Abstract
In this work, the fluorescence of thioflavin T (ThT) was studied in a wide range of viscosity and temperature. It was shown that ThT fluorescence quantum yield varies from 0.0001 in water at room temperature to 0.28 in rigid isotropic solution (T/η→0). The deviation of the fluorescence quantum yield from unity in rigid isotropic solution suggests that fluorescence quantum yield depends not only on the ultra-fast oscillation of ThT fragments relative to each other in an excited state as was suggested earlier, but also depends on the molecular configuration in the ground state. This means that the fluorescence quantum yield of the dye incorporated into amyloid fibrils must depend on its conformation, which, in turn, depends on the ThT environment. Therefore, the fluorescence quantum yield of ThT incorporated into amyloid fibrils can differ from that in the rigid isotropic solution. In particular, the fluorescence quantum yield of ThT incorporated into insulin fibrils was determined to be 0.43. Consequently, the ThT fluorescence quantum yield could be used to characterize the peculiarities of the fibrillar structure, which opens some new possibilities in the ThT use for structural characterization of the amyloid fibrils.
Introduction
The deposition of proteins in the form of regular amyloid fibrils represents a pathological hallmark of several human diseases [1], [2], [3], [4], [5], [6], [7]. Depending on the disease, such proteinaceous deposits can be found in the brain, vital organs such as the liver and spleen, or skeletal tissue, depending on the disease [6], [7]. The protein deposition diseases are among the most costly and debilitating health disorders. Many of them, such as Alzheimer's and Parkinson's diseases and late-onset diabetes, are age-related and are becoming increasingly prevalent in the modern world. Although fibrils from different pathologies display many common morphological and structural properties, the more than 20 proteins known to be involved in deposition diseases are structurally unrelated [6], [7], [8], [9]. These amyloidogenic proteins may be well-folded proteins or intrinsically unstructured [9]. There is an increasing belief that the ability to fibrillate is a generic property of a polypeptide chain, and that all proteins are potentially able to form amyloid fibrils under appropriate conditions [8], [9], [10], [11], [12]. It has been established that protein aggregation involves a unifying mechanism where the structural transformation of a polypeptide chain into a partially folded or misfolded conformation represents a first crucial step [8], [9]. Therefore, understanding the nature and structural features of different partially folded and misfolded conformations represents a crucial step in fundamental science, biotechnology and medicine.
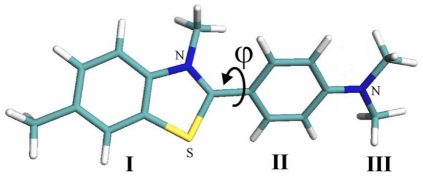
Thioflavin T (ThT, Figure 1) is a common tool for diagnostics of the amyloid fibril formation [13], [14], [15], [16], [17], [18], [19], [20]. Importantly, ThT interaction with amyloid fibrils is highly specific, as this dye does not interact with proteins in their folded, unfolded or partially folded monomeric forms or at least the formation of dye-monomeric protein complexes is not accompanied by the changes in the dye spectral properties. Therefore, due to these unique properties, ThT represents a useful and convenient diagnostic tool for the fast and reliable identification of amyloid fibrils in disease-affected tissues and organs. Furthermore, in in vitro fibrillation studies, the appearance of the specific ThT fluorescence is considered to be an indication of the amyloid fibril formation [21], [22], [23], [24]. This approach is widely accepted and the number of studies based on ThT diagnostic capabilities is rapidly growing. The current status of ThT in the investigation of amyloid fibrils is given in a recent in-depth review [25]. Although it is of great importance for studies of amyloid fibrils, the molecular mechanisms of the specific ThT binding to these structures and the reasons underlying the characteristic increase in the ThT fluorescence quantum yield accompanying the incorporation of this dye into the fibrils are not yet fully understood. A model in which ThT incorporates into fibrils in its monomeric form [26] is in agreement with the explanation of the significant (several orders of magnitude) increase in the ThT fluorescence intensity induced by restriction of torsion oscillations of its fragments [27], [28], [29], [30]. Further support for this model came from the study of the dependence of ThT fluorescence quantum yield and lifetime of the excited state on solvent viscosity and temperature [31]. However, the model analyzed in previous studies proposed that the ThT fluorescence quantum yield should be equal to unity when oscillations of the dye's fragments relative to each other are completely restricted. In this work, a significantly greater range of temperature and viscosity were analyzed, and a new interpretation of the experimental data was given that took into account a non-planar conformation of the ThT molecule in the ground state. The use of the fluorescence dye ATTO-425 with a known quantum yield and spectral characteristics similar to that of ThT has enabled a more accurate evaluation of the absolute values of the ThT fluorescence quantum yield in solutions of different viscosity and therefore, an estimation of the value of the radiative lifetime of the excited state of the dye. It is concluded that the conformation of ThT molecule in ground state affects its fluorescence quantum yield. The validity of this model was proven by the estimation of fluorescence quantum yield of ThT incorporated into the insulin amyloid fibrils.
Figure 1. Model of thioflavin T molecule.
