Abstract
Congenital disorder of glycosylation type IIc (CDG IIc) is characterized by mental retardation, slowed growth and severe immunodeficiency, attributed to the lack of fucosylated glycoproteins. While impaired Notch signaling has been implicated in some aspects of CDG IIc pathogenesis, the molecular and cellular mechanisms remain poorly understood. We have identified a zebrafish mutant slytherin (srn), which harbors a missense point mutation in GDP-mannose 4,6 dehydratase (GMDS), the rate-limiting enzyme in protein fucosylation, including that of Notch. Here we report that some of the mechanisms underlying the neural phenotypes in srn and in CGD IIc are Notch-dependent, while others are Notch-independent. We show, for the first time in a vertebrate in vivo, that defects in protein fucosylation leads to defects in neuronal differentiation, maintenance, axon branching, and synapse formation. Srn is thus a useful and important vertebrate model for human CDG IIc that has provided new insights into the neural phenotypes that are hallmarks of the human disorder and has also highlighted the role of protein fucosylation in neural development.
Introduction
Congenital disorder of glycosylation, type IIc (CDG IIc), also known as leukocyte adhesion deficiency II (LAD II) or Rambam-Hasharon syndrome (RHS), is an autosomal recessive syndrome, characterized by recurrent infections, persistent leukocytosis, severe mental retardation and slowed growth [1], [2]. The immunodeficiency that is a hallmark of these syndromes is believed to be caused by dysregulated fucose metabolism, resulting in the absence of all fucosylated glycans on the cell surface [1], [2]. The gene responsible for CDG IIc has been identified as GDP-fucose transporter (FUCT1) [3], [4], which translocates GDP-fucose from the cytosol into the Golgi lumen for fucosyltransferase-catalyzed reactions during the modification of glycans.
Several animal models have been generated to study the pathogenesis of CDG IIc: FX locus null mice, lacking an enzyme in the de novo GDP-fucose synthesis pathway [5], Gfr (homologous to FUCT1) null flies [6] and Fuct1 null mice [7]. Gfr null flies display Notch-like phenotypes during wing development and reduced Notch fucosylation, suggesting that Notch deficiency may be responsible for some of the developmental defects in CDG IIc patients [6]. However, despite the neurodevelopmental and cognitive dysfunction prominent in CDG IIc patients, the anatomical, cellular and molecular abnormalities within the nervous system have not been well documented, and the mechanisms underlying this and other neural phenotypes remain unexplored.
A large body of literature has demonstrated an important role for Notch-Delta signaling in neuronal and glial specification, neuronal maturation and learning and memory [8]. Specifically, in zebrafish, Notch-Delta signaling has been shown to regulate neurogenesis and gliogenesis. For instance, deficiency of Notch1a as in deadly seven (des) mutants resulted in increased primary motor neuron and Mauthner neuron number [9]; deficiency of Delta A as in dla mutant caused excessive primary motor neurogenesis at the expense of secondary motor neurons, some ventral interneurons and oligodendrocytes [10], [11], [12]; mutation of Mind Bomb (an E3 ubiquitin ligase for Delta) as in mib resulted in a severe neurogenic phenotype together with the loss of oligodendrocytes [12], [13]. While some studies support the involvement of Notch signaling in the balance of excitatory/inhibitory synapses in hippocampus [14] and during synaptic plasticity [15], whether Notch-Delta signaling modulates synaptogenesis is unknown.
Here we report the genetic, cellular and molecular characterization of a zebrafish mutant slytherin (srn). Previously, we have identified srn as a synaptogenic mutant that exhibits abnormal swimming behavior, has increased primary motor neurons and aberrant neuromuscular synaptogenesis [16]. We have found that the srn mutation resides in GDP-mannose 4, 6-dehydratase (GMDS), the first and rate-limiting enzyme in the fucose metabolism pathway. Because dysfunction of the same pathway is responsible for human CDG IIc, we performed cellular and molecular analyses that suggest that srn has Notch-Delta dependent and independent defects, consistent with a general defect in protein fucosylation that affects several aspects of neural development.
Materials and Methods
Zebrafish maintenance and mutants
Zebrafish were raised and maintained under standard conditions. The srn allele was previously described [16]. The desb420 allele was obtained from Dr. Christine Beattie, Tg(hsp70l:GAL4) and Tg(UAS:myc-notch1a-intra) [17] from Dr. Bruce Appel, and dlahi781 and mibhi904 alleles from Zebrafish International Resource Center, University of Oregon.
Positional cloning of srn
Genetic mapping of mutant loci was performed as described [18]. New simple sequence repeat (SSR) markers DKEY-25E12-SSR2 (forward, 5′-gcacacatgcatacgttcag-3′; reverse, 5′-tcccaaagtgaaagggtgag-3′) and DKEY-177P2-SSR4 (forward, 5′-cctgagggtcaggagagtaatg-3′; reverse, 5′-gaactaacactttcacaaacaccaa-3′) were used to define the interval that contained the mutation. PCR products containing the entire ORF of gmds (accession # NM_200489) were generated with the primers 5′-cggatgtgtttgcatccgta-3′ and 5′-tcacatgaattaaacggcat-3′ for both mutant and WT (WT) cDNAs, cloned into pCR 4-TOPO (Invitrogen), and sequenced for validation.
RNA extraction and quantitative RT-PCR (qRT-PCR)
RNA was extracted (20 embryos) with the RNeasy kit (Qiagen, Inc.). hes5 was amplified with primers 5′-gaaagccagtggtggaaaag-3′ and 5′-gaaagccagtggtggaaaag-3′. her4 was amplified with primers 5′-cctggagatgacgcttgatt-3′ and 5′-cactgggcactgagacagaa-3′. heyl was amplified with primers 5′- gcgatacctcagctctttgg-3′ and 5′-ggagaggatccagctcactg-3′. β-actin1 was amplified with primers 5′-tgaatcccaaagccaacagagaga-3′ and 5′-tcacgaccagctagatccagacg-3′. qRT-PCR was performed with the SuperScript® III Platinum® SYBR® Green One-Step qPCR Kit w/ROX (Invitrogen) and data was analyzed with 7500 Real-Time PCR System software (Applied Biosystems) using the 2-ΔCT method.
Whole mount in situ hybridization
gmds cDNA was cloned into pBluescript (Stratagene). The plasmid was linearized and anti-sense and sense probes were made with the Dig RNA labeling kit SP/T7 (Roche). hes5 in situ probe was generated with primers 5′-tggctcctgcgtatatgactgaat-3′ and 5′-gcggctcctgcttgatgtgt-3′. her4 in situ probe was generated with primers 5′-tctgatcctgacggagaactg-3′and 5′-ttcagtccatgccaatctca-3′ [19]. heyl in situ probe was generated with primers 5′-tcaaccacagcctgtcagag-3′ and 5′-caggggaatgctgttgaagt-3′ [20]. In situ hybridization was performed as described previously [16].
GDP-fucose rescue and gmds mRNA and morpholino injection
GDP-fucose (50 mM in water (pH = 7) with 0.1% phenol red as a tracer) was injected directly into 1–2 cell stage embryos collected from crosses of srn carriers. Gmds-gfp mRNAs (WT and srn) were injected into embryos from WT and srn incrosses at the 1–2 cell stage at ∼200 pg. The morpholino antisense oligonucleotide (Gene Tools) targeting the gmds exon5-intron5 junction (CGTATGTTTGCTGACCATAAGGCGA) was injected at the 1–2 cell stage at ∼4 ng.
Expression of Notch1a by heat-shock induction and rescue of gmds morphant phenotypes
To induce expression of constitutively active Notch1a (Notch1a intracellular domain, NICD), embryos were collected from matings of heterozygous Tg(hsp70l:GAL4) and Tg(UAS:myc-notch1a-intra) adults and raised at 28.5°C. At 11 hpf, embryos were heat-shocked at 39°C for 30 minutes and then returned to 28.5°C until the desired stage of development [21]. To determine whether NICD rescues srn phenotypes, gmds MO was injected into NICD transgenic embryos and the phenotypes were compared to NICD transgenic embryos alone, WT, srn and gmds MO embryos.
DAPT treatment
Embryos were dechorionated with forceps at 6 hpf and placed in DAPT (N-[N-(3,5-Difluorophenacetyl-l-alanyl]-S-phenylglycine-t-butyl ester; Calbiochem) solution at 28.5°C until the appropriate stage, as previously described [22]. For experiments, 50 µM (medium dose) and 100 µM (high dose) DAPT in embryo medium containing 1% DMSO was used. Control embryos were incubated in an equivalent concentration (1%) of DMSO.
Immunostaining, AAL staining and labeling of retinotectal projections
Embryos were anesthetized, fixed and immunostained as described previously [16] using antibodies against SV2, Zn5, 3A10, Islet1/2, F59 (all from Developmental Studies Hybridoma Bank, Univ. of Iowa) and/or goldfish GFAP [23] (gift from Drs. S. Nona and J. Scholes, Univ. of Sussex, United Kingdom) and fluorescently conjugated secondary antibodies (Jackson Labs, Inc.). Fluorescently conjugated α-bungarotoxin (Molecular Probes, Inc.) was used to label AChRs [16]. TUNEL staining was performed according to the manufacturer's instructions (Chemicon, Inc.). Fucosylated proteins were visualized in 48 hpf embryos using a biotinylated fucose-specific lectin, Aleuria Aurantia lectin (AAL; 20 µg/ml; Vector Labs; [24], [25] followed by Alexa 488 conjugated strepavidin (20 µg/mL; Molecular Probes). The number of Zn5+ cells was counted at 20 µm intervals along the rostral-caudal axis of several spinal cord hemisegments and compared statistically using Kolmogorov-Smirnov test. Retinal ganglion cell axon projections to the optic tectum were labeled as described [26].
Unless otherwise stated, each immunostaining or dye labeled figure panel is a single plane projection of a confocal z-stack of 20–160 1 µm thick planes (Leica TCS 4D). Presynaptic vesicles, AChR clusters and the co-localization of these two markers were measured from using interactive software (Metamorph).
