Abstract
Hypercapnia has been shown to impair alveolar fluid reabsorption (AFR) by decreasing Na,K-ATPase activity. Extracellular signal-regulated kinase pathway (ERK) is activated under conditions of cellular stress and has been known to regulate the Na,K-ATPase. Here, we show that hypercapnia leads to ERK activation in a time-dependent manner in alveolar epithelial cells (AEC). Inhibition of ERK by U0126 or siRNA prevented both the hypercapnia-induced Na,K-ATPase endocytosis and impairment of AFR. Moreover, ERK inhibition prevented AMPK activation, a known modulator of hypercapnia-induced Na,K-ATPase endocytosis. Accordingly, these data suggest that hypercapnia-induced Na,K-ATPase endocytosis is dependent on ERK activation in AEC and that ERK plays an important role in hypercapnia-induced impairment of AFR in rat lungs.
Keywords: Na,K-ATPase; Extracellular signal-regulated kinase; Hypercapnia; Alveolar epithelium
1. Introduction
For normal gas exchange to occur, it is essential to maintain the lungs free of edema. The alveolar epithelium reabsorbs fluid from the alveolar spaces mostly via active Na+ transport where Na+ enters the cells via apical sodium channels and it is extruded through active transport driven by the basolateral Na,K-ATPase [1-4]. The active Na+ transport across the alveolar epithelium creates an osmotic gradient responsible for the clearance of lung edema [1-3]. We and others have reported that alveolar fluid reabsorption (AFR) is highly dependent on the Na,K-ATPase activity [1,5,6].
Hypercapnia (high levels of CO2 in the blood) occurs in patients with chronic obstructive pulmonary disease [7,8], uncontrolled asthma [9] and also in patients with lung injury who are mechanically ventilated with the “permissive hypercapnia” strategy [10,11]. We have previously shown that AFR is impaired during hypercapnia and that cells exposed to high pCO2 have decreased Na,K-ATPase activity due to a reduction in the Na,K-ATPase protein abundance at the plasma membrane, suggesting hypercapnia-induced Na,K-ATPase endocytosis [5,12,13].
Extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK2) are major members of the mitogen-activated protein kinase signaling family and they regulate essential cellular processes [14,15]. For ERK 1/2 to be fully activated, dual phosphorylation of threonine 202 and tyrosine 204 of ERK1 and also threonine 183 and tyrosine 185 of ERK2 are required by an upstream kinase [15-18]. It has been described that activation of ERK 1/2 plays a role in the trafficking of the Na,K-ATPase [19,20]. Here, we investigated whether hypercapnia activates ERK 1/2 signaling pathways in alveolar epithelial cells (AEC) and whether this activation regulates the Na,K-ATPase and AFR as a measure of epithelial function.
2. Materials and methods
2.1. Reagents
All cell culture reagents were from Mediatech Inc., except for the Ham’s F-12 which was from Sigma–Aldrich. 22Na+, 3H-mannitol, and γ32P-ATP were from Perkin–Elmer. Ouabain was from ICN Biomedical Inc. U0126 was from Promega. Na,K-ATPase α1-subunit monoclonal antibody (clone 464.6) and AMPKα1-rabbit polyclonal antibody were purchased from Upstate Biotechnology. Phospho p44/42 MAPK and phospho-AMPKα rabbit monoclonal antibodies, p44/42 MAPK mouse monoclonal antibody, AMPKα rabbit polyclonal antibody, anti-rabbit and anti-mouse IgG HRP-linked, 10X Cell Lysis Buffer and SignalSilence p44/42 MAPK (ERK1/2) siRNA were from Cell Signaling Technology. AMPKα1 siRNA was from Santa Cruz Biotechnology. Silencer Negative Control #1 siRNA was from Ambion.
2.2. Animals
Pathogen-free adult male Sprague Dawley rats (300–325 g) were used for isolated perfused rat lung model and male Sprague Dawley rats (200–225 g) were used for alveolar epithelial type II cell isolation (Harlan). Animals were provided with food and water ad libitum, were maintained on a 12-h light/12-h dark cycle and were handled according to NIH guidelines and the Northwestern University Animal Care and Use Committee-approved experimental protocols.