Results and Discussion
ThT fluorescence in solvents with different viscosity
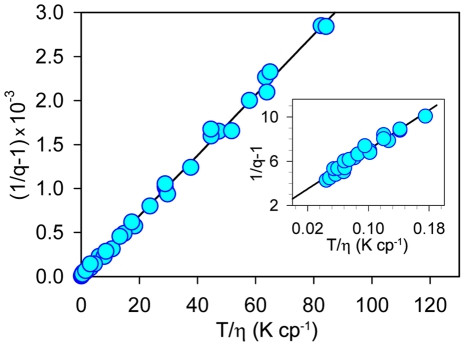
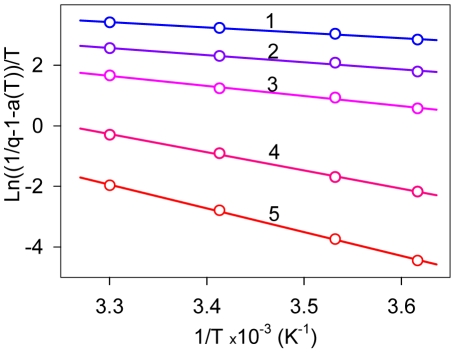
The dependence of ThT fluorescence quantum yield and excited state lifetime on solvent viscosity and temperature was determined in water-glycerol mixtures. Glycerol content was varied from 13 to 99% by weight (wt) and solvent temperature ranged from 3 to 50°C. The experimental data in the form of 1/q−1 vs. T/η plot forms a straight line (Figure 2). The intercept on the ordinate axis is larger than 2.0 (Figure 2; Insert). Figure 3 represents the dependence of the ThT fluorescence quantum yield on temperature in water-glycerol mixtures with different glycerol contents in the 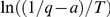 vs. 1/T coordinates. The choice of the coordinates for the presentation of experimental data in Figures 2 and 3 is explained below. Experimental values for fluorescence quantum yield and the excited states lifetimes of ThT in 96–99% wt glycerol at different temperatures are summarized in Table 1.
vs. 1/T coordinates. The choice of the coordinates for the presentation of experimental data in Figures 2 and 3 is explained below. Experimental values for fluorescence quantum yield and the excited states lifetimes of ThT in 96–99% wt glycerol at different temperatures are summarized in Table 1.
Figure 2. Dependence of thioflavin T fluorescence quantum yield on solvent viscosity and temperature in coordinates 1/q vs. T/η.
Solvent viscosity was changed by variation of glycerol content from 13 to 99% in water-glycerol mixtures. Solvent temperature was changed from 3 to 50°C. Insert. The section of the plot corresponding to solutions of high viscosity glycerol content from 96 to 99% and low temperature from 3 to 10°C is given in the extended scale.
Figure 3. Dependence of thioflavin T fluorescence quantum yield on solvent temperature in the coordinates 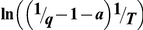 vs.
vs. 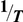 for solutions of different viscosity.
for solutions of different viscosity.
Curves 1–5 correspond to 13, 35, 56, 83 and 99% wt of glycerol content, respectively.
Table 1. Emission Quantum Yield, Excited-State Lifetime and Radiative Lifetime of Thioflavin T in Solutions with Different Glycerol Content at Different Temperatures.
| glycerol content, % wt | t, °C | q | τ, ns | τ r, ns |
| 99 | 5 | 0.158 | 1.14 | 7.18 |
| 7 | 0.156 | 1.02 | 6.51 | |
| 10 | 0.125 | 0.91 | 7.29 | |
| 20 | 0.066 | 0.48 | 7.27 | |
| 98 | 5 | 0.142 | 0.98 | 6.89 |
| 7 | 0.131 | 0.91 | 6.96 | |
| 10 | 0.113 | 0.82 | 7.30 | |
| 96 | 5 | 0.119 | 0.90 | 7.54 |
| 7 | 0.106 | 0.77 | 7.28 | |
| 10 | 0.091 | 0.73 | 8.05 | |
| 20 | 0.046 | 0.35 | 7.61 | |
| 0 | 20 | ∼0.0001a | ∼0.0008c | – |
| η→∞ | T→0 | 0.28a | 2.2b | 7.8 |
There are three structural fragments in the thioflavin T molecule: the benzothiazole ring (I), the benzyl rings (II) and the dimethylamino group (III) (Figure 1). Photophysical properties of this dye are substantially determined by the methyl group at N5 atom of benzothiazole ring [28], [30]. Van der Waals repulsion between this group and hydrogen atoms of the benzyl ring makes the planar conformation energetically unfavorable (Figure 1) and causes an energy barrier for torsional oscillation of benzothiazole and aminobenzoyl rings relative each other at ϕ = 0 (180)°. The other internal rotation barrier at ϕ = 90 (270)° is due to the disturbance of the uniform system of π-conjugated bonds of the benzothiazole and aminobenzoyl rings. The presence of the methyl group at N5 atom of benzothiazole ring not only prevents a planar configuration of the ThT molecule but also diminishes the energy barrier at ϕ = 90 (270)° between states corresponding to energy minima at ϕ = 37 and 145° and at ϕ = 217 and 325°. Different approaches evaluate this barrier as 2.0 or 3.4–4.3 kcal·mol−1. A ThT analog with a methyl group at the N5 atom of the benzothiazole ring replaced by a hydrogen atom has an energy minimum at ϕ = 0 (180)° separated by energy barriers of 11.4 kcal·mol−1 at ϕ = 90 (270)°.