Results
External phenotype, genetic cloning and mRNA rescue of slytherin
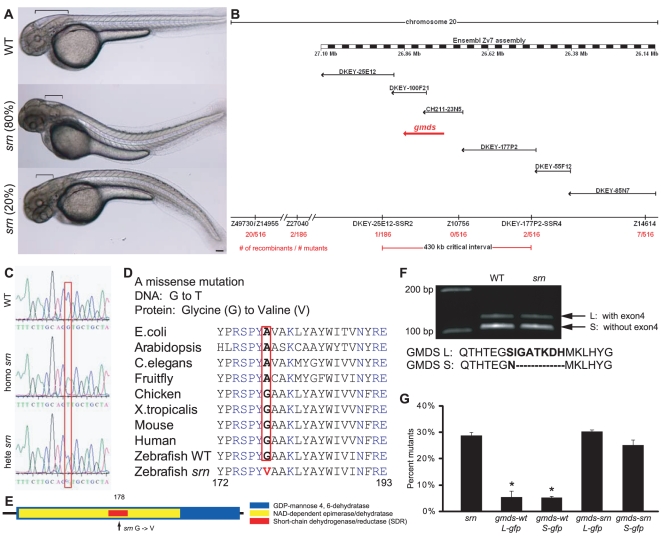
Externally, srn mutants exhibit a bent tail as early as 24 hpf, a phenotype that becomes progressively more severe (Fig. 1A), as well as a malformation of the hindbrain, which becomes apparent at 48 hpf (Fig. 1A, brackets). The srn locus was mapped between SSLP markers z49730/z14955 and z14614 on chromosome 20, with marker z10756 having no recombinants (Fig. 1B). Gmds was found to contain a G to T transversion in the nucleotide sequence that produces a nonconservative glycine (G) to valine (V) substitution of amino acid 178 (G178V) in the short-chain dehydrogenase/reductase (SDR) domain (Fig. 1C, D, E). GMDS is highly conserved at the amino acid level; the fish and human proteins are 87% identical.
Figure 1. Slytherin external phenotype, genotype, cloning and mRNA rescue of srn mutants.
A. External srn phenotypes at 48 hpf include a bent tail (80% dorsal (534 embryos, 8 carrier pairs)) and aberrant hindbrain formation (brackets). Scale bar = 100 µm. B. Genetic and physical map of the srn locus (red arrow), including SSLP markers, number of recombinants, BAC clones and megabase positions from Ensembl Zv7. C, D. In srn, Gmds mutation is G to T (C, red box) resulting in a Glycine to Valine conversion (D, red box). GMDS amino acid sequence is highly conserved. E. Schematic of srn mutation in the short-chain dehydrogenase/reductase (SDR) domain of GMDS. F. Two splice variants exist in gmds mRNA, with (gmds-L, 377 aa) or without (gmds-S, 370 aa) exon 4. Gmds alternative splicing is not altered in srn mutants. G. Injection of gmds mRNA rescues srn mutants. Compared to uninjected embryos, 28.6±1.2% of embryos were mutant when scored by external phenotypes (3413 embryos, 27 carrier pairs). In embryos injected with WT gmds-gfp mRNA, the percentage of mutants scored by external phenotypes was significantly decreased, to ca. 5% (gmds-wtL-gfp 5.4±2.5%, 401 embryos, 3 carrier pairs; gmds-wtS-gfp 5.1±0.6%, 587 embryos, 4 carrier pairs; one-way ANOVA, followed by Dunn's pairwise comparison, p<0.05). The percentage of embryos with mutant external phenotypes was unchanged in embryos injected with mutant gmds-gfp mRNA (gmds-srnL-gfp 30.2±0.9%, 387 embryos, 3 carrier pairs; gmds-wtS-gfp 25.1±1.9%, 516 embryos, 4 carrier pairs). This mRNA rescue experiment confirms that gmds is the gene responsible for srn mutation.
In situ hybridization showed that from 6 to 12 hpf, gmds transcripts are expressed throughout the embryo (Fig. S1A). By 24 hpf, gmds transcripts are enriched in the CNS and are also present in somites (Fig. S1B). Gmds mRNA expression is present in the CNS at 48 and 72 hpf, with transcripts more abundant in brain than spinal cord (Fig. S1C, D). Gmds mRNA is also expressed in the PNS at 72 hpf, including in lateral line neuromasts (data not shown; [27]).
RT-PCR analyses suggested that at least two splice variants exist in zebrafish gmds, with or without exon 4, which we name gmds-L and gmds-S respectively. Both splice variants are expressed in srn mutants and WT embryos (Fig. 1F). To confirm that gmds is the gene responsible for srn phenotypes, both splice variants of the WT and mutant gmds cDNAs were fused with gfp and were in vitro transcribed into mRNA and were injected into 1–2 cell stage embryos collected from srn incrosses. In embryos injected with WT gmds-gfp mRNAs, 5% were mutant scored by external phenotypes compared to uninjected embryos (29%) or embryos injected with mutant gmds-gfp mRNAs (Fig. 1G; one-way ANOVA, followed by Dunn's pairwise comparison, p<0.05). Moreover, when GMDS function was perturbed in WT embryos with a splice-blocking morpholino, all defects seen in srn mutants were phenocopied (see below and Fig. S3). These experiments confirm that gmds is the gene mutated in srn.
Slytherin mutants exhibit reduced protein fucosylation
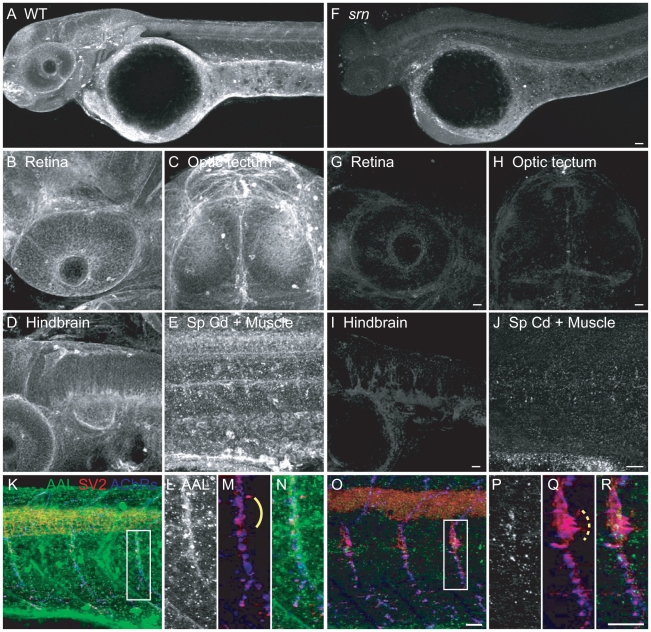
GMDS is the first enzyme in the de novo fucose metabolism pathway, catalyzing the conversion of GDP-D-mannose to GDP-4-keto-6-D-deoxymannose, which is further processed into GDP-fucose [5] and transported into the Golgi where it is used to fucosylate proteins, including Selectins, Notch and many others [5], [28], [29]. Thus AAL staining for fucosylated proteins was performed in 48 hpf WT and mutant embryos (Fig. 2).
Figure 2. slytherin mutants exhibit reduced protein fucosylation as measured by AAL staining.
A. AAL staining of WT embryos at 48 hpf showed that protein fucosylation is present throughout the embryo (10–15 embryos/2–3 adult pairs for all analyses). B–E. Protein fucosylation is prominent in several neural tissues including retina (lateral view), optic tectum (dorsal view), hindbrain (lateral view), spinal cord (lateral view) and neuromuscular synapses (lateral view of axial muscle). F. Protein fucosylation is dramatically reduced in srn mutants. Scale bar = 20 µm. G–J. Reduced protein fucosylation in several neural tissues. Scale bar = 20 µm. K. Protein fucosylation at neuromuscular synapses in WT embryos at 48 hpf, as shown by the colocalization of AAL staining (green) with markers for presynaptic axons and nerve terminals (SV2, red) and postsynaptic AChR clusters (α-bungarotoxin, blue). L–N. Higher magnification of boxed region in K. O. Protein fucosylation is reduced at srn neuromuscular synapses. Scale bar = 20 µm. P–R. Higher magnification of boxed region in O. Synapse area is significantly increased in srn mutants, e.g., at the choice point (compare dashed bracket in Q to solid bracket in M). Scale bar = 20 µm.
In WT embryos, AAL staining was detected in many tissues (Fig. 2A), including olfactory bulb, retina, optic tectum, hindbrain and spinal cord (Fig. 2B–E), which prompted us to examine the potential phenotypes in these structures in srn. Moreover, at neuromuscular junctions (NMJ), AAL staining co-localizes with markers for pre- and postsynaptic specializations, such as SV2 and acetylcholine receptors (AChRs) (Fig. 2K–N). In contrast, AAL staining is strongly reduced in srn mutants (Fig. 2F–J, O–R), consistent with analyses of cells from CDG IIc patients [3], [4], and of Drosophila Gfr mutants [6]. These studies show that protein fucosylation is dramatically reduced in the CNS and other tissues in srn, consistent with a loss of function of GMDS, confirming a prediction based on the modeling of the protein crystal structure (see Fig. S2).
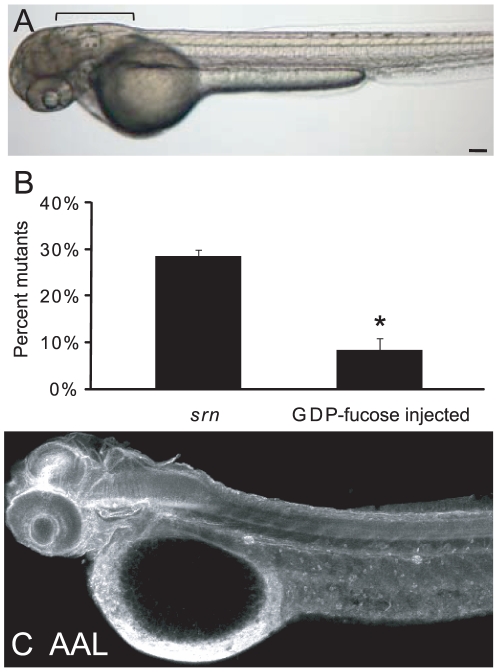
Supplementation with GDP-fucose rescues slytherin phenotypes
Since GMDS functions early in the fucose metabolism pathway, we reasoned that exogenous supply of downstream products may circumvent the genetic defect in srn. Therefore, 50 mM GDP-fucose was injected into 1–2 cell stage embryos collected from srn incrosses. Compared to uninjected embryos, the percentage of mutant embryos, as scored by external phenotypes (Fig. 3A), was dramatically reduced in GDP-fucose injected embryos (Fig. 3B). Moreover, AAL staining was similar to that in WT embryos at 48 hpf in many if not all tissues (Fig. 3C). Detailed phenotypic analyses further showed that GDP-fucose supplementation is sufficient to rescue neural defects in srn mutants (see below and Fig. S3). These strongly suggest that the absence of GDP-fucose, as a result of GMDS dysfunction, is the cause of the srn mutant phenotypes, rather than the accumulation of the substrate, GDP-mannose. Thus srn mutants display dysregulated protein fucosylation, as is seen in human CDG IIc patients, and that GDP-fucose supplementation restores fucosylation and rescues defects in srn.
