Abstract
Background
The area-at-risk (AAR) is a key determinant of myocardial infarction (MI) size. We investigated whether MRI measurement of AAR would correlate with an angiographic AAR risk score in patients with acute MI.
Methods and Results
Bright blood T2-prepared steady-state free-precession MRI (T2-SSFP MRI) was used to depict the AAR in 50 consecutive acute MI patients while infarct size was measured on gadolinium late contrast enhancement images. AAR was also estimated using the APPROACH and DUKE angiographic jeopardy scores and ST elevation score. Myocardial salvage was AAR minus infarct size. Results are mean±SD unless specified. Patients were of 61±12 years of age; 76% had ST elevation MI; 20% had prior MI. All underwent MRI 4±2 days following initial presentation. The relationship between MRI and the APPROACH angiographic estimates of AAR were similar (overall size relative to LV mass (32±12% vs. 30±12% respectively, p=0.33)), correlated well (r=0.78; p<0.0001) and had 2.5% bias in Bland Altman analysis. The DUKE Jeopardy Score underestimated AAR relative to infarct size and correlated less well with MRI (r=0.39; p=0.0055). ST elevation score underestimated infarct size in 19(50%) subjects and did not correlate with MRI (r=0.27; p=0.06). Myocardial salvage varied according to TIMI flow grade at the end of angiography/PCI (p=0.04) and TIMI flow grade was a univariable predictor of myocardial salvage (p=0.011). In multivariable analyses, infarct size was predicted by T2-SSFP MRI (p<0.0001).
Conclusions
T2-SSFP MRI delineates the AAR and enables estimation of myocardial salvage when coupled with a measurement of infarct size.
Keywords: myocardial infarction, magnetic resonance imaging, edema, myocardial ischemia
Introduction
Salvaging threatened myocardium during acute coronary occlusion is a key therapeutic objective. The extent of myocardium subject to ischemia, also known as the myocardial area-at-risk (AAR), is a determinant of infarct size and prognosis (1;2). Therefore, identification of the ischemic AAR may provide useful information for clinical and research purposes. Although it is possible to image the AAR with SPECT in research studies, determination of initial AAR and myocardial salvage in routine clinical practice has not been feasible (3). Preclinical validation studies have shown that cardiac magnetic resonance imaging (MRI) may now enable AAR delineation and estimation of salvage (4). Since changes in myocardial water content and mobility are an early consequence of ischemia (5), alterations in proton transverse relaxation times (T2) enable depiction of myocardial edema by MRI (6). Given edema formation corresponds to the ischemic AAR (7), which is typically greater than infarct size, edema imaging by MRI represents a non-invasive approach to AAR estimation.
T2 weighted MRI has considerable potential to guide management following AMI (8-11). Since several variables beyond AAR modulate infarct size, we hypothesized that multivariable analysis of clinical, angiographic, and MRI parameters would reveal the relative strength of these factors in predicting AAR, infarct size and myocardial salvage. Thus, the specific aim of this study was to validate that the ischemic AAR can be measured in patients using T2 weighted MRI. Our first hypothesis was that AAR derived by T2 weighted MRI should correlate with angiographic estimates of AAR. Our second hypothesis was that T2 weighted MRI can delineate the ischemic AAR in MI patients, including in those with prior MI, as determined by history and catheter and electrocardiographic data determining the infarct-related artery. Thus, we used T2 weighted MRI to determine AAR in 50 consecutive patients with acute MI.
Methods
Patient Population and AMI Management
Fifty consecutive patients who underwent invasive management for acute MI at a community hospital and who also had T2-weighted MRI were analyzed. No patients were excluded due to poor image quality. Exclusion criteria represented standard contra-indications to MRI or an estimated glomerular filtration rate < 30 ml/min/1.73 m2. This research was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, Bethesda, MD.
MI was defined according to a history of symptoms consistent with acute myocardial ischemia, with or without ST-segment elevation on the electrocardiogram (ECG) associated with a typical rise of troponin I (TnI) concentration (12). Cardiogenic shock was determined based on the following criteria: acute heart failure consistent with Killip class IV and/or a systolic blood pressure ≤90 mmHg despite a fluid challenge together with signs of tissue hypoperfusion (13). Acute MI management followed contemporary guidelines (12). Aspiration thrombectomy, direct stenting, anti-thrombotic drugs and other therapies were administered according to clinical judgement. Opening pulse pressure was recorded at the beginning of the catheter laboratory procedure.