Quantum-chemical calculations revealed that the isolated ThT molecule energy in the excited state is monotonously decreases with the ϕ increase from 0 to 90°, where it reaches its minimal value [29], [30]. Thus, the conformation with the disturbed π-conjugated bond system became energetically favorable. Calculations also showed that for the isolated ThT molecule, there was no energy barrier preventing the molecule from transfer to the state, which, caused the non-radiative deactivation of the excited state as postulated [29], [30]. For ThT molecules in water and alcohol solutions, there are at least two factors preventing the transition from the radiative to the non-radiative state with the disturbed system of π-conjugated bonds: electrostatic interaction with the solvent and solvent viscosity. The obtained experimental data (Figure 2 and Table 1) showed that solvent heating or a decrease in its viscosity (by a change in the water-glycerol content) is accompanied by a dramatic decrease in ThT fluorescence quantum yield and average fluorescence decay time. This means that there is a deactivation process of the excited state of ThT molecule, the rate constant of which depended on solvent temperature and viscosity. As previously suggested [29], [30], such a deactivating process could be torsional oscillations of the benzothiazole and aminobenzyl rings relative to each other. As the minimum of ThT molecule energy in excited state is at ϕ = 90 (270)°, this process will lead to the change of the initial distribution of ϕ angle causing an increase in the fraction of molecules with larger ϕ. We propose that this process, which is accompanied by a decrease in the conjugation of the π-electron system of the benzothiazole and aminobenzyl rings of ThT molecule, can lead to the increase in the non-radiative deactivation of the ThT excited state. According to the Debye-Stokes-Einstein law, the rate constant of this process will be proportional to the ration T/η (see e.g. [32]):
| (1) |
where T is the absolute temperature and η is the solvent viscosity. Taking into account that even in the ground state the ϕ angle between benzothiazole and aminobenzyl rings does not equal to zero, we have:
| (2) |
and
| (3) |
where 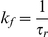 is the rate constant of the deactivation process of the excited state with radiation,
is the rate constant of the deactivation process of the excited state with radiation, 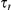 is the radiative lifetime of the excited state,
is the radiative lifetime of the excited state, 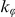 is the rate constant of the ThT molecules reaching the non-fluorescent state with ϕ = 90 (270)°, which usually called TICT (twisted internal charge transfer) and
is the rate constant of the ThT molecules reaching the non-fluorescent state with ϕ = 90 (270)°, which usually called TICT (twisted internal charge transfer) and 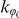 is the rate constant of the excited state deactivation when T→0, η→∞, i.e. when
is the rate constant of the excited state deactivation when T→0, η→∞, i.e. when 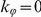 . This state is known as the LE state (local excited). It is likely that deviation of the ThT fluorescence quantum yield from unity in the absence of torsional oscillations of the benzothiazole and aminobenzyl rings relative to each other is caused by the non-planar conformation of ThT molecules in Franck-Condon excited state just after excitation, with a maximum ϕ angle distribution close to ϕ
0 = 37° [29]. We suggest that the ϕ angle distribution of ThT molecules incorporated in amyloid fibrils can differ from that in rigid isotropic solutions and this can be an important factor in determining the fluorescence quantum yield of bound ThT. Within the frame of the given assumption for ThT in solution, we have:
. This state is known as the LE state (local excited). It is likely that deviation of the ThT fluorescence quantum yield from unity in the absence of torsional oscillations of the benzothiazole and aminobenzyl rings relative to each other is caused by the non-planar conformation of ThT molecules in Franck-Condon excited state just after excitation, with a maximum ϕ angle distribution close to ϕ
0 = 37° [29]. We suggest that the ϕ angle distribution of ThT molecules incorporated in amyloid fibrils can differ from that in rigid isotropic solutions and this can be an important factor in determining the fluorescence quantum yield of bound ThT. Within the frame of the given assumption for ThT in solution, we have:
| (4) |
where 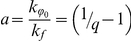 at T→0 и η→∞. Our experimental data showed that the value 1/q determined for ThT in water-glycerol mixtures with different glycerol content and at different temperatures is linearly dependent on T/η (Figure 3). This provides strong support for the model of ThT non-radiative deactivation described above. A relationship similar to (7) was used to describe the ThT fluorescence quantum yield dependence on microviscosity in inverted AOT micelles with different ratios of w0 = [H2O]/[AOT] [33].
at T→0 и η→∞. Our experimental data showed that the value 1/q determined for ThT in water-glycerol mixtures with different glycerol content and at different temperatures is linearly dependent on T/η (Figure 3). This provides strong support for the model of ThT non-radiative deactivation described above. A relationship similar to (7) was used to describe the ThT fluorescence quantum yield dependence on microviscosity in inverted AOT micelles with different ratios of w0 = [H2O]/[AOT] [33].