Figure 3. Supplementation with exogenous GDP-fucose rescues srn external phenotypes and restores AAL staining.
A. External srn phenotypes including bent tail and aberrant hindbrain (bracket) are rescued by GDP-fucose supplementation (3 embryos). Scale bar = 100 µm. B. GDP-fucose injection significantly reduced the percentage of mutants from 28.6±1.2% to 8.4±2.7% (576 embryos, 4 carrier pairs; Student's test, p<0.0001). C. After GDP-fucose supplementation (2 embryos), protein fucosylation as assessed by AAL staining at 48 hpf is rescued throughout srn embryos, to levels similar to those in WT embryos. Scale bar = 100 µm.
Slytherin mutants exhibit defects in neuron and glia number, identity, patterning and axon outgrowth due to Notch-Delta signaling reduction
Our previous work suggested that srn exhibited a neurogenic phenotype, specifically an increased number of primary motor neurons [16], similar to that observed in mutants in the Notch-Delta pathway. Analyses of Drosophila Gfr mutants suggested that Notch fucosylation is reduced, and that a reduction in Notch signaling might contribute to the pathogenesis in CDG IIc [6]. Therefore, we asked which if any neural defects in srn were similar to those observed in mutants in the Notch-Delta pathway or in embryos treated with the γ-secretase inhibitor DAPT to reduce Notch signaling.
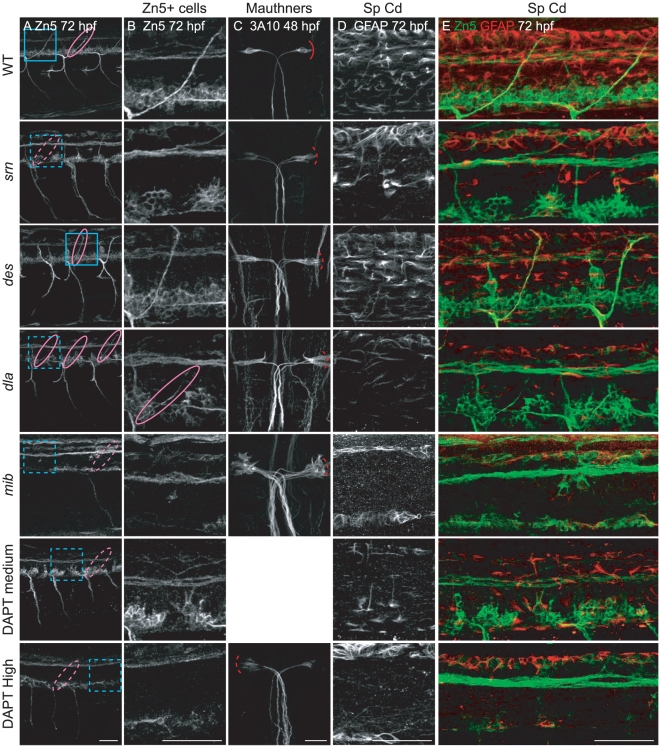
We compared srn phenotypes with known mutants in the Notch-Delta pathway, desb420 (deadly seven, a nonsense mutation in notch1a yielding a truncated protein; Gray et al., 2007), dlahi781 (delta A, an insertion in delta A, predicted to result in a truncated protein [30]) and mibhi904 (mind bomb, an insertion in an E3 ligase that targets Delta and other proteins for ubiquitination [13], predicted to result in a truncated protein [31]). Below we describe phenotypes in each mutant in order of increasing disruption of Notch-Delta signaling.
First, we examined secondary motor neuron cell body number and patterning in the spinal cord, and axon projections in muscle using Zn5 immunostaining. In srn mutants at 48 hpf and 72 hpf, while the number of Zn5+ cells is similar between srn mutant and WT embryos (Fig. S4), the patterning of these cells is aberrant. Cell bodies are clumped in srn mutants (Fig. 4A, B, second panel, dashed blue bracket), compared to evenly spaced cell bodies in WT embryos (Fig. 4A, B, top, solid blue bracket). The dorsally projecting nerve also is absent in srn mutants (Fig. 4A, B, second panel, dashed pink oval), consistent with increased Zn5+ cell death [16]. des mutants do not have defects in Zn5+ cell number or patterning, but do have motor axon pathfinding errors, possibly due to aberrant formation of somite boundary (Fig. 4A; [9]). dla mutants do not have defects in Zn5+ cell number, but have similar aberrant patterning as in srn mutants, without the loss of the dorsal projecting nerve (Fig. 4A, B, dashed blue bracket and solid pink oval, respectively). mib mutants have aberrant Zn5+ cell number and patterning that is apparent at 48 and 72 hpf, as well as loss of the dorsal nerve.
Figure 4. Reduction in Notch-Delta signaling accounts for some srn phenotypes.
A, B. Secondary motor neuron cell body number and patterning assayed with Zn5 immunostaining (18 embryos/3 carrier pairs for each). B. Higher magnification of boxed region in A. At 48–72 hpf, Zn5+ cell number is similar in srn and WT (Fig. S4), but the patterning of these cells is aberrant in srn embryos. Zn5+ cells are clumped in srn mutants (dashed blue bracket) compared to WT embryos (solid blue bracket). dla mutants do not have defects in Zn5+ cell number (Fig. S4), but have aberrant Zn5+ cell patterning as in srn mutants (dashed blue bracket). mib mutants and high dose DAPT treated embryos have aberrant Zn5+ cell number (Fig. S4) and patterning (dashed blue bracket). Medium dose DAPT treated embryos show aberrant Zn5+ cell patterning defects (dashed blue bracket), without an obvious change in cell number (Fig. S4), as in srn. The dorsal projecting nerve is absent in srn mutants (dashed pink oval) compared to WT (solid pink oval), consistent with increased cell death; this nerve is present in dla and des mutants (solid pink oval); des also has other motor axon pathfinding errors. In mib mutants and high and medium dose DAPT treated embryos, the dorsal projecting nerve is absent (dashed pink oval). C. In WT embryos at 48 hpf, two Mauthner neurons are present (dorsal view of hindbrain). In srn, des, dla, mib and high dose DAPT treated embryos, Mauthner neuron number is increased (dashed red brackets), with the largest increase observed in mib (12 embryos, 3 carrier pairs for each). D. In the spinal cord, the number of GFAP+ glial cells is reduced in srn and dla mutants and medium dose DAPT treated embryos compared to WT and des embryos at 48–72 hpf. In mib and high dose DAPT treated embryos, a more dramatic reduction is observed. The GFAP labeling that remains in mib mutants is likely to be in Rohon-Beard neurons dorsally and secondary motor neurons ventrally and is easily separated morphologically and based on its location from glial processes, thus does not interfere with analyses of glial defects (18 embryos, 3 carrier pairs for each). E. Overlay showing both the Zn5 and GFAP staining in the spinal cord. Scale bars = 40 µm.
To analyze the Zn5+ cell patterning defects quantitatively, we counted the number of Zn5+ cells at every 20 µm interval along the rostral-caudal axis of several spinal cord hemisegments. This analysis showed that, while there are 3–5 Zn5+ cells every 20 µm in WT and des mutants, there are 1–9 in srn and dla, and 0–3 in mib, confirming our visual impression that patterning is aberrant (Fig. S5E–F). Moreover, while Islet1/2+ cells are dramatically increased in srn mutants at 24 hpf, consistent with increased primary motor neurons (Panzer et al., 2005), these cells are decreased at 48 hpf and the majority of Zn5+ cells lack Islet1/2 expression in srn mutants (Fig. S5). As Zn5 is expressed in secondary motor neurons and is colocalized with Islet1/2 in wild type embryos, and that Islet1/2 is reduced in Zn5+ cells in srn, our results suggest the patterning defects in Zn5+ cells may be correlated with the aberrant Islet1/2 expression. There may be a defect in secondary motor neuron specification in srn, consistent with a role for Islet1 and Islet2 in secondary motor neuron formation and axonogenesis [32].
We also found that in the spinal cord, the number of Rohon-Beard neurons is also significantly increased in srn mutants at 24 and 48 hpf (Fig. S5A–B), similar to dla mutants [33], consistent with reduced Notch-Delta signaling in srn mutants.
In the hindbrain and retina, similar defects in neuron number and patterning are present. In the hindbrain at 48 hpf, an increase in Mauthner neurons is observed in srn, des (as previously reported, Gray et al., 2001), dla and mib, with the largest increase in Mauthner neuron number observed in mib (Fig. 4C, red brackets). Moreover, neuronal patterning in the hindbrain is severely perturbed in srn and in mib (data not shown). In the retina at 72 hpf, cell number and patterning appear grossly normal in srn, des and dla, but in mib, retinal ganglion cell number is reduced (Fig. S6A), probably due to increased cell death, as previously reported [34]. These data suggest that reduced Notch-Delta signaling may account for some of the CNS and PNS phenotypes observed in srn.
Because deficiencies in Notch-Delta signaling have been shown to result in reduced gliogenesis [10], [11], [12], [13], we examined glial cells in the spinal cord, hindbrain and retina with GFAP immunostaining. In the spinal cord and hindbrain, the number of GFAP+ glial cells is reduced in srn mutants compared to WT embryos at 48–72 hpf (Fig. 4D, 4E and data not shown). A similar reduction in GFAP+ glial cells is also observed in dla and mib, but not in des (Fig. 4D, 4E and data not shown). In the retina, the number of radially oriented GFAP+ Muller cells is decreased in srn and mib, but not in des or dla (Fig. S6B). These results suggest that a reduction in Notch-Delta signaling may account for the reduction in glia observed in srn mutants.