2.3. Alveolar epithelial type II cell isolation and cell culture
Rat type II alveolar epithelial (ATII) cells were isolated as previously described [21,22]. The day of isolation and plating was designated cultured day 0. All experimental conditions were tested in day 2 cells. Rat ATII, human A549 cells (ATCC #CCL-185) and clones expressing the GFP-rat NKA-α1 (A549-GFP-α1) [23] were grown in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 20 mM Hepes. For the A549-GFP-α1 cells, 3 μM ouabain was added to the medium to suppress the endogenous Na,K-ATPase α1-subunit. Cells were incubated in a humidified atmosphere of 5% CO2/95% air at 37 °C.
2.4. CO2 exposure
Solutions and exposure of cells in a C-Chamber in which the atmosphere was controlled with a PRO-CO2 carbon dioxide controller (Biospherix) was performed as previously described [13].
2.5. ERK 1/2 and AMPKα phosphorylation
For the detection of ERK 1/2 and AMPKα phosphorylation, after exposure to hypercapnia for the appropriate times, cells were washed in ice-cold phosphate-buffered saline and solubilized in lysis buffer. The lysates were cleared by centrifugation for 10 min at 14,000×g. Samples containing equal amounts (25–75 μg) of proteins were resuspended in Laemmli sample buffer, boiled for 5 min, and subjected to Western blot analysis with specific antibodies as described below.
2.6. Western blot
Protein concentration was quantified using Protein Assay Dye (Bio-Rad). Proteins were then resolved in 10–12.5% polyacrylamide gels (SDS–PAGE) as described previously [13].
2.7. Determination of Na,K-ATPase activity
After the treatments, Na-K-ATPase activity was determined by [γ-32P] ATP hydrolysis as previously described [5,24]. Na-K-ATPase activity was calculated as the difference between test samples (total ATPase activity) and samples assayed in the same medium, but devoid of Na+ and K+ and in the presence of 1 mM ouabain (ouabain-insensitive ATPase activity).
2.8. Biotinylation of cell surface proteins
After the treatments, cells were labeled for 20 min using 1 mg/ml EZ-link NHS-SS-biotin (Pierce Biotechnology) in 10 mM Triethanolamine [pH 8], 2 mM CaCl2 150 mM NaCl as previously described [5]. Proteins were analyzed by SDS–PAGE and Western blot as described above.
2.9. Transient transfection
A549 or A549-GFP-α1 cells were grown on 35 mm plates at a density of 2 × 105 cells per plate in DMEM with 10% fetal bovine serum and 20 mM Hepes without antibiotics. Cells were transfected with SignalSilence p44/42 MAPK (ERK1/2) siRNA (10 picomol) or AMPKα1 siRNA (100 picomol) by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. Hypercapnia exposure was performed after 48 h. A non-silencing siRNA (10 picomol) was used as a negative control.
2.10. Isolated-perfused rat lung model
The isolated lung preparation has been described in detail previously [5,13].
2.11. Data analysis
Data are represented as means ± S.E.M. Multiple comparisons were made using a one-way analysis of variance followed by a multiple comparison test (Dunnett) when the F statistic indicated significance. Results were considered significant when p < 0.05.
3. Results
3.1. ERK 1/2 is activated in a time-dependent manner in AEC exposed to hypercapnia
To investigate whether ERK 1/2 was activated during exposure to hypercapnia, ATII cells were exposed for up to 30 min to 40 mmHg pCO2 (control) and 120 mmHg pCO2 (hypercapnia), while buffering the pH to 7.4. As shown in Fig. 1, exposure of the ATII cells to hypercapnia for as little as 1 minute stimulated ERK 1/2 phosphorylation.
Fig. 1.
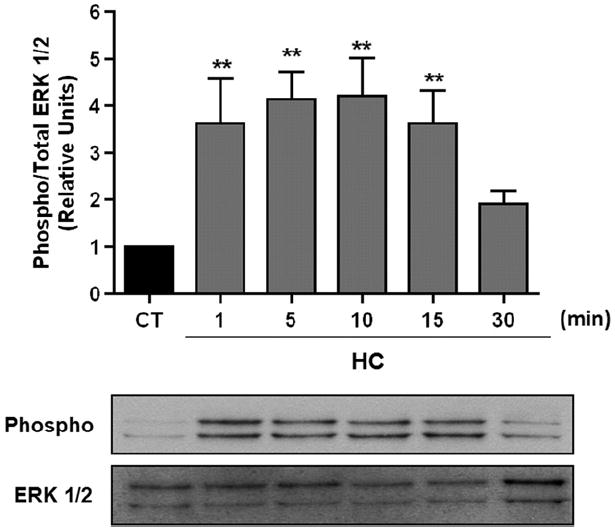
ERK 1/2 is activated in a time-dependent manner in AEC exposed to high pCO2. ATII cells were exposed to 40 (black bars) or 120 (grey bars) mmHg pCO2 for 1–30 min. Phospho-ERK 1/2 and total amount of ERK 1/2 were measured by Western blot analysis. Graph represents the phospho/total ERK 1/2 ratio. Values are expressed as mean ± S.E.M., n = 4. Representative Western blots are shown. **P < 0.01.