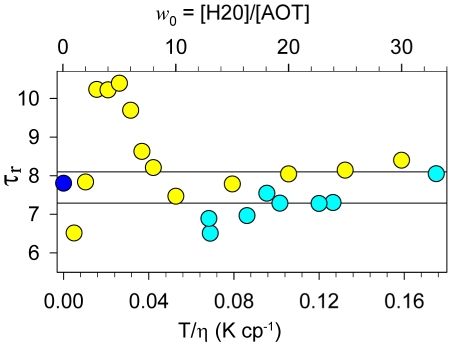
The radiative lifetime, determined as an average value of the ratio of fluorescence quantum yield and fluorescence lifetime of the excited state of ThT in solutions with different viscosity and temperature 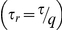 , was estimated as τr = 7.2 ns (Figure 4). The radiative lifetime for ThT in rigid environment was further evaluated based on the value of the fluorescence quantum yield at T/η→0 (qT
/η→0 = 0.28, Figure 3, Insert) and on the value of the ThT excited state lifetime in rigid solutions of glycerol and n-propanol at T = 77K (τ = 2.2 ns) determined earlier [31]. Using these parameters, the radiation lifetime was determined to be τr = 7.8 ns (Figure 4, closed circle). This evaluation is close to the radiative lifetime value obtained above (7.2 ns) and the value derived from the data obtained for the ThT in inverted AOT micelles with different ratios H2O and AOT (8.1 ns) [33]. In Figure 4, the data from [33] are presented in terms of τr (open squares). Average τ
r value was determined based on the data corresponding to w0 in the range from 30 to 10. The w0 decrease from 10 to 5 leads to the dramatic increase in the τr values. This is probably due to the specific interaction of AOT with the dye, which make the dye molecule more planar. The further decrease of w0 leads to the τr decrease practically to the values characteristic to the water solutions. Probably this means that ThT molecules do not incorporate into such micelles. Interestingly, the range of w0 from 30 to 10 corresponds to the range of T/η values from 21 to 12 K·cp−1, whereas in experiments with water-glycerol mixtures, the τr values were determined for ThT in solutions with T/η in the range from 0.18 to 0.07 K·cp−1 (Figure 4).
, was estimated as τr = 7.2 ns (Figure 4). The radiative lifetime for ThT in rigid environment was further evaluated based on the value of the fluorescence quantum yield at T/η→0 (qT
/η→0 = 0.28, Figure 3, Insert) and on the value of the ThT excited state lifetime in rigid solutions of glycerol and n-propanol at T = 77K (τ = 2.2 ns) determined earlier [31]. Using these parameters, the radiation lifetime was determined to be τr = 7.8 ns (Figure 4, closed circle). This evaluation is close to the radiative lifetime value obtained above (7.2 ns) and the value derived from the data obtained for the ThT in inverted AOT micelles with different ratios H2O and AOT (8.1 ns) [33]. In Figure 4, the data from [33] are presented in terms of τr (open squares). Average τ
r value was determined based on the data corresponding to w0 in the range from 30 to 10. The w0 decrease from 10 to 5 leads to the dramatic increase in the τr values. This is probably due to the specific interaction of AOT with the dye, which make the dye molecule more planar. The further decrease of w0 leads to the τr decrease practically to the values characteristic to the water solutions. Probably this means that ThT molecules do not incorporate into such micelles. Interestingly, the range of w0 from 30 to 10 corresponds to the range of T/η values from 21 to 12 K·cp−1, whereas in experiments with water-glycerol mixtures, the τr values were determined for ThT in solutions with T/η in the range from 0.18 to 0.07 K·cp−1 (Figure 4).
Figure 4. Radiative lifetime determined for thioflavin T in solutions of different viscosity and temperature (see Table 1 ) (open circles, bottom axis) and for the dye in the reverse micelles (open squares, top axis).
Radiative lifetime for the dye in the reverse micelles were determined on the basis of the data given in the work [33], where w 0 = [H2O]/[AOT] is the water to surfactant molar ratio. Radiative lifetime for the dye in rigid solution (T/η→0) (closed circle) was determined on the basis of the experimental dependence of 1/q on T/η and the value of fluorescence lifetime determined previously [31].
Extrapolation of the (1/ q−1) vs. T/η dependence gave a ThT fluorescent quantum yield in water at room temperature of q≈0.0001. Taking into account the determined radiative lifetime (7.8 ns), the lifetime of the ThT excited state under these conditions is shorter than 0.001 ns. Therefore, we can conclude that the data obtained based on the experiment with water-glycerol mixtures are in good agreement with the results obtained for the inverse micelles [33], [34].
Temperature-dependence of the ThT fluorescence quantum yield
The main factor used in analysis of the dependence of ThT fluorescence quantum yield on temperature is the temperature dependence of the solvent viscosity, which is described by the equation [32]:
| (5) |
where ΔEη is the activation energy of the solvent viscous flow. For water, this value is 3.7 kcal·mol−1 and data for water-glycerol mixtures with high content of glycerol are given in Table 2. These values were obtained on the basis of data from the study [31]. Combination of equations (4) and (5) resulted in the following equation:
| (6) |
where ΔEq is the activation energy of non-radiative deactivation of the excited state. Therefore, the experimental data were fitted to linear dependence in a 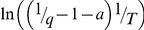 vs
vs 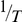 plot (Figure 3). The activation energy of the process of non-radiative deactivation of ThT determined based on the temperature dependence of fluorescence quantum yield ΔEq is slightly larger than the activation energy of the solvent viscous flow ΔEη (Table 2). A possible explanation for this phenomenon is the existence of some other factors (along with viscosity) preventing transition of the ThT molecule from its fluorescent to non-fluorescent state with the disturbed π-conjugated system of bonds. One of these factors is the electrostatic interaction of ThT molecule in the excited state with the molecules of the polar solvent.
plot (Figure 3). The activation energy of the process of non-radiative deactivation of ThT determined based on the temperature dependence of fluorescence quantum yield ΔEq is slightly larger than the activation energy of the solvent viscous flow ΔEη (Table 2). A possible explanation for this phenomenon is the existence of some other factors (along with viscosity) preventing transition of the ThT molecule from its fluorescent to non-fluorescent state with the disturbed π-conjugated system of bonds. One of these factors is the electrostatic interaction of ThT molecule in the excited state with the molecules of the polar solvent.