We then compared srn phenotypes with those caused by Notch signaling inhibitor DAPT, a γ-secretase inhibitor, that prevents intramembrane proteolysis of Notch and thus decreases the downstream signaling dependent on the Notch intracellular domain [22]. While high dose of DAPT treatment resulted in phenotypes resembling those seen in mib (Fig. 4 and Fig. S6), medium dose DAPT treatment closely recapitulated srn phenotypes, including the Zn5+ cell patterning defects and the reduction of GFAP+ glial cells in the spinal cord and retina (Fig. 4; Figs. S5, Fig. S6). These results substantiate the conclusion that a reduction in Notch-Delta signaling may account for the observed neural defects in srn mutants.
In order to test the synergy between srn and Notch-Delta deficiency, we initially sought to examine embryos double heterozygous for srn and mib, but these embryos did not show any obvious defects, likely because both single heterozygous embryos are haploid sufficient. We also examined embryos double homozygous for srn and mib, reasoning since Notch signaling is mostly if not completely absent in mib [13], if srn defects are also caused by Notch signaling deficiency, introducing srn into mib background would not result in addictive effects, i.e. would not be more severe then mib. Indeed, srn and mib double mutants showed reduced Zn5+ cells and GFAP+ glial cells in the spinal cord, closely resembling those seen in mib (Fig. 5). Furthermore, using the same reasoning, we tested the synergy between srn and DAPT treatment. Similarly, in DAPT high dose treated embryos, in which Notch signaling is mostly if not completely blocked, srn did not add to the defects caused by DAPT alone, i.e. DAPT treated srn mutants resembled DAPT treated WT embryos showing similar reduced Zn5+ cells and GFAP+ glial cells in the spinal cord (Fig. 5). These results are consistent with the hypothesis that Notch signaling deficiency underlies the neurogenesis and gliogenesis defects in srn.
Figure 5. mib and DAPT treatment exclude srn phenotypes.
A–C. mib excludes srn phenotypes. A. AAL staining is reduced in srn and srn + mib double mutants, but not in mib. B. srn + mib double mutants showed reduction of secondary motor neurons, more severe than srn but similar to mib alone. C. srn + mib double mutants have reduced GFAP+ glia, more severe than srn, but similar to mib alone (15 embryos, 2 carrier pairs for each). Scale bar = 40 µm. D–F. DAPT treatment excludes srn phenotypes. D. AAL staining is reduced in srn and srn mutants treated with DAPT, but not in DAPT treated embryos. E. srn mutants treated with DAPT showed reduction of secondary motor neurons, more severe than srn but similar to DAPT treated embryos. F. srn mutants treated with DAPT showed reduction of GFAP+ glia, more severe than srn, but similar to DAPT treated WT embryos (10 embryos, 2 carrier pairs for each). Scale bar = 40 µm.
If the observed neural defects in srn results from reduced Notch signaling, then overexpressing constitutively active Notch would rescue these phenotypes. We utilized transgenic lines in which a constitutively active form of Notch, Notch1a intracellular domain (NICD) is overexpressed under the heat-shock promoter (Tg(hsp70l:GAL4); Tg(UAS:myc-notch1a-intra)) [17], recapitulated srn phenotypes in these embryos by morpholino knockdown of gmds transcripts, and examined whether NICD rescued the neural defects. Indeed, NICD overexpression rescued the Zn5+ cell patterning and reduced GFAP+ glial cells phenotypes in gmds morphants (Fig. 6). Moreover, NICD overexpression suppressed the increased mauthner neuron phenotype in gmds morphants (Fig. S7). These results strongly suggest that Notch signaling deficiency underlies the neurogenesis and gliogenesis defects in srn.
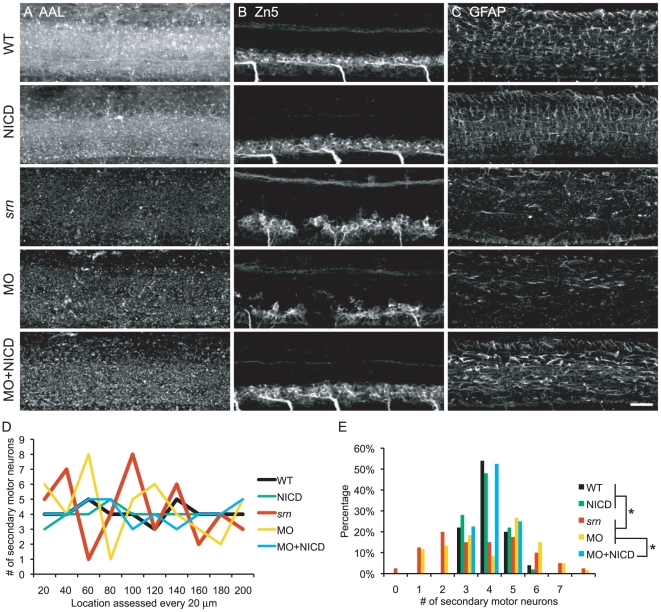
Figure 6. NICD rescues srn neuro- and gliogenesis phenotypes.
A. AAL staining is reduced in srn, gmds morphants and gmds morphants overexpressing NICD, but not in WT embryos or WT embryos overexpressing NICD. B. WT and WT overexpressing NICD had normal Zn5+ cell patterning. srn and gmds MO showed Zn5+ cell patterning defects which was rescued by NICD overexpression in gmds morphants. C. WT and WT overexpressing NICD had normal GFAP+ glia cells in spinal cord. srn and gmds morphants had reduced GFAP+ glia cells, rescued by NICD overexpression in gmds morphants (>10 embryos in each experiment). Scale bar = 40 µm. D–E. Quantification of Zn5+ cell patterning defects. There are 3–5 Zn5+ cells every 20 µm in WT, WT overexpressing NICD and gmds MO overexpressing NICD; compared to 1–8 in srn and gmds MO embryos. D, data from a representative embryo; E, distribution of all embryos (4–6 embryos; Kolmogorov-Smirnov test, * p<0.05).
To further assess whether Notch-Delta signaling is deficient in srn mutants, we examined the expression of several Notch effector genes, including hes5, her4 and heyl as direct readout of Notch transcriptional activation, using real time quantitative RT-PCR and in situ hybridization. mib embryos display a strong reduction in Notch signaling [13] and hes5, her4 and heyl were collectively shown to be reduced in mib mutant fish and/or mice [35], [36], [37], [38], [39]. We found that, at 48 hpf, hes5, her4 and heyl expression were significantly reduced in srn mutants, similar as in mib mutants, although to a lesser extent (Fig. 7A, B). Because these data show that defects in neuron and glia number, patterning and Notch effector genes expression in srn mutants are similar to those observed in mutants in the Notch-Delta pathway, a reduction in Notch-Delta signaling caused by the lack of fucosylation accounts for these srn phenotypes.
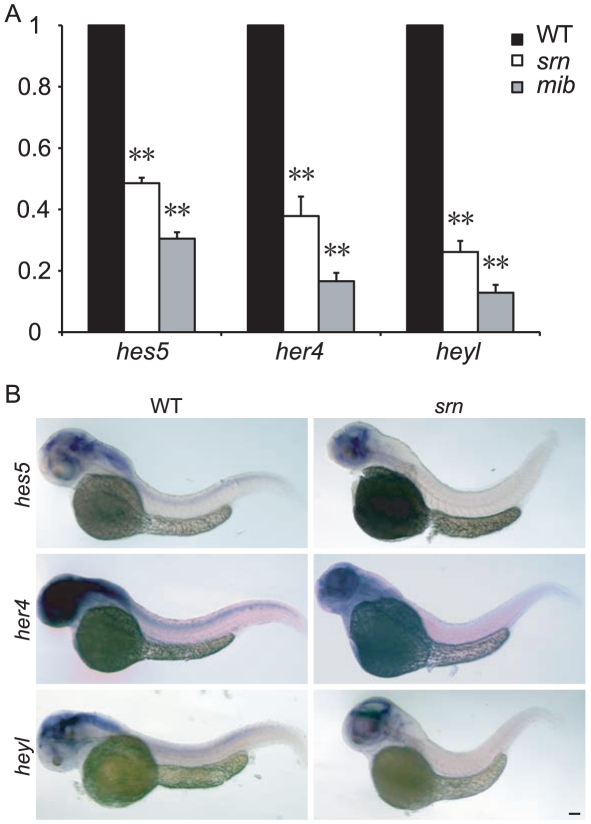
Figure 7. srn mutants showed aberrant expression of Notch responsive genes similar to mib mutants.
A. qRT-PCR assessment of fold change in hes5, her4 and heyl expression in WT, srn and mib mutant embryos at 48 hpf, normalized to β-actin1. hes5, her4 and heyl expression is dramatically reduced in srn, similar to those in mib, but to a lesser extent. (3–5 experiments, 20 embryos each, one-way ANOVA, Bonferroni's Multiple Comparison Test, ** p<0.001, * p<0.5). B. hes5, her4 and heyl in situ hybridization at 48 hpf confirm reduced expression in the brain and spinal cord in srn mutants compared to WT (>30 embryos for each). Scale bar = 100 µm.
Slytherin mutants exhibit defects in neuromuscular synaptogenesis due to Notch-Delta signaling reduction
Because srn was first identified in a screen for mutants with defects in neuromuscular synaptogenesis, we assessed the role of protein fucosylation and Notch-Delta signaling in neuromuscular synapse formation, particularly at the choice point where the first neuromuscular synapses are made [16]. Choice point neuromuscular synapse size was increased at 24 hpf in srn, des, dla, mib and DAPT treated embryos (Fig. 8). At 48 hpf, mib and DAPT treated embryos showed no enlargement of choice point neuromuscular synapses, likely due to a reduced number of secondary motor neurons (Fig. S4). These defects are not due to defects in muscle fiber integrity or number (Fig. S8 and [16]). These results show that dysregulated protein fucosylation in srn mutants resulted in an aberrant neuromuscular synaptogenesis that was phenocopied in Notch-Delta signaling deficient embryos, suggesting that Notch-Delta signaling plays an important and previously unappreciated role in neuromuscular synapse formation.