3.2. Inhibition of ERK 1/2 prevents the hypercapnia-induced Na,K-ATPase downregulation
Previously, we have reported that AEC were sensitive to elevated increasing levels of pCO2 [5,12,13]. We set out to determine whether Na,K-ATPase activity and endocytosis were dependent on ERK activation. ATII cells pre-treated with 10 μM U0126 for 30 min were exposed to hypercapnia for 30 min and as shown in Fig. 2A and B the ERK inhibitor U0126 prevented the hypercapnia-induced decrease in Na,K-ATPase activity and protein abundance at the plasma membrane. To further confirm that the hypercapnia-induced Na,K-ATPase endocytosis is dependent on ERK, A549-GFP-α1 cells were transfected with ERK 1/2 siRNA (~60% knockdown, Fig. 2C). Forty-eight hours post-transfection cells were exposed to hypercapnia for 30 min and as shown in Fig. 2C, the hypercapnia-induced downregulation of the Na, K-ATPase was prevented.
Fig. 2.
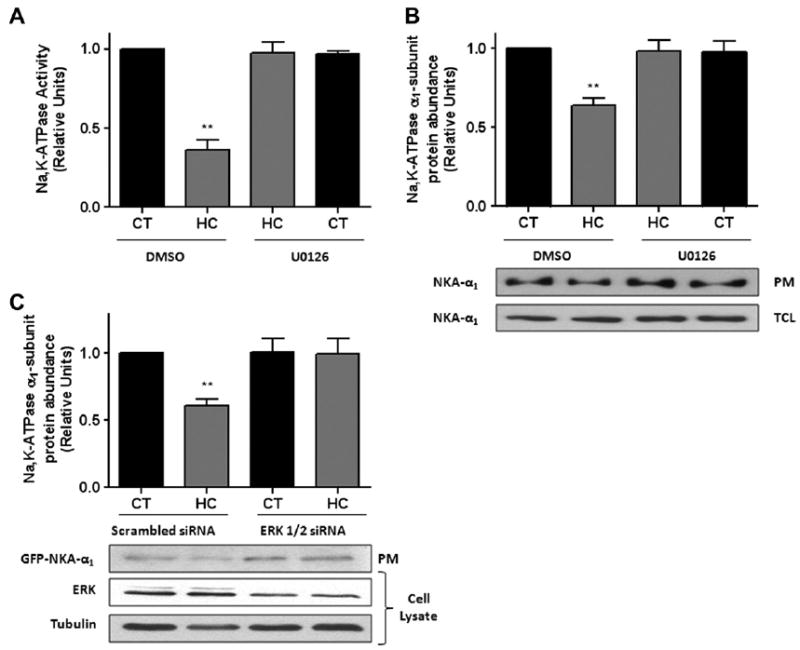
Inhibition of ERK 1/2 prevents the hypercapnia-induced Na,K-ATPase downregulation. (A) ATII cells were pre-treated with U0126 (10 μM) or DMSO for 30 min and then exposed to 40 (black bars) or 120 (grey bars) mmHg pCO2 for 30 min in the presence or absence of U0126. Na,K-ATPase activity was measured as [γ-32P] ATP hydrolysis. (B) ATII cells treated as in (A) and Na,K-ATPase protein abundance at the plasma membrane (PM) was determined by biotin-streptavidin pull down and subsequent Western blot against Na,K-ATPase α1-subunit. Total cell lysates are also shown. (C) A549-GFP-α1 cells were transiently transfected with scrambled or ERK 1/2 siRNA and 48 h later exposed to 40 or 120 mmHg pCO2 for 30 min. The Na,K-ATPase protein abundance at the plasma membrane (PM) was determined as in (B). Representative Western blots of total cell lysates for total ERK 1/2 and tubulin are shown. Graph represents the mean ± S.E.M, n = 5. **P < 0.01.