Table 2. Comparison of activation energy of the solvent viscous flow (ΔEη) with the activation energy of the non-radiative deactivation of excited state (ΔEq) (determined by the temperature dependence of fluorescence quantum yield at high glycerol content in solution).
| glycerol content, % wt | ΔEη, kcal· mol−1 | ΔEq, kcal· mol−1 |
| 96.1 | 16.4 | 20.3 |
| 97.1 | 16.7 | 19.6 |
| 97.8 | 16.8 | 24.2 |
| 98.4 | 17.0 | 21.4 |
| 98.8 | 17.1 | 22.7 |
The ThT molecule has a positive charge (Z = +1e) that is non-uniformly distributed between the molecule fragments. Furthermore, this distribution depends on the angle ϕ between the molecule fragments and changes dramatically on molecule transition to the excited state [29]. The charged molecule with total charge Z can be represented as a molecule with a uniformly distributed charge Z/2 on each fragment and a dipole with the charge |Z 1−Z 2|/2. The larger the charge variation between the molecule fragments, the larger the dipole moment (|Z 1−Z 2|/2)×r, where r is the vector connecting geometrical centers of positive and negative charge. For the ThT molecule in the ground state, the charge allocation on the benzothiazole ring is +0.6, +0.7 and +0.8 and on the aminobenzyl ring, it is +0.4, +0.3 and +0.2. These three values correspond to the angle between the planes of the rings (ϕ equal to 0, 37 and 90°, respectively). For ThT in the excited state, the charge distribution is +0.5, +0.3 and −0.1 on the benzothiazole ring and +0.5, +0.7 and +1.1 on the aminobenzyl ring. This means that the transition to the excited state is accompanied by a reversal in the dipole direction. Therefore, in polar solution, the transition of the molecule to the excited state causes a state of significant non-equilibrium with the solvent. This non-equilibrium will decrease with ϕ→0 and increase with ϕ→90° and is equivalent to the existence of the energy barrier when ThT in the excited state transforms from the fluorescent to non-fluorescent state (ϕ→90°). Consequently, in the presence of polar solution, the transition of the molecule to the non-fluorescent state with ϕ close to 90° will be hindered in comparison to this transition for the isolated molecule.
Fluorescence quantum yield of ThT incorporated into the insulin amyloid fibrils
The fluorescence quantum yield of ThT bound to the amyloid fibrils, qb, was determined using the following equation:
| (7) |
where IThT is the ThT fluorescence in the solution containing amyloid fibrils, IATTO is the ATTO-425 fluorescence intensity in the solution with the optical density determined at the excitation wavelength to be equal to DATTO = Db+Df, (where Db and Df are the optical densities of the bound and free ThT in solution containing amyloid fibrils). The quantum yield of the fibril-bound dye was measured in the solutions of amyloid fibrils and ThT prepared by the equilibrium dialysis. Db and Df values were determined from the absorption spectra of free and fibril-bound dye as described in legend to Figure 5. The fluorescence quantum yield of ThT incorporated into the amyloid fibrils was equal to 0.43. The noticeably higher values of the fluorescence quantum yield of ThT incorporated into the amyloid fibrils in comparison with the corresponding values measured for the dye in the rigid isotropic solution can be explained by the model where the ThT configuration became more planar at the dye embedding into the fibrils.
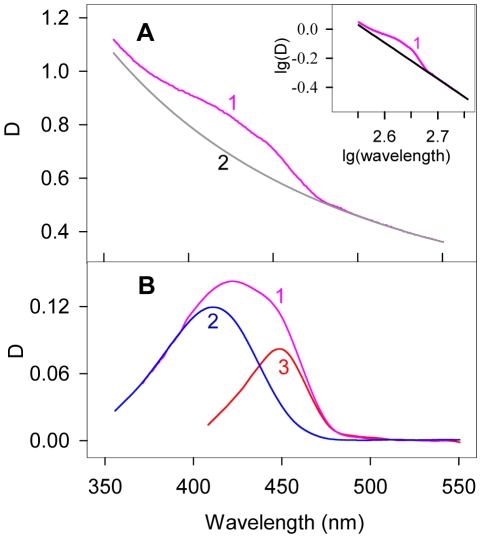
Figure 5. Absorption spectra of ThT incorporated into the insulin amyloid fibrils.
Panel a. Curve 1 – Superposition of the absorption spectra of free ThT (at concentration Cf), ThT bound to fibrils (at dye concentration Cb) and the apparent absorption caused by fibrils light scattering; Curve 2 – Apparent absorption caused by fibrils light scattering. Insert: Determination of the apparent absorption caused by fibrils light scattering: Dscat = aλ−m. Coefficients a and m were determined from the linear part of the curve 1 plotted in logarithmic coordinates lg(Dscat) = f(lg(λ)). Panel b. Curve 1 is the total absorption of free and bound ThT after light scattering subtraction. Curve 2 is the absorption spectra of free ThT. Curve 3 is the absorption spectra of the ThT incorporated into the amyloid fibrils – the difference between 1 and 2 spectra. Insulin concentration was 6.9·10−5 M.