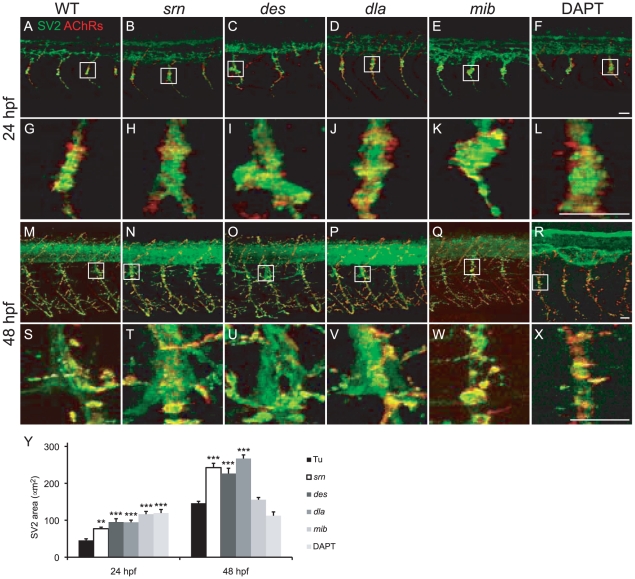
Figure 8. Slytherin mutants exhibit defects in neuromuscular synaptogenesis due in part to reduction in Notch-Delta signaling.
A–X. Presynaptic terminals (green) and postsynaptic AChR clusters (red) in 24 and 48 embryos from WT (A, G, M, S), srn (B, H, N, T), des (C, I, O, U), dla (D, J, P, V), mib (E, K, Q, W) and DAPT treated embryos (F, L, R, X). Boxed regions are shown at higher magnification at 24 (G–L) and 48 hpf (S–X; 3 hemisegments in each of 20 embryos, 3 carrier pairs for each). Scale bar = 20 µm. Y. Presynaptic terminal, axon and synapse area at the choice point was significantly increased in all mutants, except in mib and DAPT treated embryos at 48 hpf, compared to WT (one-way ANOVA, Bonferroni's Multiple Comparison Test, ** p<0.01, *** p<0.001).
Slytherin mutants exhibit defects in CNS axon branching and synaptic connectivity that are independent of Notch-Delta signaling
Phenotypic analyses showed that srn has several defects that are not present in mutants in the Notch-Delta pathway des, dla or mib, or DAPT treated embryos. In the retina, while overall cellular lamination is grossly normal in srn mutants (Fig. 9E, bottom left panel), neuropil in the outer and inner plexiform layers (OPL and IPL) are dramatically altered (Fig. 9A, arrowheads and arrows). In srn mutants at 48–72 hpf, the OPL and IPL synaptic layers are disorganized, and this is not seen in des, dla or medium dose DAPT treated embryos (Fig. 9A). In mib and high dose DAPT treated embryos, retinal ganglion and other cells die, resulting in a reduction in synapses throughout the retina (Fig. 9A). Thus srn displayed unique defects in CNS synaptic connectivity that are not phenocopied by Notch signaling deficient embryos. These data are consistent with one of two possibilities. First, fucosylation of proteins other than those involved in Notch-Delta signaling may be required to shape CNS synaptic connectivity. Alternatively, Notch-Delta signaling may contribute, in a specific spatio-temporal context, to these defects. Resolution of these possibilities will require identification of protein targets of srn-mediated fucosylation and exploration of their role in CNS synaptic connectivity, and/or analyses of mutants with more precise spatial and temporal disruption of Notch-Delta signaling than are currently available.
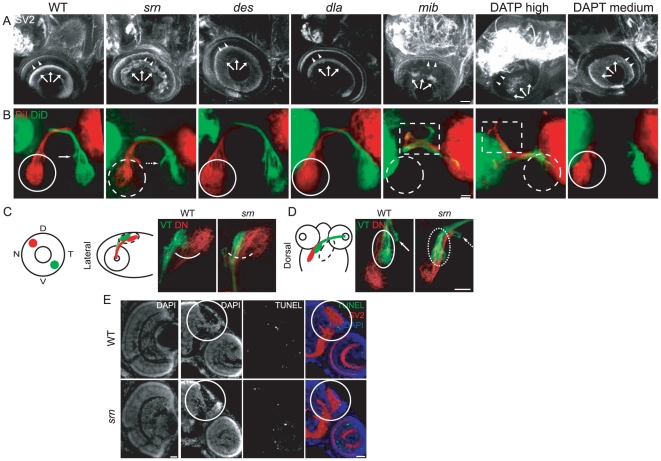
Figure 9. Slytherin mutants exhibit defects in axon branching and CNS synaptic connectivity that are independent of Notch-Delta signaling.
A. In srn mutants at 72 hpf, the OPL (arrowheads) and IPL (arrows) are disorganized; this is not seen in des or dla mutants. In mib mutants, retinal ganglion and other cells die, resulting in decreased retina neuropil (rightmost panel; 8 embryos, 2 carrier pairs for each). Scale bar = 20 µm. B. In srn mutants, retinal ganglion cell axons grow out to the optic chiasm and to optic tectum, but axon branches are aberrantly distributed within tectum (dashed white circle) and medial axon projections are shifted towards the midline (compare solid arrow and dashed arrow). Virtually all retina was dye labeled, and the labeling pattern was consistent across experiments, thus these defecs aren't due to incomplete dye uptake or labeling in srn mutants. These phenotypes are not present in des or dla mutants, and are also different from mib mutants, in which retinal ganglion cell axonal projections to optic tectum are dramatically reduced, as a consequence of retinal ganglion cell death. Mib mutants also displayed axon pathfinding errors at the optic chiasm; axons branched anterior to the optic chiasm (dashed square), while branching within tectum was dramatically reduced (dashed white circle; 15 embryos, 3 carrier pairs for each). Scale bar = 20 µm. C, D. Topographic mapping of axon projections to optic tectum; dorsonasal (DN) and ventrotemporal (VT) axons were with DiI or DiD. DN and VT axon projections within tectum are aberrant in srn mutants, as is overlap dorsally (C) and laterally (D; 8 embryos, 2 carrier pairs for each). Scale bar = 20 µm. E. In retina and optic tectum (white circle), the overall cellular lamination pattern as assessed by DAPI staining is grossly normal in srn mutants (compare bottom left panels, WT and srn). TUNEL staining showed that increased cell death was observed in the retina of srn mutants compared to WT embryos at 72 hpf; no difference in cell death in the optic tectum was observed in srn mutants compared to WT embryos at 72 hpf (color overlay, right most panels; 2–3 embryos, 1 carrier pair). Scale bar = 20 µm.
Given that AAL staining showed high levels of protein fucosylation in optic tectum (Fig. 2), we examined whether retinal ganglion cell axon outgrowth to and branching within the optic tectum was affected in srn and other mutants. In srn mutants, retinal ganglion axons grow to the correct location (Fig. 9B), but their axons are aberrantly branched within the optic tectum (Fig. 9B, dashed white circle) and medial axon projections are shifted towards the midline (Fig. 9B, compare solid arrow and dashed arrow). These phenotypes are not present in des, dla or medium dose DAPT treated embryos (Fig. 9B). In mib and high dose DAPT treated embryos, the retinal ganglion cell axon projection to optic tectum is dramatically reduced due to retinal ganglion cell death (Fig. 9B). Mib and high dose DAPT treated embryos also displayed retinal ganglion axon pathfinding errors at the optic chiasm (Fig. 9B, dashed square) and decreased branching within the optic tectum (Fig. 9B, dashed white circle). Furthermore, topographic mapping analyses, in which the dorsonasal (DN) and ventrotemporal (VT) retinal ganglion cell projections were differentially labeled (Fig. 9C, D) showed that, in srn mutants, the location of the DN and VT axon projections in the optic tectum is aberrant, and that these projections overlap aberrantly dorsally and laterally (Fig. 9D). Moreover, the cellular lamination and cell viability in the optic tectum was similar between srn and WT embryos at 72 hpf (Fig. 9E, middle left panels). These results suggest that signaling independent of the Notch-Delta pathway, but requiring protein fucosylation, modulates axon branching and synaptic patterning in the CNS.
Discussion
We report that the srn mutation causes a loss of GMDS function, leading to a severe reduction in protein fucosylation, including that of Notch among many others. Srn displays increased neurogenesis, decreased gliogenesis, increased neuronal cell death, abnormal neuronal patterning, abnormal axon arborization, and abnormal neuromuscular and CNS synaptic connectivity, indicating that protein fucosylation plays an important role in several aspects of neural development.
Notch-Delta signaling reduction underlies some but not all srn neural phenotypes
Our results suggest that both Notch-dependent and -independent mechanisms contribute to the neural phenotypes observed in srn. Srn mutants showed reduced Notch transcriptional activity, as assayed by hes5, her4 and heyl expression, increased primary motor neuron, Rohon-Beard neuron and Mauthner neuron number, decreased gliogenesis and abnormal neural patterning. These defects are phenocopied by mutants in the Notch-Delta pathway and in embryos with reduced Notch signaling. That mib and Notch signaling inhibition by DAPT occlude srn defects, and that NICD overexpression rescues these srn phenotypes, strongly suggest that the dysregulated fucosylation of proteins in the Notch-Delta pathway accounts for these prominent neural defects in srn mutants. While the lack of anti-zebrafish Notch antibodies prevented direct analysis of Notch fucosylation, Notch is known to be fucosylated, and other proteins in the Notch-Delta pathway, including Delta, Serrate and Jagged, contain consensus sequence(s) for O-linked fucose modification [40], [41], [42]. Notch is also N-fucosylated, in which fucose is added to N-linked glycan side chains [6], [43], [44]. Notch O- and N-fucosylation has been shown to be reduced in the Drosophila Gfr null [6]. It thus seems highly likely that the fucosylation of proteins in the Notch-Delta pathway is aberrant in srn mutants and that this accounts for some, but not all, srn neural phenotypes.
Interestingly, there is a hierarchy in the spectrum of phenotypes among srn and mutant in the Notch-Delta pathway. Phenotypes in des, except for the axon pathfinding errors, are weaker than those in dla, and both of these are weaker than srn. This is consistent with the hypothesis that many Notch-Delta factors, including Notch, Delta, Serrate and Jagged, require proper protein fucosylation and compromised fucosylation of these proteins may account for the wider spectrum of defects seen in srn. Mib mutants also displayed a wide range of defects, not seen in the other three mutants, both due to the fact that mib regulates a large spectrum of Notch signaling, as it interacts with various Notch ligands and is broadly required for Notch signaling in many tissues, and also it interacts with a number of proteins besides Delta and may serve as an integrator of multiple neuronal developmental pathways [45].
