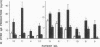Abstract
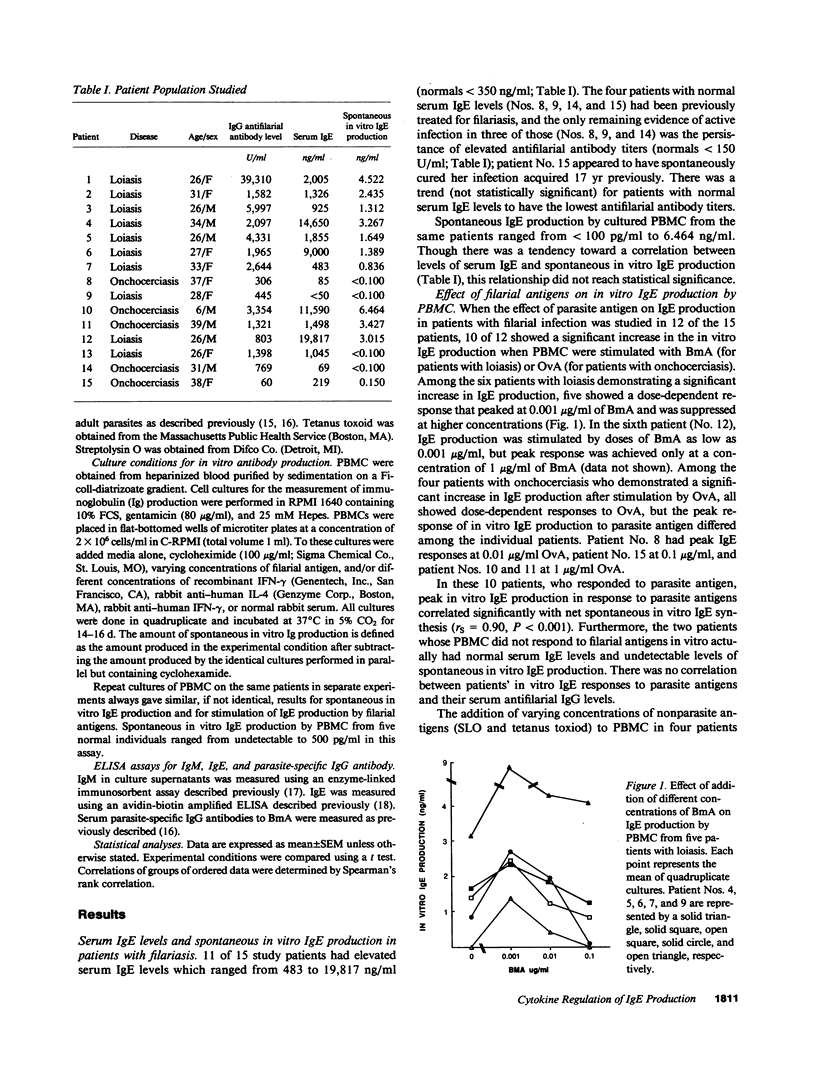
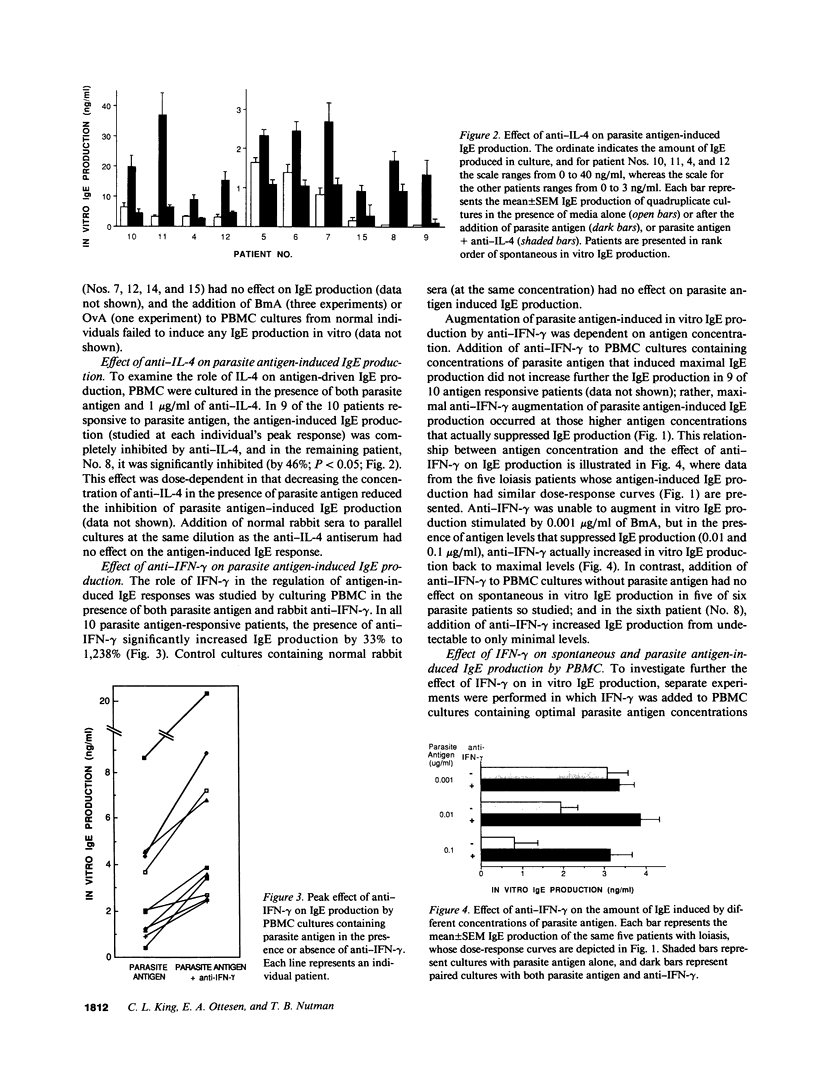
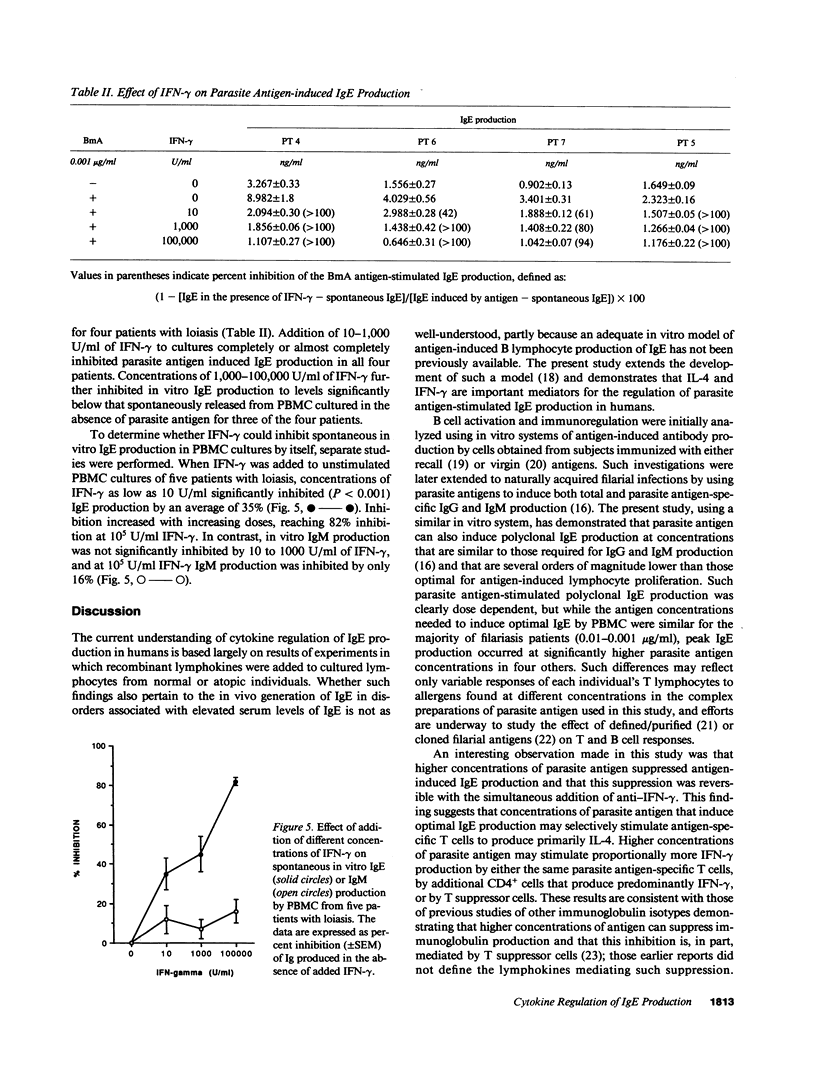
To define the immunoregulatory mechanisms underlying serum IgE levels found in patients with filariasis, we studied polyclonal IgE production by peripheral blood mononuclear cells (PBMC) from 15 patients with filarial infections, with a focus on the role of interleukin-4 (IL-4) and interferon-gamma (IFN-gamma) in the generation and regulation of the response. Spontaneous in vitro IgE production was elevated in 10 of the 15 patients (836-6,464 pg/ml; normals, less than 500 pg/ml). Addition of filarial parasite antigen to PBMC cultures significantly stimulated polyclonal IgE production in an antigen dose-dependent manner in 10 of 12 patients tested (P less than 0.001). The essential role of IL-4 in the generation of this response was demonstrated when simultaneous addition of anti-IL-4 completely inhibited antigen-stimulated IgE production in all 10 patients studied. An inhibitory role of endogenously produced IFN-gamma was also indicated when the addition of anti-IFN-gamma to the cultures significantly augmented filarial antigen-stimulated IgE production by 33-1,238% in these same patients. Addition of 10-1,000 U/ml of recombinant human IFN-gamma to PBMC completely inhibited parasite antigen-induced IgE production. This study demonstrates that filarial antigen-stimulated IgE production in patients with filariasis is mediated by IL-4 and down regulated by IFN-gamma and suggests that the amount of IgE produced depends on the relative quantity of IL-4 and IFN-gamma