Conclusions
Earlier [31], the dependence of ThT fluorescence quantum yield on solvent viscosity was explained assuming the existence of the unique way of the ThT excited state deactivation, namely the existence of the ultrafast intramolecular twisting of the dye fragments relative to each other. According to this model, the ThT fluorescence quantum yield in rigid solution was expected to be equal to unity. In this study, we used the fluorescence dye ATTO-425 with spectral characteristics close to that of ThT as a reference. This permitted the determination of the ThT fluorescence quantum yield in a wide range of viscosity and temperature. The study demonstrated that the dependence (1/q−1) on T/η is linear and extrapolation of this dependence allowed determination of the fluorescence quantum yield in water at room temperature (q≈0.0001) and showed that ThT fluorescence quantum yield in rigid solution is lower than unity: qT/η →0 = 0.28. This means that along with non-radiative deactivation of excited state by torsional oscillations of the ThT fragments relative to each other, leading to a twisted conformation (ϕ = 90°), there is some other cause of deactivation. We propose that this deactivation is due to the non-planar conformation of the ThT molecule in the ground state and consequently, in the Franck-Condon excited states. Determination of the fluorescence quantum yield and the lifetime of the excited state (τ) for ThT in solutions of different viscosity and temperature allowed the determination of the radiative lifetime (τ r = 7.8 ns) and the calculation of excited state lifetime for ThT in water at room temperature (τ≈0.001 ns). Our results suggested that the fluorescence quantum yield of ThT incorporated into amyloid fibrils is determined not only by the steric restrictions of the ultra-fast twisting of the ThT fragments relative to each other, but also by the conformation of the bound ThT molecule.
It is evident now that fibrils formed by different proteins, or even by the same protein but at different conditions, are not structurally identical [35]. This potentially might result in the noticeable differences in the bound ThT fluorescence intensities. Furthermore, even in a single amyloid fibril, there could be several geometrically different binding sites [36], [37], [38] that are able to interact with different conformations of the dye and therefore resulting in differently bound ThT molecules having dissimilar fluorescence quantum yields. We showed that the ThT bound to the insulin amyloid fibrils is characterized by the fluorescence quantum yield which was noticeably higher than that measured in the rigid isotropic solution (q = 0.43 vs. q = 0.28). We believe that this difference is determined by the conformational difference between the dye molecule in the fibril-bound form and in the rigid isotropic solution, where the fibril-bound ThT is characterized by more planar structure. Consequently, the ThT fluorescence quantum yield can be used to characterize the peculiarities of the fibrillar structure. These observations clearly open new perspectives for the utilization of ThT in structural characterization of amyloid fibrils.
Materials and Methods
Materials
ThT from Sigma-Aldrich (USA) and Fluka (Switzerland) was purified by crystallization from 3∶1 (v/v) acetonitrile with ethanol [27]. ThT “Ultra Pure Grade” from AnaSpec, (USA), glycerol from Merck, (Germany) and fluorescence dye ATTO-425 from ATTO-TEC, (Germany) were used without further purification.
The samples of insulin and buffer components from Sigma (USA) were used without additional purification. Insulin amyloid fibrils were generated according to the standard protocol described earlier [39]. The concentration of insulin in the fibrillar form was determined based on the concentrations of the protein prior to fibrillation. Viscosity of the water-glycerol mixtures was estimated on the basis of glycerol concentrations determined by Abbe refractometer, LOMO, (Russia) at 23°C. The temperature dependence of the viscosity of different water-glycerol mixtures was taken from the literature [40]. ThT concentration in solutions was 1.4×10−5M.
Steady-state and time-resolved fluorescence
Measurements were taken using the spectrofluorometers described earlier [41]. Absorption spectra were analyzed using a EPS-3T (Hitachi, Japan) spectrophotometer.
Fluorescence quantum yield determination
When determining fluorescence quantum yield, the dependence of the ThT molar extinction coefficient on the content of glycerol in the water-glycerol solution was taken into account (Figure 6). The recorded fluorescence intensity was corrected on a solvent refractive index [42]. The independence of the ThT fluorescence spectrum from glycerol content in water-glycerol mixtures suggested that the fluorescence quantum yield is proportional to the fluorescence intensity (λem = 480 nm). An aqueous solution of fluorescent dye ATTO-425 with known quantum yield (q = 0.9) was used as a reference. Fluorescence was excited at 435 nm and recorded at 480 nm.
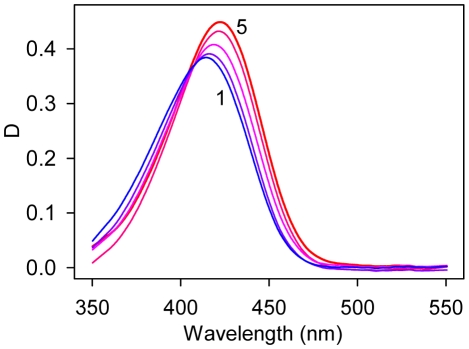
Figure 6. Absorption spectra of thioflavin T in water-glycerol mixtures.