Moreover, our observation that srn and mutants in the Notch-Delta pathway have increased neuromuscular synapses supports a previously underappreciated role for Notch-Delta signaling during synaptogenesis. Because primary motor neuron number is increased in srn, it is difficult to separate direct effects of Notch-Delta signaling on presynaptic differentiation from indirect effects on neurogenesis. The total number of motor neurons innervating trunk muscles actually decreases due to secondary motor neuron cell death [16], while the increase in neuromuscular synapse number and size persists. This strongly suggests that Notch-Delta signaling plays a role in synaptogenesis, independent of its role in neurogenesis.
Recent work has shown that reduced protein fucosylation, as a result of gmds mutation in twohead (twd) mutants, results in defects in the migration of vagus motor neuron progenitors [46]. However, they argued that Notch signaling is unaltered, based on several lines of evidence. First, they concluded from semi-quantitative RT-PCR analyses, that expression of her4, a downstream effector in the Notch pathway, was unchanged, but their data suggests that her4 expression may indeed be decreased. On the other hand, we show using quantitative RT-PCR in our Fig. 7 that her4 is reduced in srn mutants. Second, Ohata et al. analyzed motor neuron number and patterning by in situ for islet1 and islet2 and concluded that motor neuron number and patterning are unaltered in twd. On the other hand, we show that the number of neurons assayed by islet1 and islet2 in situ [16] and by islet1/2 immunostaining at 24 hpf (Fig. S5) is increased in srn mutants. Thus, detailed analyses of neural and glial phenotypes and analyses of additional Notch target genes in twd mutants may help resolve this apparent discrepancy.
Previous work suggested that Fringe, a glycosyltransferase that glycosylates specific sites on the Notch extracellular domain during its intracellular processing, modulates Notch activity [29], [47], [48], [49]. In srn, both O- and N-fucosylation are compromised due to reduced production of fucose moieties. Fringe acts one step downstream of O-fucosylation, adding N-glycans onto fucosylated sites. We speculate that Fringe loss of function may result in similar, but milder, deficits than in srn mutants. Indeed, recent work suggested that lunatic fringe (Lfng), a known modifier of Notch, promotes the lateral inhibition of neurogenesis, that Lfng loss of function by morpholino knockdown leads to increased expression of proneural genes and increased neurogenesis, and that transgenic overexpression of Lfng decreases neurogenesis [50]. These observations are consistent with our results, and further support our conclusion that dysregulated glycosylation of Notch and its ligands results in Notch signaling deficiency and leads to increased neurogenesis.
While deficiencies in Notch-Delta signaling underlie some srn phenotypes, other srn phenotypes are likely to be independent of this pathway. Srn mutants exhibit prominent defects in retinotectal connectivity that are quite different from those observed in mutants in the Notch-Delta pathway such as des and dla in which no defects in retinotectal axon branching are observed, and from the dramatic reduction in retinal ganglion cell number and axon pathfinding observed in mib. We present several lines of evidence that support the conclusion that some, but clearly not all, of the mechanisms underlying the neural phenotypes in srn are Notch-dependent. Future work will focus on identifying the fucosylated proteins that mediate the neural deficits that are independent of Notch-Delta signaling.
It seems likely that the regulation of Notch signaling by fucosylation is context dependent, i.e. different aspects of neural development require specific types and extent of fucosylation and other modifications of Notch receptors and/or ligands, in a particular spatiotemporal fashion. Our results do not completely rule out the possibility that Notch signaling may contribute, in a specific spatiotemporal context, to the synaptic defects and retinal ganglion cell arborization defects in srn, and this will only be resolved once the relevant fucosylation targets are identified.
Srn as a zebrafish model for congenital disorders of glycosylation
Over the last decade, a large number of human genetic diseases with aberrant glycoprotein synthesis have been identified and grouped as congenital disorders of glycosylation (CDG). Since glycosylation is essential for the function of many proteins, it is not surprising that disruption of glycosylation can lead to severe, multi-systemic phenotypes, including neurodevelopmental and cognitive disorders. In srn mutants, the gmds mutation largely abolishes the synthesis of GDP-fucose, resulting in reduction or elimination of both O-linked and N-linked fucosylation of Notch and many other proteins. Thus it is possible that disruption of O- as well as N-linked glycosylation of Notch and other proteins contributes to CDG IIc pathogenesis, although this has not been examined extensively in humans.
There are several reports of neural deficits in CDGIIc patients, including severe mental retardation, microcephaly, cortical atrophy, seizures, psychomotor retardation and hypotonia [2], [4], [51]. These clinical observations are consistent with the CNS and PNS cellular phenotypes observed in srn. Giving the advantage of performing imaging, genetic and pharmacological manipulations in zebrafish, srn will be a useful tool to guide future analyses in human CDG IIc patients and contribute to a better understanding of the mechanisms responsible for this devastating disorder that affects nervous system and other organ development.
Supporting Information
Gmds mRNA localization by in situ hybridization in wild type zebrafish embryos from 12 to 72 hpf. In situ hybridization was performed as described previously (Panzer et al., 2005), with anti-sense (A–E) gmds probe; sense probe was used as a control (F). Several hundred embryos from several carrier pairs were used from 6 to 72 hpf. A. From 6 to 12 hpf, gmds transcripts are expressed throughout the embryo. B. By 24 hpf, gmds transcripts are highly expressed in the CNS and are also expressed in somites at lower levels. C, D. Gmds mRNA expression is present in the CNS at 48 (C) and 72 (D) hpf, with transcripts more abundant in brain than spinal cord.
(2.49 MB EPS)
Modeling of zebrafish GMDS protein structure. Because of the high degree of amino acid sequence conservation between zebrafish and human GMDS, we reasoned that it would be informative to superimpose the zebrafish GMDS sequence onto the human GMDS crystal structure; this was done using a search of the using a search of the Protein Data Bank database (www.pdb.org) and MODELLER and PYMOL software. The wild type (brown rods) and srn (blue rods) primary amino acid sequence was modeled onto the human GMDS protein crystal structure. A: As in the srn mutation, Valine was substituted for Glycine at residue 178 and an energy minimization calculation was performed. When the srn mutation is present, the Valine deforms a nearby Glutamate residue, Glu155. This change is predicted to push away the substrate GDP-manose, resulting in loss of function. B: To understand how the movement of Glu155 could affect surrounding amino acids, the wild type structure (brown sticks) was examined in more detail. Three ordered H2O molecules exist between the negatively charged group on Glu155 and the negatively charged phosphate group on GDP. The bond lengths between water oxygens and phosphate or carboxylic acid oxygens are appropriate to form hydrogen bonds to coordinate GDP to Glu155.
(0.83 MB EPS)
GDP-fucose rescue of srn and morpholino knockdown of gmds. A. RT-PCR showed >80% of gmds transcript was mis-spliced after gmds morpholino (4 ng) injection. B–E. External phenotypes in srn and gmds morphants (E) include tail bend (compare B, wild type with C, srn) which is rescued after GDP-fucose supplementation (D). F–I. srn (G) and gmds morphants (I) showed reduced AAL staining compared to wild type (F) which is rescued after GDP-fucose supplementation (H). J–M. srn (K) and gmds morphants (M) showed increased Mauthner neuron number compared to wild type (J) a phenotype that is rescued after GDP-fucose supplementation (L). N–Q. srn (O) and gmds morphants (Q) showed reduced GFAP+ glia in the spinal cord compared to wild type (N), a phenotype that is rescued after GDP-fucose supplementation (P). R–U. srn (S) and gmds morphants (U) showed increased neuromuscular synapses compared to wild type (R), a phenotype that is rescued after GDP-fucose supplementation (T). Scale bar = 40 µm. In each experiment, at least 10 srn, normal siblings, gmds morphants or GDP-fucose rescued srn mutant embryos were assessed at 48 hpf. These results show that GDP-fucose rescues external and neural defects in srn mutants and that gmds knockdown by morpholino phenocopies srn phenotypes. Together, these further support the conclusions that gmds is the gene mutated in srn, that the fucose metabolism pathway is deficient in srn mutants, and that the resulting lack GDP-fucose is the cause of the srn mutant phenotypes, rather than the accumulation of the substrate, GDP-mannose.
(6.55 MB EPS)
Zn5+ cell number is reduced in mib but not srn, des or dla compared to wild type embryos. The number of Zn5+ cells was counted from embryos at 48 and 72 hpf after immunostaining with Zn5 antibody and confocal reconstruction of the motor neuron pool. At 48 hpf, Zn5+ cell number per hemisegment was similar among wild type (46±2), srn (49±1), des (52±2) and dla (46±2) embryos, and is significantly reduced in mib mutant embryos (27±2) (1 hemisegment in each of 6–9 48 hpf embryos counted of each genotype; one-way ANOVA, Bonferroni's Multiple Comparison Test, only mib is significantly different compared to other mutants and wild type, p<0.001). At 72 hpf, Zn5+ cell number per hemisegment was similar among wild type (63±2), srn (62±1), des (63±2) and dla (61±2) embryos, and is significantly reduced in mib mutant embryos (36±2) (1 hemisegment in each of 6–10 72 hpf embryos counted of each genotype; one-way ANOVA, Bonferroni's Multiple Comparison Test; only mib is significantly different compared to other mutants and wild type, * p<0.001).
(0.37 MB EPS)
Immunostaining of Zn5, Islet1/2 and GFAP, and quantification of Zn5+ cell patterning defects. A. At 24 hpf, Islet1/2 staining is increased in srn mutants compared to WT embryos, in primary motor neurons and Rohon-Beard neurons, identified based on their morphology and location in the spinal cord. Dashed lines indicate segment boundaries. B. At 48 hpf, in WT embryos, Zn5+ cells are also Islet1/2+. In srn mutants, Islet1/2 expression is reduced and majority of Zn5+ cells are not Islet1/2+. C. Zn5 and GFAP immunostaining in WT and srn mutants at 48 hpf, showing the spatial relationship between these markers. D. GFAP staining in HuC:GFP embryos at 48 hpf, showing the spatial relationship between neuron cell bodies and GFAP+ processes in the spinal cord. Scale bar = 40 µm. E–H. Quantification of Zn5+ cell patterning defects. There are 3–5 Zn5+ cell at every 20 µm interval in WT and des mutants, 1–9 in srn, dla mutants and medium dose DAPT treated embryos, and 0–3 in mib and high dose DAPT treated embryos, consistent with clumping and gaps in the spinal cord. Data from a representative embryos is shown in E and G. The distribution of all embryos is shown in F and H (4–9 embryos for each; Kolmogorov-Smirnov test, p<0.05).