3.3. Inhibition of ERK 1/2 prevents the hypercapnia-induced AMP-activated protein kinase (AMPK) activation
We have previously reported that AMPK is necessary for hypercapnia-induced Na,K-ATPase endocytosis [13]. To determine whether ERK 1/2 is upstream of AMPK activation, we used specific siRNA to knockdown ERK (~60% knockdown) and studied AMPK activation after exposure for 10 min to hypercapnia. As shown in Fig. 3A, ERK knockdown completely abolished AMPK activation. However, the opposite experiment (siRNA for AMPKα1, ~80% knockdown) did not prevent the hypercapnia-induced ERK activation (Fig. 3B).
Fig. 3.
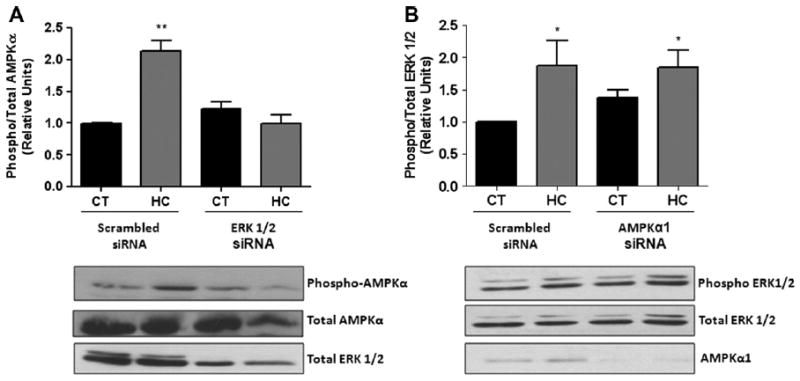
Inhibition of ERK1/2 prevents the hypercapnia-induced AMPK activation. (A) A549 cells transiently transfected with scrambled or ERK 1/2 siRNA and then exposed to 40 (black bars) or 120 (grey bars) mmHg pCO2 for 10 min. Phospho-AMPKα and total amount of AMPKa were measured by Western blot analysis. A representative Western blot is shown for total ERK 1/2. (B) A549 cells transiently transfected with scrambled or AMPKα1 siRNA and then exposed to 40 or 120 mmHg pCO2 for 10 min. Phospho-ERK 1/2 and total amount of ERK 1/2 were measured by Western blot analysis. A representative Western blot of total cell lysates for total AMPKα1 is shown. Data represent the mean ± S.E.M., n = 5. *P < 0.05, **P < 0.01.
3.4. ERK 1/2 regulates AFR in rat lungs exposed to hypercapnia
We have shown that high levels of pCO2 reduced AFR by ~50% [5,12,13]. To determine whether ERK 1/2 mediated the hypercapnia induced impairment of AFR, anesthetized Sprague Dawley rats were pre-treated with 5 mg/kg U0126 or DMSO (I.V.) 30 minutes prior to isolating the lungs and measuring AFR using our ex vivo isolated-perfused rat lung model. As depicted in Fig. 4A and B, pre-treatment with U0126 prevented the hypercapnia induced decrease in AFR, without affecting the epithelial barrier permeability.
Fig. 4.
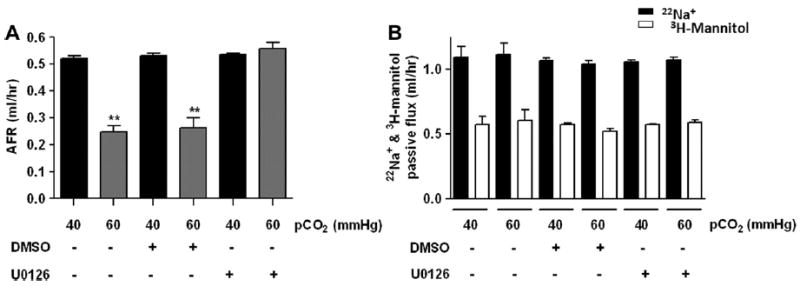
ERK 1/2 regulates AFR in rat lungs exposed to high pCO2. Anesthetized Sprague Dawley rats were pre-treated with 5 mg/kg U0126 or DMSO (I.V.) 30 min prior to the isolated-perfused rat lung model. (A) Isolated rats lungs were perfused for 1 h with 40 mmHg pCO2 (black bars) or with approximately 60 mmHg pCO2 (grey bars) and AFR was measured. (B) Passive fluxes of 22Na+ (black bars) and 3H-mannitol (white bars) were measured. Bars represent the mean ± S.E.M, n = 5. **P < 0.01.