generated by parasite-specific T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Dessein A. J., Parker W. L., James S. L., David J. R. IgE antibody and resistance to infection. I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J Exp Med. 1981 Feb 1;153(2):423–436. doi: 10.1084/jem.153.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Hamilton R. G., Hussain R., Ottesen E. A., Adkinson N. F., Jr The quantitation of parasite-specific human IgG and IgE in sera: evaluation of solid-phase RIA and ELISA methodology. J Immunol Methods. 1981;44(1):101–114. doi: 10.1016/0022-1759(81)90111-3. [DOI] [PubMed] [Google Scholar]
- Hussain R., Hamilton R. G., Kumaraswami V., Adkinson N. F., Jr, Ottesen E. A. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981 Oct;127(4):1623–1629. [PubMed] [Google Scholar]
- King C. L., Gallin J. I., Malech H. L., Abramson S. L., Nutman T. B. Regulation of immunoglobulin production in hyperimmunoglobulin E recurrent-infection syndrome by interferon gamma. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10085–10089. doi: 10.1073/pnas.86.24.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal R. B., Lynch T. J., Nutman T. B. Brugia malayi antigens associated with lymphocyte activation in filariasis. J Immunol. 1987 Sep 1;139(5):1652–1657. [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman T. B., Hussain R., Ottesen E. A. IgE production in vitro by peripheral blood mononuclear cells of patients with parasitic helminth infections. Clin Exp Immunol. 1984 Oct;58(1):174–182. [PMC free article] [PubMed] [Google Scholar]
