Abstract
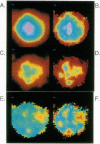
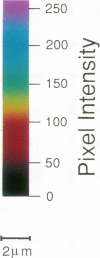
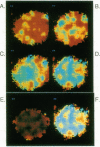
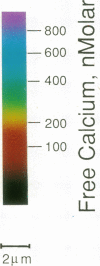
We have previously shown that the intracellular free Ca2+ increase induced by erythropoietin is likely related to differentiation rather than proliferation in human BFU-E-derived erythroblasts (1989. Blood. 73:1188-1194). Since cell differentiation involves transcription of specific regions of the genome, and since nuclear endonucleases responsible for single strand DNA breaks observed in cells undergoing differentiation are Ca2+ dependent, we investigated whether the erythropoietin-induced calcium signal is transmitted from cytosol to nucleus in this study. To elucidate subcellular Ca2+ gradients, the technique of optical sectioning microscopy was used. After determining the empirical three-dimensional point spread function of the video imaging system, contaminating light signals from optical planes above and below the focal plane of interest were removed by deconvolution using the nearest neighboring approach. Processed images did not reveal any discernible subcellular Ca2+ gradients in unstimulated erythroblasts. By contrast, with erythropoietin stimulation, there was a two- to threefold higher Ca2+ concentration in the nucleus compared to the surrounding cytoplasm. We suggest that the rise in nuclear Ca2+ may activate Ca2(+)-dependent endonucleases and initiate differentiation. The approach described here offers the opportunity to follow subcellular Ca2+ changes in response to a wide range of stimuli, allowing new insights into the role of regional Ca2+ changes in regulation of cell function.
Full text
PDF


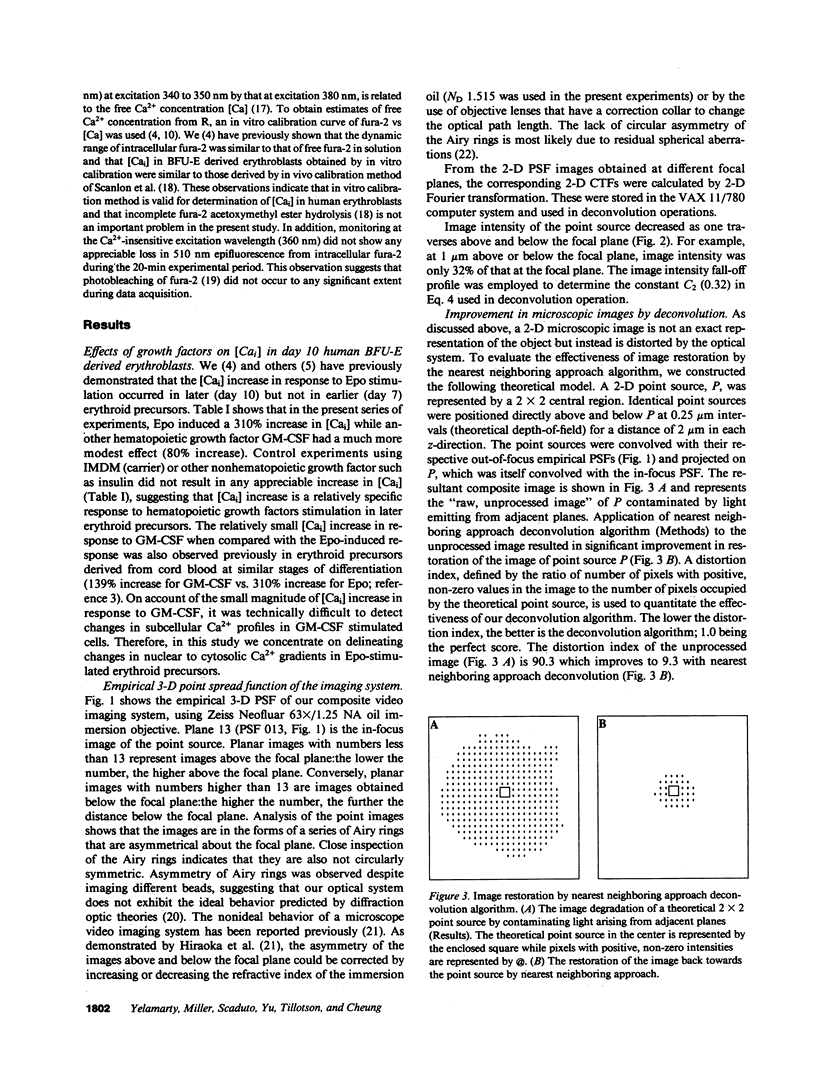

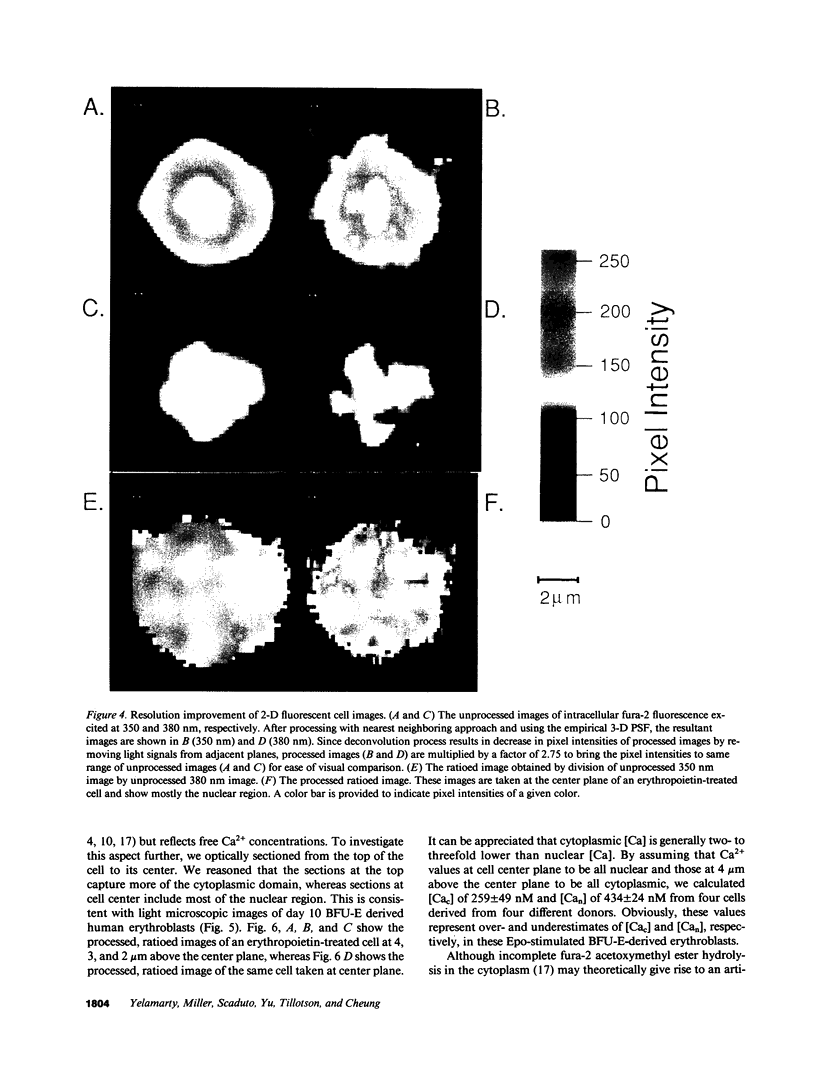