Curves 1–5 correspond to 13, 35, 56, 83 and 99% wt glycerol content, respectively.
Analysis of the fluorescence decay
Decay curves were fitted using the non-linear least-squares method. Minimization was accomplished according to Marquardt [43]. Experimental data were analyzed using the multiexponential approach:
| (8) |
where αi and τi are amplitude and lifetime of component i, 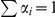 . The root-mean square value of fluorescent lifetimes,
. The root-mean square value of fluorescent lifetimes, 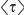 , for biexponential decay is determined as:
, for biexponential decay is determined as:
| (9) |
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by the Belorussian Foundation of Basic Research (grant X10P-227), the Russian Foundation of Basic Research (grant 10-04-90038_Bel), Program “Molecular and Cell Biology,” Russian Academy of Sciences, and Dmitry Zimin's Russian Charitable Foundation “Dynasty.” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harper JD, Lieber CM, Lansbury PT., Jr Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer's disease amyloid-beta protein. Chem Biol. 1997;4:951–959. doi: 10.1016/s1074-5521(97)90303-3. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JW. Amyloid fibril formation and protein misassembly: a structural quest for insights into amyloid and prion diseases. Structure. 1997;5:595–600. doi: 10.1016/s0969-2126(97)00215-3. [DOI] [PubMed] [Google Scholar]
- 3.Carrell RW, Gooptu B. Conformational changes and disease-serpins, prions and Alzheimer's. Curr Opin Struct Biol. 1998;8:799–809. doi: 10.1016/s0959-440x(98)80101-2. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto M, Masliah E. Alpha-synuclein in Lewy body disease and Alzheimer's disease. Brain Pathol. 1999;9:707–720. doi: 10.1111/j.1750-3639.1999.tb00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo EH, Lansbury PT, Jr, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci U S A. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uversky VN, Talapatra A, Gillespie JR, Fink AL. Protein deposits as the molecular basis of amyloidosis. Part I. Systemic amyloidoses. Med Sci Monitor. 1999;5:1001–1012. [Google Scholar]
- 7.Uversky VN, Talapatra A, Gillespie JR, Fink AL. Protein deposits as the molecular basis of amyloidosis. II. Localized amyloidosis and neurodegenerative disorders. Med Sci Monitor. 1999;5:1238–1254. [Google Scholar]
- 8.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 9.Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta. 2004;1698:131–153. doi: 10.1016/j.bbapap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 11.Fandrich M, Fletcher MA, Dobson CM. Amyloid fibrils from muscle myoglobin. Nature. 2001;410:165–166. doi: 10.1038/35065514. [DOI] [PubMed] [Google Scholar]
- 12.Pertinhez TA, Bouchard M, Tomlinson EJ, Wain R, Ferguson SJ, et al. Amyloid fibril formation by a helical cytochrome. FEBS Lett. 2001;495:184–186. doi: 10.1016/s0014-5793(01)02384-5. [DOI] [PubMed] [Google Scholar]
- 13.Naiki H, Higuchi K, Hosokawa M, Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 1989;177:244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 14.Naiki H, Higuchi K, Matsushima K, Shimada A, Chen WH, et al. Fluorometric examination of tissue amyloid fibrils in murine senile amyloidosis: use of the fluorescent indicator, thioflavine T. Lab Invest. 1990;62:768–773. [PubMed] [Google Scholar]
- 15.LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeVine H., 3rd Thioflavine T interaction with amyloid-sheet structures. Amyloid: Int J Exp Clin Invest. 1995;2:1–6. [Google Scholar]
- 17.LeVine H., 3rd Stopped-flow kinetics reveal multiple phases of thioflavin T binding to Alzheimer beta (1–40) amyloid fibrils. Arch Biochem Biophys. 1997;342:306–316. doi: 10.1006/abbi.1997.0137. [DOI] [PubMed] [Google Scholar]
- 18.LeVine H., 3rd Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 19.Allsop D, Swanson L, Moore S, Davies Y, York A, et al. Fluorescence anisotropy: a method for early detection of Alzheimer beta-peptide (Abeta) aggregation. Biochem Biophys Res Commun. 2001;285:58–63. doi: 10.1006/bbrc.2001.5123. [DOI] [PubMed] [Google Scholar]
- 20.Yoshiike Y, Chui DH, Akagi T, Tanaka N, Takashima A. Specific compositions of amyloid-beta peptides as the determinant of toxic beta-aggregation. J Biol Chem. 2003;278:23648–23655. doi: 10.1074/jbc.M212785200. [DOI] [PubMed] [Google Scholar]
- 21.Goers J, Permyakov SE, Permyakov EA, Uversky VN, Fink AL. Conformational prerequisites for alpha-lactalbumin fibrillation. Biochemistry. 2002;41:12546–12551. doi: 10.1021/bi0262698. [DOI] [PubMed] [Google Scholar]