MRI Acquisition and Analyses
MRI was performed on a Siemens Magnetom Espree (Erlangen, Germany) 1.5-T scanner with 12 surface-coil elements. The MRI protocol included steady state free precession (SSFP) cine MRI, T2-prepared SSFP (T2p-SSFP) edema MRI (4), and delayed enhancement phase sensitive inversion recovery (PSIR) sequences (14). Sample images are shown in Figure 1. We used bright blood T2-weighted MRI (4) to avoid bright rim artifacts associated with black blood T2-STIR MRI (4). A T2-prepared single-shot SSFP sequence with parallel techniques to reduce imaging duration was used to repetitively acquire an interleaved T2 weighted image and a proton density weighted reference mid-diastolic image every 2 R-R intervals (4) using prospective ECG gating. The proton density-weighted image was used for surface coil correction. Typical imaging parameters were: bandwidth = 977 Hz/pixel, TE/TR = 1.6/3.2 ms, flip angle 60° - 90°, and T2 preparation TE = 60 ms. Parallel imaging (rate 2) was used. Temporal resolution within the cardiac cycle was 175 ms. The in-plane resolution was typically 1.9 × 2.5 mm2 with a 6 mm slice thickness. Eight respiratory motion corrected images were obtained per acquisition.
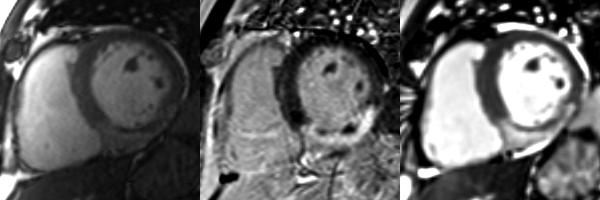
Figure 1.
Matched diastolic cardiac MRI (left - cine MRI; middle - phase sensitive inversion recovery image; right - T2-weighted steady state free precession [SSFP]) obtained in a 57 year-old man one week after primary PCI to the right coronary artery for an acute inferior STEMI. Post-PCI culprit artery flow was reduced (TIMI flow grade 1). Transmural infarction (as revealed by late gadolinium enhancement, middle) corresponds with transmural edema (bright blood T2-weighted SSFP, right). Absolute AAR and salvage in this patient were 29% and 6%, respectively. The central dark zones within the infarct territory represent MVO complicated by hemorrhage (28).
Microvascular obstruction (MVO) was defined as a dark zone on early delayed enhancement imaging 1, 3, 5 and 7 minutes post-contrast injection and within an area of late gadolinium enhancement. MI was imaged using segmented phase-sensitive inversion recovery (PSIR) turbo fast low-angle shot (14), starting about 9 minutes after intravenous injection of 0.15 mmol/kg of gadolinium diethyltriaminepenta-acetic acid (Gd-DTPA, Magnevist, Berlex). Typical imaging parameters were bandwidth = 140 Hz/pixel, TE/TR = 4.2/8.7 ms, readout flip angle = 25°, FOV = 360 × 270 mm, in-plane spatial resolution = 1.4 × 2.2 mm (matrix = 256 × 125), views per segment = 25 and slice thickness 6 mm.
MR Image Analyses
All MR images were analyzed on a Siemens Leonardo workstation by a level 3 trained cardiologist blinded to the patients’ history and outcomes. Left ventricular dimensions and volumes and ejection fraction were quantified using computer assisted planimetry.
Standardized Measurements of T2-weighted Area at Risk
Hyperintense zones on T2-weighted MRI images were first reviewed by two cardiologists who were both blinded to the angiographic data and clinical history to ensure consensus agreement on the affected territory. Each observer measured AAR independently. Left ventricular endo- and epicardial borders were delineated. The window setting was defined as the sum of the mean myocardial signal intensity (SI) of the unaffected area plus 2 standard deviations (SDs) for this area. The level setting was set at the mean SI of the unaffected area. The jeopardized LV AAR was defined as the percentage of LV volume delineated by the hyperintense zone on T2-weighted images. Inter-observer variability in AAR measurement was evaluated using data from 8 (≈15% of overall cohort) randomly selected subjects.