(5.80 MB EPS)
Reduction in Notch-Delta signaling accounts for some srn phenotypes in the retina. A. Retina patterning was examined with immunostaining using antibody Zn5 at 72 hpf. Retina cell patterning appears grossly normal in srn, des, dla and medium dose DAPT treated embryos, but in mib and high does DAPT treated embryos retinal ganglion cell number is reduced, probably due to increased cell death, as previously reported (Bernardos et al., 2005) (8 embryos, 2 carrier pairs were examined). Scale bar = 40 µm. B. Glial cells in the retina were examined after immunostaining with anti-GFAP antibody. In the retina, the number of radially oriented GFAP+ Muller cells is decreased in srn and mib and medium dose DAPT treated embryos, but not in des or dla (8 embryos, 2 carrier pairs were examined). Scale bar = 40 µm. These results suggest that a reduction in Notch-Delta signaling may account for the glial defects observed in srn mutants.
(2.16 MB EPS)
NICD overexpression suppresses the increased Mauthner neuron phenotype in gmds morphants. A. AAL staining is reduced in gmds morphants overexpressing NICD, but not in WT embryos or WT embryos overexpressing NICD. B. WT embryos have a pair of Mauthner neurons. WT overexpressing NICD showed dramatic hindbrain patterning defects, resulting in an almost complete loss of Mauthner neurons. gmds morphants overexpressing NICD showed similar reduction of Mauthner neurons. This result suggests NICD overexpression suppresses the increase of Mauthner neurons observed in gmds morphants and thus supports our conclusion that reduction of Notch-Delta signaling in srn mutants is responsible for the neurogenesis defects (3–4 embryos assessed for each manipulation). Scale bar = 20 µm.
(2.95 MB EPS)
Muscle patterning is grossly normal in srn mutants. Slow muscle fibers were examined with F59 antibody and glia cells in the spinal cord were examined with GFAP antibody. While there is an obvious reduction of GFAP+ glia cells in the spinal cord in srn mutants, the patterning of slow muscle fibers is similar in srn and wild type embryos at 48 hpf. Previous work showed that fast muscle fiber number and patterning are unaltered in srn compared to wild type embryos at 48 hpf (Panzer et al., 2005; 3 embryos, 1 carrier pair were examined). Scale bar = 200 µm.
(1.86 MB EPS)
Acknowledgments
We thank Dr. Christine Beattie for providing the deadly seven mutant line, Dr. Bruce Appel for providing the Tg(hsp70l:GAL4) and Tg(UAS:myc-notch1a-intra) transgenic lines, Dr. Chi-Bin Chien for help with analyses of retinal ganglion cell axon targeting and branching, Drs. Sam Nona and John Scholes for providing the goldfish GFAP antibody, Ethan Hughes for help with quantification of neuromuscular synapses, Mrs. Marion Scott for technical assistance, and members of the Balice-Gordon lab for helpful discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Supported by National Institutes of Health grant NS050524 to R. B.-G. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Becker DJ, Lowe JB. Leukocyte adhesion deficiency type II. Biochim Biophys Acta. 1999;1455:193–204. doi: 10.1016/s0925-4439(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 2.Etzioni A, Sturla L, Antonellis A, Green ED, Gershoni-Baruch R, et al. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am J Med Genet. 2002;110:131–135. doi: 10.1002/ajmg.10423. [DOI] [PubMed] [Google Scholar]
- 3.Luhn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28:69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 4.Lubke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, et al. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28:73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- 5.Smith PL, Myers JT, Rogers CE, Zhou L, Petryniak B, et al. Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J Cell Biol. 2002;158:801–815. doi: 10.1083/jcb.200203125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa HO, Higashi S, Ayukawa T, Sasamura T, Kitagawa M, et al. Notch deficiency implicated in the pathogenesis of congenital disorder of glycosylation IIc. Proc Natl Acad Sci U S A. 2005;102:18532–18537. doi: 10.1073/pnas.0504115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellbusch CC, Sperandio M, Frommhold D, Yakubenia S, Wild MK, et al. Golgi GDP-fucose transporter-deficient mice mimic congenital disorder of glycosylation IIc/leukocyte adhesion deficiency II. J Biol Chem. 2007;282:10762–10772. doi: 10.1074/jbc.M700314200. [DOI] [PubMed] [Google Scholar]
- 8.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 9.Gray M, Moens CB, Amacher SL, Eisen JS, Beattie CE. Zebrafish deadly seven functions in neurogenesis. Dev Biol. 2001;237:306–323. doi: 10.1006/dbio.2001.0381. [DOI] [PubMed] [Google Scholar]
- 10.Appel B, Eisen JS. Regulation of neuronal specification in the zebrafish spinal cord by Delta function. Development. 1998;125:371–380. doi: 10.1242/dev.125.3.371. [DOI] [PubMed] [Google Scholar]
- 11.Appel B, Givan LA, Eisen JS. Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Dev Biol. 2001;1:13. doi: 10.1186/1471-213X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HC, Appel B. Delta-Notch signaling regulates oligodendrocyte specification. Development. 2003;130:3747–3755. doi: 10.1242/dev.00576. [DOI] [PubMed] [Google Scholar]
- 13.Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 14.Salama-Cohen P, Arevalo MA, Grantyn R, Rodriguez-Tebar A. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J Neurochem. 2006;97:1269–1278. doi: 10.1111/j.1471-4159.2006.03783.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panzer JA, Gibbs SM, Dosch R, Wagner D, Mullins MC, et al. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev Biol. 2005;285:340–357. doi: 10.1016/j.ydbio.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Scheer N, Riedl I, Warren JT, Kuwada JY, Campos-Ortega JA. A quantitative analysis of the kinetics of Gal4 activator and effector gene expression in the zebrafish. Mech Dev. 2002;112:9–14. doi: 10.1016/s0925-4773(01)00621-9. [DOI] [PubMed] [Google Scholar]
- 18.Willer GB, Lee VM, Gregg RG, Link BA. Analysis of the Zebrafish perplexed mutation reveals tissue-specific roles for de novo pyrimidine synthesis during development. Genetics. 2005;170:1827–1837. doi: 10.1534/genetics.105.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thisse B, Pflumio S, Fürthauer M, Loppin B, Heyer V, et al. Expression of the zebrafish genome during embryogenesis. ZFIN Direct Data Submission 2001 [Google Scholar]
- 20.Thisse B, Thisse C. High Throughput Expression Analysis of ZF-Models Consortium Clones. ZFIN Direct Data Submission 2005 [Google Scholar]
- 21.Shin J, Poling J, Park HC, Appel B. Notch signaling regulates neural precursor allocation and binary neuronal fate decisions in zebrafish. Development. 2007;134:1911–1920. doi: 10.1242/dev.001602. [DOI] [PubMed] [Google Scholar]
- 22.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nona SN, Shehab SA, Stafford CA, Cronly-Dillon JR. Glial fibrillary acidic protein (GFAP) from goldfish: its localisation in visual pathway. Glia. 1989;2:189–200. doi: 10.1002/glia.440020308. [DOI] [PubMed] [Google Scholar]
- 24.Luhn K, Laskowska A, Pielage J, Klambt C, Ipe U, et al. Identification and molecular cloning of a functional GDP-fucose transporter in Drosophila melanogaster. Exp Cell Res. 2004;301:242–250. doi: 10.1016/j.yexcr.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 25.Kochibe N, Furukawa K. Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry. 1980;19:2841–2846. doi: 10.1021/bi00554a004. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, et al. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer). Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Thisse B, Thisse C. Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission 2004 [Google Scholar]
- 28.Lowe JB. Glycosylation, immunity, and autoimmunity. Cell. 2001;104:809–812. doi: 10.1016/s0092-8674(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 29.Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 30.Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, et al. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135–140. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- 32.Hutchinson SA, Eisen JS. Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development. 2006;133:2137–2147. doi: 10.1242/dev.02355. [DOI] [PubMed] [Google Scholar]
- 33.Cornell RA, Eisen JS. Delta signaling mediates segregation of neural crest and spinal sensory neurons from zebrafish lateral neural plate. Development. 2000;127:2873–2882. doi: 10.1242/dev.127.13.2873. [DOI] [PubMed] [Google Scholar]
- 34.Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina. Dev Biol. 2005;278:381–395. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Bae YK, Shimizu T, Hibi M. Patterning of proneuronal and inter-proneuronal domains by hairy- and enhancer of split-related genes in zebrafish neuroectoderm. Development. 2005;132:1375–1385. doi: 10.1242/dev.01710. [DOI] [PubMed] [Google Scholar]
- 36.Zecchin E, Filippi A, Biemar F, Tiso N, Pauls S, et al. Distinct delta and jagged genes control sequential segregation of pancreatic cell types from precursor pools in zebrafish. Dev Biol. 2007;301:192–204. doi: 10.1016/j.ydbio.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 37.Hegde A, Qiu NC, Qiu X, Ho SH, Tay KQ, et al. Genomewide expression analysis in zebrafish mind bomb alleles with pancreas defects of different severity identifies putative Notch responsive genes. PLoS ONE. 2008;3:e1479. doi: 10.1371/journal.pone.0001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- 39.Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev. 2003;17:1213–1218. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris RJ, Spellman MW. O-linked fucose and other post-translational modifications unique to EGF modules. Glycobiology. 1993;3:219–224. doi: 10.1093/glycob/3.3.219. [DOI] [PubMed] [Google Scholar]
- 41.Harris RJ, van Halbeek H, Glushka J, Basa LJ, Ling VT, et al. Identification and structural analysis of the tetrasaccharide NeuAc alpha(2—>6)Gal beta(1—>4)GlcNAc beta(1—>3)Fuc alpha 1—>O-linked to serine 61 of human factor IX. Biochemistry. 1993;32:6539–6547. doi: 10.1021/bi00077a007. [DOI] [PubMed] [Google Scholar]
- 42.Moloney DJ, Haltiwanger RS. The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-beta1,3-glucosyltransferase activity from Chinese hamster ovary cells. Glycobiology. 1999;9:679–687. doi: 10.1093/glycob/9.7.679. [DOI] [PubMed] [Google Scholar]
- 43.Jaeken J, Matthijs G. Congenital disorders of glycosylation: a rapidly expanding disease family. Annu Rev Genomics Hum Genet. 2007;8:261–278. doi: 10.1146/annurev.genom.8.080706.092327. [DOI] [PubMed] [Google Scholar]
- 44.Sturla L, Rampal R, Haltiwanger RS, Fruscione F, Etzioni A, et al. Differential terminal fucosylation of N-linked glycans versus protein O-fucosylation in leukocyte adhesion deficiency type II (CDG IIc). J Biol Chem. 2003;278:26727–26733. doi: 10.1074/jbc.M304068200. [DOI] [PubMed] [Google Scholar]
- 45.Choe EA, Liao L, Zhou JY, Cheng D, Duong DM, et al. Neuronal morphogenesis is regulated by the interplay between cyclin-dependent kinase 5 and the ubiquitin ligase mind bomb 1. J Neurosci. 2007;27:9503–9512. doi: 10.1523/JNEUROSCI.1408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, et al. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 2009;136:1653–1663. doi: 10.1242/dev.033290. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Irvine KD, Carroll SB. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell. 1995;82:795–802. doi: 10.1016/0092-8674(95)90476-x. [DOI] [PubMed] [Google Scholar]
- 48.Rauskolb C, Correia T, Irvine KD. Fringe-dependent separation of dorsal and ventral cells in the Drosophila wing. Nature. 1999;401:476–480. doi: 10.1038/46786. [DOI] [PubMed] [Google Scholar]
- 49.Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–912. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaou N, Watanabe-Asaka T, Gerety S, Distel M, Koster RW, et al. Lunatic fringe promotes the lateral inhibition of neurogenesis. Development. 2009;136:2523–2533. doi: 10.1242/dev.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frydman M, Etzioni A, Eidlitz-Markus T, Avidor I, Varsano I, et al. Rambam-Hasharon syndrome of psychomotor retardation, short stature, defective neutrophil motility, and Bombay phenotype. Am J Med Genet. 1992;44:297–302. doi: 10.1002/ajmg.1320440307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gmds mRNA localization by in situ hybridization in wild type zebrafish embryos from 12 to 72 hpf. In situ hybridization was performed as described previously (Panzer et al., 2005), with anti-sense (A–E) gmds probe; sense probe was used as a control (F). Several hundred embryos from several carrier pairs were used from 6 to 72 hpf. A. From 6 to 12 hpf, gmds transcripts are expressed throughout the embryo. B. By 24 hpf, gmds transcripts are highly expressed in the CNS and are also expressed in somites at lower levels. C, D. Gmds mRNA expression is present in the CNS at 48 (C) and 72 (D) hpf, with transcripts more abundant in brain than spinal cord.