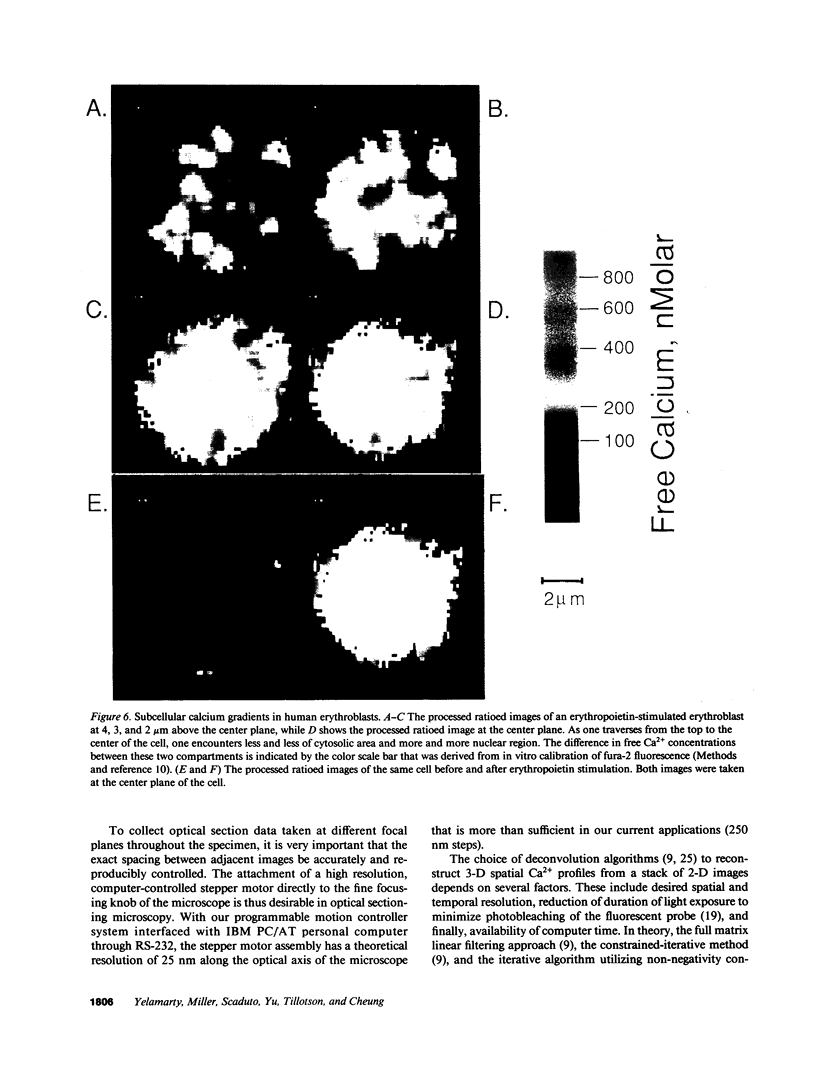



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Fay F. S. Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am J Physiol. 1987 Oct;253(4 Pt 1):C613–C618. doi: 10.1152/ajpcell.1987.253.4.C613. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Skorecki K. L., Kreisberg J. I., Cheung J. Y. Vasopressin increases cytosolic free calcium concentration in glomerular mesangial cells. Am J Physiol. 1986 Jul;251(1 Pt 2):F94–102. doi: 10.1152/ajprenal.1986.251.1.F94. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Constantine J. M., Bonventre J. V. Regulation of cytosolic free calcium concentration in cultured renal epithelial cells. Am J Physiol. 1986 Oct;251(4 Pt 2):F690–F701. doi: 10.1152/ajprenal.1986.251.4.F690. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Fay F. S., Carrington W., Fogarty K. E. Three-dimensional molecular distribution in single cells analysed using the digital imaging microscope. J Microsc. 1989 Feb;153(Pt 2):133–149. [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Hochstrasser M., Mathog D., Saumweber H., Agard D. A., Sedat J. W. Spatial organization of the Drosophila nucleus: a three-dimensional cytogenetic study. J Cell Sci Suppl. 1984;1:223–234. doi: 10.1242/jcs.1984.supplement_1.14. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. The use of a charge-coupled device for quantitative optical microscopy of biological structures. Science. 1987 Oct 2;238(4823):36–41. doi: 10.1126/science.3116667. [DOI] [PubMed] [Google Scholar]
- Imagawa S., Smith B. R., Palmer-Crocker R., Bunn H. F. The effect of recombinant erythropoietin on intracellular free calcium in erythropoietin-responsive cells. Blood. 1989 May 1;73(6):1452–1457. [PubMed] [Google Scholar]