Infarct Size
Infarct size was measured on contrast enhanced images using validated software (15;16) and expressed as a percentage of total LV mass. MVO regions were included within the infarct area. Transmural extent of infarction was categorized qualitatively on a typical 5 point scale.
Myocardial Salvage
Myocardial salvage, as estimated by MRI, was calculated by subtraction of percent infarct size from percent AAR.
Angiographic Jeopardy Scores and Coronary and Collateral Flow Grades
The Jeopardy Score from Duke University (1) and the Lesion Score from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) (2) provided angiographic estimates of the AAR (17).
All angiograms were analyzed by two interventional cardiologists independent of the MRI analyses. The percentage of jeopardized myocardium distal to the infarct-related artery on the index angiogram was calculated for the APPROACH Lesion Score and Duke Jeopardy Score. The Rentrop classification was used to evaluate coronary collateral supply (18). The Thrombolysis in Myocardial Infarction (TIMI) flow classification was used to grade culprit artery flow at initial angiography and at the end of the procedure (19).
Biochemical Assessment of Infarct Size
Peak TnI (AccuTnI®; Beckman Coulter) was used as a biochemical measure of infarct size.
Electrocardiographic Assessment of Infarct Size
Since the extent of ST-elevation at presentation is a reasonable predictor of the extent of myocardial injury (20), we also used the admission electrocardiogram (ECG) with the most extensive ST elevation as a reference measure for percent jeopardized myocardium (20).
Statistical Analyses
Normality was confirmed or excluded using the Shapiro-Francia test. Mean (SD) values and medians (interquartile range) were calculated. Correlations (r) between normally and non-normally distributed variables were tested by Pearson's or Spearman's methods, respectively. All tests were two-tailed. Between-group comparisons of normally distributed continuous data were undertaken using a Student's t test or with analysis of variance (ANOVA). Between-group comparisons of non-normally distributed data were performed with a Mann Whitney test. A Fisher's exact test was used to assess the difference in proportions.
Univariable regression models were constructed to determine the predictors of MRI-derived AAR, IS and salvage. Linearity assumptions were satisfied for variables entered into the univariable and multivariable models. Relationships were described using the correlation coefficient (r) and beta-estimate with 95% confidence intervals. A multivariable model was constructed for predictors of IS. Variables which were measures of IS (e.g. TnI) or which are consequences of MI (e.g. LV volumes and function) were not included in the multivariable model for IS. Univariable predictors significant at a level of P<0.1 were entered into the multivariable model. In the multivariable model for IS, the proportion of variability accounted for by each variable was expressed as the coefficient of determination (R2) and beta-coefficient (continuous data) for a given increment of the covariate are reported along with the associated P-value.
A significance level of 5% was used in all tests. No adjustments were made to p-values to account for multiple testing. All statistical analyses were performed using STATA version 7 (Statacorp, College Station, TX).
Results
Fifty acute MI patients (mean±SD age 61±12 years; 76% ST elevation MI) underwent MRI 4±2 days following initial management between January 23, 2006 and February 12, 2008 (Tables 1, 2, and 3).
Table 1.
Clinical characteristics of acute MI patients who underwent invasive management and in whom MRI was performed during the index admission.
