML, Ward MG, Howett MK. Overexpression of cyclooxygenase 2 in hamartomatous polyps of Peutz–Jeghers syndrome. Am. J. Gastroenterol. 2003;98:671–678. doi: 10.1111/j.1572-0241.2003.07328.x. [DOI] [PubMed] [Google Scholar]
- [42].Wei C, Amos CI, Rashid A, Sabripour M, Nations L, McGarrity TJ, Frazier ML. Correlation of staining for LKB1 and COX-2 in hamartomatous polyps and carcinomas from patients with Peutz–Jeghers syndrome. J. Histochem. Cytochem. 2003;51:1665–1672. doi: 10.1177/002215540305101210. [DOI] [PubMed] [Google Scholar]
- [43].Takeda H, Miyoshi H, Tamai Y, Oshima M, Taketo MM. Simultaneous expression of COX-2 and mPGES-1 in mouse gastrointestinal hamartomas. Br. J. Cancer. 2004;90:701–704. doi: 10.1038/sj.bjc.6601584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Leng WW, Westerman AM, Weterman MA, de Rooij FW, Dekken HH, de Goeij AF, Gruber SB, Wilson JH, Offerhaus GJ, Giardiello FM, Keller JJ. Cyclooxygenase 2 expression and molecular alterations in Peutz–Jeghers hamartomas and carcinomas. Clin. Cancer Res. 2003;9:3065–3072. [PubMed] [Google Scholar]
- [45].Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr. Opin. Oncol. 2004;16:564–575. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- [46].Vignot S, Faivre S, Aguirre D, Raymond E. MTOR-targeted therapy of cancer with rapamycin derivatives. Ann. Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- [47].Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol. Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [48].Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem. Biophys. Res. Commun. 2005;331:295–302. doi: 10.1016/j.bbrc.2005.03.166. [DOI] [PubMed] [Google Scholar]
- [49].Ito D, Fujimoto K, Mori T, Kami K, Koizumi M, Toyoda E, Kawaguchi Y, Doi R. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int. J. Cancer. 2006;118:2337–2343. doi: 10.1002/ijc.21532. [DOI] [PubMed] [Google Scholar]