4. Discussion
Hypercapnia (increased pCO2) occurs in patients with many pulmonary diseases as a result of respiratory dysfunction. We and others have reported that hypercapnia has deleterious effects on the alveolar epithelium [5,12,13,25-27], independent of pH and carbonic anhydrase [5,12]. Here, we provide first evidence that hypercapnia leads to a rapid ERK 1/2 phosphorylation, which has a role in Na,K-ATPase endocytosis and decreased AFR.
We found that ERK was activated in AEC exposed to hypercapnia as early as 1 min, peaking between 10 and 15 min and returning almost to baseline by 30 min. There have been several reports describing the role of ERK in the trafficking of the Na,K-ATPase. It has previously been shown that Na,K-ATPase was upregulated via ERK-dependent pathways in AEC treated with either dopamine or β-adrenergic agonists [28,29]; alternatively, a role for ERK regulating the Na,K-ATPase endocytosis has also been shown in opossum kidney cells [19]. We show that the hypercapnia-induced Na,K-ATPase endocytosis was prevented by using the MEK inhibitor, U0126 and siRNA against ERK 1/2, allowing us to conclude that ERK activation is necessary for Na,K-ATPase downregulation during hypercapnia.
We have previously demonstrated the critical role that AMPK plays in the hypercapnia-induced Na,K-ATPase endocytosis [13]. Surprisingly, we found that ERK is involved in the hypercapnia activation of AMPK. This could indicate a role for ERK in the regulation of the upstream events that lead to AMPK phosphorylation which includes several kinases and phosphatases [30].
We show that when the MEK inhibitor U0126 is administered to rats in vivo, it attenuated the hypercapnia-induced decrease in AFR (see Fig. 4). U0126 has been used to study in vivo the role of ERK in several models, such as the influx of eosinophils in an animal model of asthma [31,32], and in the cerulein-induced pancreatitis in rats [33]. Additionally, a recent study by Schuh and Pahl found in a lipopolysaccharide-induced lung injury model that U0126 attenuated the effects of acute lung injury by reducing the inflammatory cell influx in the bronchoalveolar lavage of the mice [34]. Our study suggests that inhibition of the ERK 1/2 signaling pathway may have therapeutic potential for the treatment patients with hypercapnia.
In summary, we provide evidence that AEC exposed to hypercapnia stimulated ERK 1/2 phosphorylation and caused Na, K-ATPase endocytosis. Inhibition of ERK 1/2 prevented the hypercapnia-induced Na,K-ATPase downregulation. In addition, rats pre-treated with ERK inhibitor U0126 protected the lungs from hypercapnia-induced impairment of AFR and warrants further studies to understand the molecular pathways involved in alveolar epithelial dysfunction during hypercapnia.
Acknowledgments
The authors wish to acknowledge the valuable insights to this manuscript by Istvan Vadasz and Aileen Kelly. This research was supported in part by NIH Grants HL-85534, T32-HL076139 and AHA 09POST2250970 (H.E.T.).