| All n=50 | STEMI n=38 (76%) | NSTEMI n=12 (24%) | P | |
|---|---|---|---|---|
| Mean±SD age, yrs | 61±12 | 58±11 | 66±12 | 0.050 |
| Male gender | 38 (76) | 29 (76) | 9 (75) | 0.93 |
| History, n (%) | ||||
| History of myocardial infarction | 10 (20) | 5 (13) | 5 (42) | 0.031 |
| Diabetes mellitus | 11 (22) | 9 (24) | 3 (25) | 0.61 |
| Hypertension | 21 (42) | 14 (37) | 7 (58) | 0.19 |
| History of cigarette smoking | 13 (26) | 11 (29) | 2 (17) | 0.40 |
| History of previous angina | 12 (24) | 6 (16) | 6 (50) | 0.016 |
| History of chronic heart failure | 4 (8) | 1 (3) | 3 (25) | 0.013 |
| Dyslipidemia | 37 (74) | 27 (71) | 10 (83) | 0.40 |
| History of PCI | 3 (6) | 1 (3) | 2 (17) | 0.074 |
| History of CABG | 4 (8) | 1 (3) | 3 (25) | 0.013 |
| Presenting characteristics | ||||
| Cardiogenic shock | 4 (8) | 4 (11) | 0 | 0.24 |
| Mean±SD pulse pressure, mmHg | 49±22 | 47±21 | 54±23 | 0.35 |
| Median (IQR) peak troponin I, μg/L | 30.1 (8.8, 76.4) | 41.0 (18.7, 95.1) | 6.0 (4.2, 13.0) | 0.0005 |
| Median (IQR) time from onset of symptoms to PCI, hrs | 5.4 (3.1, 15.4) | 4.6 (3.0, 11.8) | 32 (8.4, 72) | 0.0057 |
| Median (IQR) door-to-balloon time, hrs | 1.8 (1.0, 3.2) | 1.6 (0.9, 2.2) | 6.5 (3.4, 48) | 0.0034 |
| Electrocardiogram on admission | ||||
| Sum of ST segment elevation | - | 4 (6, 10) | - | - |
| ST elevation jeopardy score | - | 18.2±7.3 | - | - |
| Drug therapy, n (%) | ||||
| Aspirin | 50 (100) | 38 (100) | 12 (100) | 1.00 |
| Glycoprotein IIbIIIa inhibitor | 26 (52) | 23 (60) | 3 (25) | 0.032 |
| Prior clopidogrel therapy | 12 (24) | 10 (26) | 2 (17) | 0.50 |
| Beta-blocker | 50 (100) | 12 (100) | 38 (100) | 1.00 |
STEMI = ST elevation myocardial infarction; NSTEMI = Non-ST elevation myocardial infarction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft.
Table 2.
Cardiac catheter laboratory findings and outcomes.
| All n=50 | STEMI n=38 (76%) | NSTEMI n=12 (24%) | P | |
|---|---|---|---|---|
| Angiographic characteristics, n (%) | ||||
| Multivessel coronary artery disease* | 29 (58) | 22 (58) | 7 (58) | 0.98 |
| Emergent procedure | 41 (80) | 34 (89) | 7 (58) | 0.014 |
| Infarct-related territory | ||||
| Right | 15 (30) | 14 (36) | 1 (8) | 0.11 |
| Left anterior descending | 31 (62) | 22 (58) | 9 (75) | |
| Circumflex | 4 (8) | 2 (5) | 2 (17) | |
| Rentrop's collateral grade | ||||
| 0 | 32 (64) | 24 (63) | 8 (67) | |
| 1 | 5 (10) | 4 (10) | 1 (8) | 0.80 |
| 2 | 11 (22) | 9 (24) | 2 (17) | |
| 3 | 2 (4) | 1 (3) | 1 (8) | |
| TIMI flow grade (first view) | ||||
| 0 | 31 (62) | 25 (66) | 6 (50) | |
| 1 | 4 (8) | 4 (10) | 0 | |
| 2 | 6 (12) | 3 (8) | 3 (25) | 0.23 |
| 3 | 9 (18) | 6 (16) | 3 (25) | |
| TIMI flow grade post-procedure | ||||
| 0 | 5 (10) | 2 (5) | 3 (25) | |
| 1 | 3 (6) | 3 (8) | 0 | |
| 2 | 15 (30) | 12 (32) | 3 (25) | 0.20 |
| 3 | 27 (54) | 21 (55) | 6 (50) | |
| Invasive management | ||||
| PCI | 43 (86) | 34 (89) | 9 (75) | |
| CABG | 3 (6) | 2 (5) | 1 (8) | 0.062 |
| PCI and CABG | 2 (4) | 2 (5) | 0 | |
| Angiography without intervention | 2 (4) | 0 | 2 (17) | |
| Aspiration thrombectomy | 13 (26) | 11 (29) | 2 (17) | 0.40 |
| Angiographic risk scores | ||||
| Mean±SD APPROACH Lesion Score | 30±12 | 31±12 | 26±10 | 0.16 |
| Median (IQR) Duke Jeopardy Score | 17 (17, 33) | 17 (17, 33) | 21 (17, 33) | 0.83 |
Multivessel coronary artery disease was defined according to the presence of 2 or more arteries with stenoses of at least 50% of the reference vessel diameter, by visual assessment.