- Nutman T. B., Kumaraswami V., Pao L., Narayanan P. R., Ottesen E. A. An analysis of in vitro B cell immune responsiveness in human lymphatic filariasis. J Immunol. 1987 Jun 1;138(11):3954–3959. [PubMed] [Google Scholar]
- Nutman T. B., Miller K. D., Mulligan M., Ottesen E. A. Loa loa infection in temporary residents of endemic regions: recognition of a hyperresponsive syndrome with characteristic clinical manifestations. J Infect Dis. 1986 Jul;154(1):10–18. doi: 10.1093/infdis/154.1.10. [DOI] [PubMed] [Google Scholar]
- Nutman T. B., Volkman D. J., Hussain R., Fauci A. S., Ottesen E. A. Filarial parasite-specific T cell lines: induction of IgE synthesis. J Immunol. 1985 Feb;134(2):1178–1184. [PubMed] [Google Scholar]
- Nutman T. B., Withers A. S., Ottesen E. A. In vitro parasite antigen-induced antibody responses in human helminth infections. J Immunol. 1985 Oct;135(4):2794–2799. [PubMed] [Google Scholar]
- O'Hehir R. E., Bal V., Quint D., Moqbel R., Kay A. B., Zanders E. D., Lamb J. R. An in vitro model of allergen-dependent IgE synthesis by human B lymphocytes: comparison of the response of an atopic and a non-atopic individual to Dermatophagoides spp. (house dust mite). Immunology. 1989 Apr;66(4):499–504. [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. 3. Effect of thymectomy and splenectomy. J Immunol. 1971 Apr;106(4):1019–1025. [PubMed] [Google Scholar]
- Ottesen E. A., Skvaril F., Tripathy S. P., Poindexter R. W., Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985 Apr;134(4):2707–2712. [PubMed] [Google Scholar]
- Romagnani S., Maggi E., Del Prete G. F., Ricci M. IgE synthesis in vitro induced by T cell factors from patients with elevated serum IgE levels. Clin Exp Immunol. 1983 Apr;52(1):85–88. [PMC free article] [PubMed] [Google Scholar]