Abbreviations
- ERK
extracellular signal-regulated kinase
- AFR
alveolar fluid reabsorption
- AEC
alveolar epithelial cells
- ATII
type II alveolar epithelial
References
- 1.Berthiaume Y, Folkesson HG, Matthay MA. Lung edema clearance: 20 years of progress: invited review: alveolar edema fluid clearance in the injured lung. J Appl Physiol. 2002;93:2207–2213. doi: 10.1152/japplphysiol.01201.2001. [DOI] [PubMed] [Google Scholar]
- 2.Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med. 2001;163:1021–1029. doi: 10.1164/ajrccm.163.4.2006116. [DOI] [PubMed] [Google Scholar]
- 3.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol. 2001;280:L646–L658. doi: 10.1152/ajplung.2001.280.4.L646. [DOI] [PubMed] [Google Scholar]
- 4.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na(+)-K(+)-ATPase. J Appl Physiol. 2002;93:1860–1866. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- 5.Briva A, et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem. 2006;281:19892–19898. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- 7.Connors AF, Jr, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- 9.Mutlu GM, Factor P, Schwartz DE, Sznajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia. Crit Care Med. 2002;30:477–480. doi: 10.1097/00003246-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 10.Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med. 1994;150:1722–1737. doi: 10.1164/ajrccm.150.6.7952641. [DOI] [PubMed] [Google Scholar]
- 11.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill – too little of a good thing? Lancet. 1999;354:1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Lecuona E, Briva A, Welch LC, Sznajder JI. Carbonic anhydrase II and alveolar fluid reabsorption during hypercapnia. Am J Respir Cell Mol Biol. 2008;38:32–37. doi: 10.1165/rcmb.2007-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vadasz I, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 15.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 16.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 17.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 19.Khundmiri SJ, Bertorello AM, Delamere NA, Lederer ED. Clathrin-mediated endocytosis of Na, K-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the alpha-subunit. J Biol Chem. 2004;279:17418–17427. doi: 10.1074/jbc.M311715200. [DOI] [PubMed] [Google Scholar]
- 20.Zhong Z. C-peptide stimulates Na+, K+-ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci. 2004;61:2782–2790. doi: 10.1007/s00018-004-4258-x. [DOI] [PubMed] [Google Scholar]
- 21.Ridge KM, Rutschman DH, Factor P, Katz AI, Bertorello AM, Sznajder JL. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. Am J Physiol. 1997;273:L246–L255. doi: 10.1152/ajplung.1997.273.1.L246. [DOI] [PubMed] [Google Scholar]
- 22.Ridge KM, et al. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Cir Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 23.Bertorello AM, Komarova Y, Smith K, Leibiger IB, Efendiev R, Pedemonte CH, Borisy G, Sznajder JI. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell. 2003;14:1149–1157. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridge KM, Dada L, Lecuona E, Bertorello AM, Katz AI, Mochly-Rosen D, Sznajder JI. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and -delta. Mol Biol Cell. 2002;13:1381–1389. doi: 10.1091/mbc.01-07-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doerr CH, Gajic O, Berrios JC, Caples S, Abdel M, Lymp JF, Hubmayr RD. Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator injured lungs. Am J Respir Crit Care Med. 2005;171:1371–1377. doi: 10.1164/rccm.200309-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang JD, Figueroa M, Sanders KD, Aslan M, Liu Y, Chumley P, Freeman BA. Hypercapnia via reduced rate and tidal volume contributes to lipopolysaccharide-induced lung injury. Am J Respir Crit Care Med. 2005;171:147–157. doi: 10.1164/rccm.200302-305OC. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer B, Hachenberg T, Wendt M, Marshall B. Mechanical ventilation with permissive hypercapnia increases intrapulmonary shunt in septic and nonseptic patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:285–289. doi: 10.1097/00003246-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero C, Lecuona E, Pesce L, Ridge KM, Sznajder JI. Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281:L79–L85. doi: 10.1152/ajplung.2001.281.1.L79. [DOI] [PubMed] [Google Scholar]
- 29.Pesce L, Guerrero C, Comellas A, Ridge KM, Sznajder JI. Beta-agonists regulate Na,K-ATPase via novel MAPK/ERK and rapamycin-sensitive pathways. FEBS Lett. 2000;486:310–314. doi: 10.1016/s0014-5793(00)02298-5. [DOI] [PubMed] [Google Scholar]
- 30.Witczak C, Sharoff C, Goodyear L. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65:3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahl A, Zhang M, Kuss H, Szelenyi I, Brune K. Regulation of IL-13 synthesis in human lymphocytes: implications for asthma therapy. Br J Pharmacol. 2002;135:1915–1926. doi: 10.1038/sj.bjp.0704656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Antiinflammatory effects of mitogen-activated protein kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004;172:7053–7059. doi: 10.4049/jimmunol.172.11.7053. [DOI] [PubMed] [Google Scholar]
- 33.Clemons AP, Holstein DM, Galli A, Saunders C. Cerulein-induced acute pancreatitis in the rat is significantly ameliorated by treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas. 2002;25:251–259. doi: 10.1097/00006676-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Schuh K, Pahl A. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochem Pharmacol. 2009;77:1827–1834. doi: 10.1016/j.bcp.2009.03.012. [DOI] [PubMed] [Google Scholar]