Table 3.
Cardiac MRI findings.
| All n=50 | STEMI n=38 (76%) | NSTEMI n=12 (24%) | P | |
|---|---|---|---|---|
| LV dimensions and function, mean±SD | ||||
| LV ejection fraction, % | 51±11 | 51±10 | 51±15 | 0.94 |
| End-diastolic volume index, ml/m2 | 84±25 | 84±24 | 86±29 | 0.77 |
| End-systolic volume index, ml/m2 | 43±20 | 42±18 | 45±28 | 0.74 |
| Left atrial volume index, ml/m2 | 44±14 | 42±14 | 43±14 | 0.68 |
| LV mass, g/m2 | 69±18 | 69±17 | 70±22 | 0.80 |
| Late gadolinium enhancement, n (%) | ||||
| Number of affected territories per patient | ||||
| 1 | 46 (92) | 37 (97) | 9 (75) | 0.013 |
| 2 | 4 (8) | 1 (3) | 3 (25) | |
| Wall motion score index | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.6) | 1.2 (1.0, 1.9) | 0.39 |
| Transmurality, n (%) | 34 (68) | 26 (68) | 8 (67) | 0.91 |
| Mean±SD acute infarct size, % | 18.8±12.4 | 21.4±12.5 | 10.7±8.1 | 0.0016 |
| Microvascular obstruction | 42 (84%) | 32 (84%) | 10 (83%) | 0.94 |
| Edema imaging | ||||
| Mean±SD area-at-risk, % | 32.2±11.9 | 34.7±11.3 | 24.3±10.8 | 0.0074 |
| Myocardial salvage | ||||
| Mean±SD % salvage (%AAR - %IS) | 13.5±8.6 | 13.4±8.4 | 13.7±9.8 | 0.78 |
Characteristics of Selected Patients Groups
Compared to patients without prior MI, patients with prior MI (n=10; 20%) had a higher frequency of NSTEMI (50% vs. 18%; p=0.031) and prior coronary revascularization (40% vs. 8%; p=0.008). Baseline TIMI flow (p=0.06) and collateral grades (p=0.09) tended to be higher in patients with prior MI, whereas TIMI flow grades post-PCI were similar between groups. Compared to patients without a history of revascularization, patients with prior revascularization had a lower LVEF (43±16% vs. 52±9%; p=0.030) and lower TIMI flow grades post-PCI (p=0.017).
Cardiac MRI Findings
The cardiac MRI findings are summarized in Table 3. Figure 1 shows a representative MRI image of MI. The 95% limits of agreement for AAR estimation by two independent observers were –12% and 15%, respectively. There was no evidence of bias (p=0.14). Myocardial salvage was greater in patients with a history of MI (19±10%) compared to in patients without prior MI (12±8%; p=0.022). AAR, infarct size and salvage in patients with a history of prior revascularization were similar to MRI findings in patients without prior revascularization.
Agreement Between Localization of Edema and Infarction
In 48 (96%) patients, there was good agreement between bright-blood T2-weighted MRI and acute infarct localization for the culprit artery revealed by coronary angiography.
Relationships between MRI-derived AAR and other Jeopardy Scores
The relationship between MRI and the APPROACH angiographic estimates of AAR were similar (overall size relative to LV mass (32±12% vs. 30±12% respectively, p=0.33)). In all MI patients, AAR derived by T2 weighted MRI correlated strongly with the APPROACH Lesion Score (r=0.78; p<0.0001) and moderately with the DUKE jeopardy score (r=0.54; p=0.0001; Table 4). When further restricted to patients with first STEMI and no prior CABG, there was a similar correlation between AAR derived by T2 weighted MRI and the APPROACH Lesion Score (r=0.74; p<0.0001).
Table 4.