- Saryan J. A., Leung D. Y., Geha R. S. Induction of human IgE synthesis by a factor derived from T cells of patients with hyper-IgE states. J Immunol. 1983 Jan;130(1):242–247. [PubMed] [Google Scholar]
- Saxon A., Morrow C., Stevens R. H. Subpopulations of circulating B cells and regulatory T cells involved in in vitro immunoglobulin E production in atopic patients with elevted serum immunoglobulin E. J Clin Invest. 1980 Jun;65(6):1457–1468. doi: 10.1172/JCI109810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]
- Umetsu D. T., Leung D. Y., Siraganian R., Jabara H. H., Geha R. S. Differential requirements of B cells from normal and allergic subjects for the induction of IgE synthesis by an alloreactive T cell clone. J Exp Med. 1985 Jul 1;162(1):202–214. doi: 10.1084/jem.162.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnasch T. R., Gallin M. Y., Soboslay P. T., Erttmann K. D., Greene B. M. Isolation and characterization of expression cDNA clones encoding antigens of Onchocerca volvulus infective larvae. J Clin Invest. 1988 Jul;82(1):262–269. doi: 10.1172/JCI113581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Arai K., Yokota T., Geha R. S. Endogenous interleukin 6 plays an obligatory role in interleukin 4-dependent human IgE synthesis. Eur J Immunol. 1989 Aug;19(8):1419–1424. doi: 10.1002/eji.1830190811. [DOI] [PubMed] [Google Scholar]
- Volkman D. J., Allyn S. P., Fauci A. S. Antigen-induced in vitro antibody production in humans: tetanus toxoid-specific antibody synthesis. J Immunol. 1982 Jul;129(1):107–112. [PubMed] [Google Scholar]