(2.49 MB EPS)
Modeling of zebrafish GMDS protein structure. Because of the high degree of amino acid sequence conservation between zebrafish and human GMDS, we reasoned that it would be informative to superimpose the zebrafish GMDS sequence onto the human GMDS crystal structure; this was done using a search of the using a search of the Protein Data Bank database (www.pdb.org) and MODELLER and PYMOL software. The wild type (brown rods) and srn (blue rods) primary amino acid sequence was modeled onto the human GMDS protein crystal structure. A: As in the srn mutation, Valine was substituted for Glycine at residue 178 and an energy minimization calculation was performed. When the srn mutation is present, the Valine deforms a nearby Glutamate residue, Glu155. This change is predicted to push away the substrate GDP-manose, resulting in loss of function. B: To understand how the movement of Glu155 could affect surrounding amino acids, the wild type structure (brown sticks) was examined in more detail. Three ordered H2O molecules exist between the negatively charged group on Glu155 and the negatively charged phosphate group on GDP. The bond lengths between water oxygens and phosphate or carboxylic acid oxygens are appropriate to form hydrogen bonds to coordinate GDP to Glu155.
(0.83 MB EPS)
GDP-fucose rescue of srn and morpholino knockdown of gmds. A. RT-PCR showed >80% of gmds transcript was mis-spliced after gmds morpholino (4 ng) injection. B–E. External phenotypes in srn and gmds morphants (E) include tail bend (compare B, wild type with C, srn) which is rescued after GDP-fucose supplementation (D). F–I. srn (G) and gmds morphants (I) showed reduced AAL staining compared to wild type (F) which is rescued after GDP-fucose supplementation (H). J–M. srn (K) and gmds morphants (M) showed increased Mauthner neuron number compared to wild type (J) a phenotype that is rescued after GDP-fucose supplementation (L). N–Q. srn (O) and gmds morphants (Q) showed reduced GFAP+ glia in the spinal cord compared to wild type (N), a phenotype that is rescued after GDP-fucose supplementation (P). R–U. srn (S) and gmds morphants (U) showed increased neuromuscular synapses compared to wild type (R), a phenotype that is rescued after GDP-fucose supplementation (T). Scale bar = 40 µm. In each experiment, at least 10 srn, normal siblings, gmds morphants or GDP-fucose rescued srn mutant embryos were assessed at 48 hpf. These results show that GDP-fucose rescues external and neural defects in srn mutants and that gmds knockdown by morpholino phenocopies srn phenotypes. Together, these further support the conclusions that gmds is the gene mutated in srn, that the fucose metabolism pathway is deficient in srn mutants, and that the resulting lack GDP-fucose is the cause of the srn mutant phenotypes, rather than the accumulation of the substrate, GDP-mannose.
(6.55 MB EPS)
Zn5+ cell number is reduced in mib but not srn, des or dla compared to wild type embryos. The number of Zn5+ cells was counted from embryos at 48 and 72 hpf after immunostaining with Zn5 antibody and confocal reconstruction of the motor neuron pool. At 48 hpf, Zn5+ cell number per hemisegment was similar among wild type (46±2), srn (49±1), des (52±2) and dla (46±2) embryos, and is significantly reduced in mib mutant embryos (27±2) (1 hemisegment in each of 6–9 48 hpf embryos counted of each genotype; one-way ANOVA, Bonferroni's Multiple Comparison Test, only mib is significantly different compared to other mutants and wild type, p<0.001). At 72 hpf, Zn5+ cell number per hemisegment was similar among wild type (63±2), srn (62±1), des (63±2) and dla (61±2) embryos, and is significantly reduced in mib mutant embryos (36±2) (1 hemisegment in each of 6–10 72 hpf embryos counted of each genotype; one-way ANOVA, Bonferroni's Multiple Comparison Test; only mib is significantly different compared to other mutants and wild type, * p<0.001).
(0.37 MB EPS)
Immunostaining of Zn5, Islet1/2 and GFAP, and quantification of Zn5+ cell patterning defects. A. At 24 hpf, Islet1/2 staining is increased in srn mutants compared to WT embryos, in primary motor neurons and Rohon-Beard neurons, identified based on their morphology and location in the spinal cord. Dashed lines indicate segment boundaries. B. At 48 hpf, in WT embryos, Zn5+ cells are also Islet1/2+. In srn mutants, Islet1/2 expression is reduced and majority of Zn5+ cells are not Islet1/2+. C. Zn5 and GFAP immunostaining in WT and srn mutants at 48 hpf, showing the spatial relationship between these markers. D. GFAP staining in HuC:GFP embryos at 48 hpf, showing the spatial relationship between neuron cell bodies and GFAP+ processes in the spinal cord. Scale bar = 40 µm. E–H. Quantification of Zn5+ cell patterning defects. There are 3–5 Zn5+ cell at every 20 µm interval in WT and des mutants, 1–9 in srn, dla mutants and medium dose DAPT treated embryos, and 0–3 in mib and high dose DAPT treated embryos, consistent with clumping and gaps in the spinal cord. Data from a representative embryos is shown in E and G. The distribution of all embryos is shown in F and H (4–9 embryos for each; Kolmogorov-Smirnov test, p<0.05).
(5.80 MB EPS)
Reduction in Notch-Delta signaling accounts for some srn phenotypes in the retina. A. Retina patterning was examined with immunostaining using antibody Zn5 at 72 hpf. Retina cell patterning appears grossly normal in srn, des, dla and medium dose DAPT treated embryos, but in mib and high does DAPT treated embryos retinal ganglion cell number is reduced, probably due to increased cell death, as previously reported (Bernardos et al., 2005) (8 embryos, 2 carrier pairs were examined). Scale bar = 40 µm. B. Glial cells in the retina were examined after immunostaining with anti-GFAP antibody. In the retina, the number of radially oriented GFAP+ Muller cells is decreased in srn and mib and medium dose DAPT treated embryos, but not in des or dla (8 embryos, 2 carrier pairs were examined). Scale bar = 40 µm. These results suggest that a reduction in Notch-Delta signaling may account for the glial defects observed in srn mutants.
(2.16 MB EPS)
NICD overexpression suppresses the increased Mauthner neuron phenotype in gmds morphants. A. AAL staining is reduced in gmds morphants overexpressing NICD, but not in WT embryos or WT embryos overexpressing NICD. B. WT embryos have a pair of Mauthner neurons. WT overexpressing NICD showed dramatic hindbrain patterning defects, resulting in an almost complete loss of Mauthner neurons. gmds morphants overexpressing NICD showed similar reduction of Mauthner neurons. This result suggests NICD overexpression suppresses the increase of Mauthner neurons observed in gmds morphants and thus supports our conclusion that reduction of Notch-Delta signaling in srn mutants is responsible for the neurogenesis defects (3–4 embryos assessed for each manipulation). Scale bar = 20 µm.
(2.95 MB EPS)
Muscle patterning is grossly normal in srn mutants. Slow muscle fibers were examined with F59 antibody and glia cells in the spinal cord were examined with GFAP antibody. While there is an obvious reduction of GFAP+ glia cells in the spinal cord in srn mutants, the patterning of slow muscle fibers is similar in srn and wild type embryos at 48 hpf. Previous work showed that fast muscle fiber number and patterning are unaltered in srn compared to wild type embryos at 48 hpf (Panzer et al., 2005; 3 embryos, 1 carrier pair were examined). Scale bar = 200 µm.
(1.86 MB EPS)