Univariable predictors of MRI-derived percent AAR in all MI patients (n=50).
| Variable | r | Beta-estimate | 95% CI | P value |
|---|---|---|---|---|
| Univariable predictors of AAR | ||||
| APPROACH Lesion Score, % | 0.78 | 0.79 | 0.60, 0.97 | <0.0001 |
| DUKE Jeopardy Score, % | 0.54 | 0.51 | 0.28, 0.74 | 0.0001 |
| ST elevation jeopardy score, mm | 0.35 | 0.57 | 0.06, 1.07 | 0.028 |
| STEMI | 0.37 | 10.36 | 2.91, 17.82 | 0.007 |
The relationship between IS measured by contrast-enhanced MRI and AAR measured by T2 weighted MRI provides a check for internal consistency as AAR is expected to be greater or equal to infarct size. Forty six (92%), 44 (88%), 35 (70%) and 19 (50%) patients had an AAR greater or equal to the infarct size, when AAR was estimated by T2 weighted MRI, the APPROACH Lesion Score, the DUKE Jeopardy Score, and an ST elevation myocardial jeopardy score, respectively (Figure 2). The relationship between IS and AAR was most constricted when measured by MRI and least constricted when estimated by the ECG.
Figure 2.
Scatterplots with lines of identity for the relationships between infarct size measured by MRI versus 1) AAR derived with bright blood T2-weighted MRI (r=0.74; p<0.0001); 2) the APPROACH Lesion Score (r=0.66; p<0.0001); 3) the DUKE Jeopardy Score (r=0.39; P=0.0055); and 4) an ST elevation jeopardy score (r=0.27; p=0.06; n=38 patients). As infarct size is physiologically a subset of the AAR, the expected relationship between AAR and infarct size involves all points at or below the line of identity.
In STEMI patients (n=38), AAR estimated by T2 weighted MRI correlated with the APPROACH Lesion Score (r=0.79; p<0.0001) and DUKE Jeopardy Scores (r=0.54; p=0.0005). In patients with index MI (n=40; Figure 3), there was good correlation between T2-weighted MRI and the APPROACH Lesion Score AAR estimates. On Bland Altman analysis there was a 2.5% bias (p=0.1) with 95% confidence limits of +19.6% and –14.6%. T2-weighted MRI and the Duke Jeopardy Score AAR estimates correlated less well and demonstrated a bias of 6.7% (p<0.01) with 95% confidence limits of +31.6% and –18.2% on Bland Altman analysis.
Figure 3.
Relationships between AAR derived by T2 weighted MRI and the APPROACH and DUKE angiographic scores in STEMI and NSTEMI patients without a history of prior myocardial infarction (n=40). Bland Altman analysis revealed a 2.5% bias and 95% confidence limits between 19.6% and -14.6% for MRI vs APPROACH. Bland Altman analysis revealed a 6.7% bias and 95% confidence limits between 31.6% and -18.2% for MRI vs DUKE score.
Relationships between AAR, IS and salvage by MRI and other measures of infarct severity
Compared to patients with non-transmural MI, patients with transmural MI had larger mean AAR (35±11% vs. 25±12%; p=0.0037) and mean IS (23±12% vs. 11±9%; p=0.0007) by MRI, whereas myocardial salvage was similar in each group (p=0.5). Moderate correlations were also observed between MRI derived AAR and peak TnI concentration (r=0.55; p<0.0001). The ST elevation score did not correlate with MRI (r=0.27; p=0.06).
Hemodynamic and microvascular factors associated with AAR, IS and salvage
Persistent flow to the ischemic territory influences infarct size and myocardial salvage
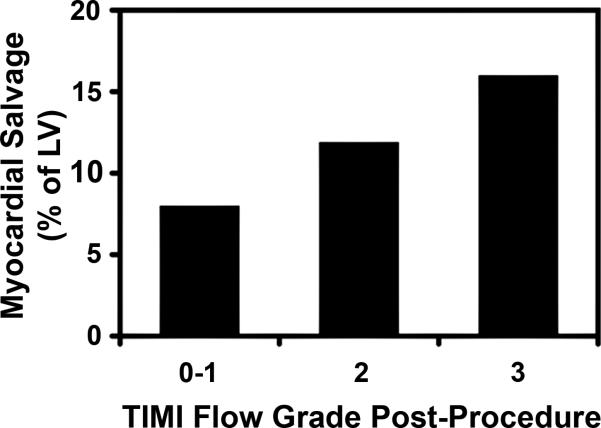
Mean infarct size fell with increasing TIMI flow grade at baseline angiography (p=0.02) and the sum of collateral and TIMI flow influenced infarct size (Table 5). TIMI flow grade post-procedure influenced myocardial salvage (Table 6 and Figure 4).