- Jones D. P., McConkey D. J., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation in rat liver nuclei. J Biol Chem. 1989 Apr 15;264(11):6398–6403. [PubMed] [Google Scholar]
- Kulikova O. G., Savost'ianov G. A., Beliavtseva L. M., Razumovskaia N. I. Issledovanie ATPaznoi aktivnosti i ATP-zavisimoi akkumuliatsii Ca2+ iadrami skeletnykh myshts. Effekty denervatsii i élektricheskoi stimuliatsii. Biokhimiia. 1982 Jul;47(7):1216–1221. [PubMed] [Google Scholar]
- Laverriere J. N., Tixier-Vidal A., Buisson N., Morin A., Martial J. A., Gourdji D. Preferential role of calcium in the regulation of prolactin gene transcription by thyrotropin-releasing hormone in GH3 pituitary cells. Endocrinology. 1988 Jan;122(1):333–340. doi: 10.1210/endo-122-1-333. [DOI] [PubMed] [Google Scholar]
- Maizels E. T., Jungmann R. A. Ca2+-calmodulin-dependent phosphorylation of soluble and nuclear proteins in the rat ovary. Endocrinology. 1983 Jun;112(6):1895–1902. doi: 10.1210/endo-112-6-1895. [DOI] [PubMed] [Google Scholar]
- McMahon G., Alsina J. L., Levy S. B. Induction of a Ca2+, Mg2+-dependent endonuclease activity during the early stages of murine erythroleukemic cell differentiation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7461–7465. doi: 10.1073/pnas.81.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. A., Cheung J. Y., Tillotson D. L., Hope S. M., Scaduto R. C., Jr Erythropoietin stimulates a rise in intracellular-free calcium concentration in single BFU-E derived erythroblasts at specific stages of differentiation. Blood. 1989 Apr;73(5):1188–1194. [PubMed] [Google Scholar]
- Miller B. A., Scaduto R. C., Jr, Tillotson D. L., Botti J. J., Cheung J. Y. Erythropoietin stimulates a rise in intracellular free calcium concentration in single early human erythroid precursors. J Clin Invest. 1988 Jul;82(1):309–315. doi: 10.1172/JCI113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Defize L. H., De Laat S. W. Ionic signalling by growth factor receptors. J Exp Biol. 1986 Sep;124:359–373. doi: 10.1242/jeb.124.1.359. [DOI] [PubMed] [Google Scholar]