- 22.Ban T, Hamada D, Hasegawa K, Naiki H, Goto Y. Direct observation of amyloid fibril growth monitored by thioflavin T fluorescence. J Biol Chem. 2003;278:16462–16465. doi: 10.1074/jbc.C300049200. [DOI] [PubMed] [Google Scholar]
- 23.Kumita JR, Weston CJ, Choo-Smith LP, Woolley GA, Smart OS. Prevention of peptide fibril formation in an aqueous environment by mutation of a single residue to Aib. Biochemistry. 2003;42:4492–4498. doi: 10.1021/bi026856t. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, Zhang XJ, Wang LY, Zhou JM, Perrett S. Relationship between stability of folding intermediates and amyloid formation for the yeast prion Ure2p: a quantitative analysis of the effects of pH and buffer system. J Mol Biol. 2003;328:235–254. doi: 10.1016/s0022-2836(03)00249-3. [DOI] [PubMed] [Google Scholar]
- 25.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J Chem Biol. 2009 doi: 10.1007/s12154-009-0027-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krebs MR, Bromley EH, Donald AM. The binding of thioflavin-T to amyloid fibrils: localisation and implications. J Struct Biol. 2005;149:30–37. doi: 10.1016/j.jsb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Voropai ES, Samtsov MP, Kaplevsky KN, Maskevich AA, Stepuro VI, et al. Spectral properties of Thioflavine T and its complexes with amyloid fibrils. J Appl Spectrosc. 2003;70:868–874. [Google Scholar]
- 28.Turoverov KK, Kuznetsova IM, Maskevich AA, Stepuro VI, Kuzmitsky VA, et al. Tht as an instrument for testing and investigation of amyloid and amyloid-like fibrils. Proc of SPIE. 2007;6733 [Google Scholar]
- 29.Stsiapura VI, Maskevich AA, Kuzmitsky VA, Turoverov KK, Kuznetsova IM. Computational study of thioflavin T torsional relaxation in the excited state. J Phys Chem A. 2007;111:4829–4835. doi: 10.1021/jp070590o. [DOI] [PubMed] [Google Scholar]
- 30.Maskevich AA, Stsiapura VI, Kuzmitsky VA, Kuznetsova IM, Povarova OI, et al. Spectral properties of thioflavin T in solvents with different dielectric properties and in a fibril-incorporated form. J Proteome Res. 2007;6:1392–1401. doi: 10.1021/pr0605567. [DOI] [PubMed] [Google Scholar]
- 31.Stsiapura VI, Maskevich AA, Kuzmitsky VA, Uversky VN, Kuznetsova IM, et al. Thioflavin T as a molecular rotor: fluorescent properties of thioflavin T in solvents with different viscosity. J Phys Chem B. 2008;112:15893–15902. doi: 10.1021/jp805822c. [DOI] [PubMed] [Google Scholar]
- 32.Loutfy RO, Arnold BA. Effect of viscosity and temperature on torsional relaxation of molecular rotors. J Phys Chem. 1982;86:4205–4211. [Google Scholar]
- 33.Singh PK, Kumbhakar M, Pal H, Nath S. Ultrafast torsional dynamics of protein binding dye thioflavin-T in nanoconfined water pool. J Phys Chem B. 2009;113:8532–8538. doi: 10.1021/jp902207k. [DOI] [PubMed] [Google Scholar]
- 34.Singh PK, Kumbhakar M, Pal H, Nath S. Viscosity effect on the ultrafast bond twisting dynamics in an amyloid fibril sensor: thioflavin-T. J Phys Chem B. 2010;114:5920–5927. doi: 10.1021/jp100371s. [DOI] [PubMed] [Google Scholar]
- 35.Loksztejn A, Dzwolak W. Chiral bifurcation in aggregating insulin: an induced circular dichroism study. J Mol Biol. 2008;379:9–16. doi: 10.1016/j.jmb.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 36.Biancalana M, Makabe K, Koide A, Koide S. Molecular mechanism of thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J Mol Biol. 2009;385:1052–1063. doi: 10.1016/j.jmb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockhart A, Ye L, Judd DB, Merritt AT, Lowe PN, et al. Evidence for the presence of three distinct binding sites for the thioflavin T class of Alzheimer's disease PET imaging agents on beta-amyloid peptide fibrils. J Biol Chem. 2005;280:7677–7684. doi: 10.1074/jbc.M412056200. [DOI] [PubMed] [Google Scholar]
- 38.Ye L, Morgenstern JL, Gee AD, Hong G, Brown J, et al. Delineation of positron emission tomography imaging agent binding sites on beta-amyloid peptide fibrils. J Biol Chem. 2005;280:23599–23604. doi: 10.1074/jbc.M501285200. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen L, Khurana R, Coats A, Frokjaer S, Brange J, et al. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40:6036–6046. doi: 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- 40.Segur JB. Physical properties of glycerol and its solutions. New York: Reinhold Publ Corp; 1953. pp. 238–334. [Google Scholar]
- 41.Turoverov KK, Biktashev AG, Dorofeiuk AV, Kuznetsova IM. [A complex of apparatus and programs for the measurement of spectral, polarization and kinetic characteristics of fluorescence in solution]. Tsitologiia. 1998;40:806–817. [PubMed] [Google Scholar]
- 42.Lakowicz JR. Principles of fluorescence spectroscopy. Springer, New York; 2006. [Google Scholar]
- 43.Marquardt DW. An algorithm for least-squares estimation of non linear parameters. J Soc Ind Appl Math. 1963;11:431–441. [Google Scholar]