- Muel A. S., Chaudun E., Courtois Y., Modak S. P., Counis M. F. Nuclear endogenous Ca2+-dependent endodeoxyribonuclease in differentiating chick embryonic lens fibers. J Cell Physiol. 1986 Apr;127(1):167–174. doi: 10.1002/jcp.1041270120. [DOI] [PubMed] [Google Scholar]
- Nasi E., Tillotson D. The rate of diffusion of Ca2+ and Ba2+ in a nerve cell body. Biophys J. 1985 May;47(5):735–738. doi: 10.1016/S0006-3495(85)83972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., McConkey D. J., Jones D. P., Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989 Jan;86(2):453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J. P., Fernández F. Effect of calcium and calmodulin on RNA synthesis in isolated nuclei from rat liver cells. FEBS Lett. 1982 Jun 21;143(1):157–160. doi: 10.1016/0014-5793(82)80295-0. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Tucker J. B. The ground substance of the living cell. Sci Am. 1981 Mar;244(3):56–67. doi: 10.1038/scientificamerican0381-56. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Simmen R. C., Dunbar B. S., Guerriero V., Chafouleas J. G., Clark J. H., Means A. R. Estrogen stimulates the transient association of calmodulin and myosin light chain kinase with the chicken liver nuclear matrix. J Cell Biol. 1984 Aug;99(2):588–593. doi: 10.1083/jcb.99.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Milligan R. A. A large particle associated with the perimeter of the nuclear pore complex. J Cell Biol. 1982 Apr;93(1):63–75. doi: 10.1083/jcb.93.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]