Table 5.
Predictors of MRI-derived percent infarct size (n=50).
| Variable | r | Beta-estimate | 95% CI | P value |
|---|---|---|---|---|
| Univariable predictors of IS | ||||
| AAR by MRI, % | 0.74 | 0.76 | 0.56, 0.96 | <0.0001 |
| APPROACH Lesion Score, % | 0.67 | 0.70 | 0.47, 0.93 | <0.0001 |
| DUKE Jeopardy Score, % | 0.39 | 0.48 | 0.23, 0.72 | 0.0055 |
| STEMI | 0.39 | 10.7 | 2.98, 18.42 | 0.008 |
| Sum of Rentrop's collateral grade and TIMI flow grade at initial angiography | -0.38 | -3.80 | -6.58, -1.01 | 0.009 |
| Pulse pressure, mmHg | -0.35 | -0.21 | -0.38, -0.04 | 0.019 |
| Insulin therapy | 0.33 | 9.7 | 0.89, 18.51 | 0.032 |
| TIMI flow grade post-procedure, 0/1, 2, 3 | -0.28 | -4.41 | -9.0, 0.18 | 0.059 |
| Multivariable predictors of IS | ||||
| AAR | - | 0.69 | 0.46, 0.92 | <0.0001 |
| Sum of collateral and baseline TIMI flow grades at initial angiography | - | -1.87 | -4.00, 0.26 | 0.084 |
| TIMI flow grade post-PCI | - | -3.83 | -7.28, -0.38 | 0.031 |
The R2 value for the multivariable model was 0.69. Variables which were prospectively determined as potential determinants of MI (e.g. AAR) rather than consequences of MI (e.g. ejection fraction, LV volumes) were tested.
Table 6.
Univariable predictors of MRI-derived percent myocardial salvage (n=50).
| Univariable predictors of myocardial salvage | r | Beta-estimate | 95% CI | P value |
|---|---|---|---|---|
| TIMI flow grade post-procedure (0/1, 2, 3) | 0.35 | 4.07 | 0.96, 7.18 | 0.011 |
| Prior MI | 0.31 | 6.06 | 0.68, 11.43 | 0.028 |
| Sum of collateral and baseline TIMI flow grades at initial angiography | 0.30 | 2.03 | 0.02, 4.03 | 0.047 |
Figure 4.
Myocardial salvage by MRI according to the TIMI flow grade post-procedure (n=50) show stepwise increases in salvage related to the increasing amounts of post-procedural TIMI flow.
AAR was greater in patients with MVO (34±11%) compared to in patients without MVO (21±10%; p=0.0047). IS was also greater in patients with MVO (21±12%) compared to in patients without MVO (9±10%; p=0.013).
Predictors of AAR, IS and salvage
The univariable predictors of %AAR, %IS and % salvage in all MI patients are shown in Tables 4, 5, and 6, respectively. AAR was predicted by angiographic and electrocardiographic jeopardy scores and by presentation with STEMI (Table 4). Myocardial salvage was predicted by coronary angiographic flow grades pre- and post-angiography/PCI and by prior history of MI (Table 6).
A multivariable model of IS was constructed using univariable predictors which were prospectively determined as potential determinants of MI rather than consequences of MI. In this model (Table 5), IS was predicted by T2 weighted MRI-derived AAR (for a 1% change: beta estimate (95% confidence interval) 0.69 (0.46, 0.92); p<0.0001) and by TIMI flow grade post-PCI (-3.83 (-7.28, -0.38); p=0.031).
Discussion
Our main findings are summarized as follows. First, AAR by T2 weighted MRI correlates well with angiographic measures of myocardial jeopardy such as the APPROACH lesion score. Second, measurement of AAR and IS in the early post-infarct period enabled estimation of myocardium salvaged in all patients, regardless of presentation type or past history of MI. Third, in a multivariable analysis, IS was predicted by MRI-derived AAR and also by the sum of coronary and collateral artery flow grades at initial angiography.
Determination of initial AAR and myocardial salvage has several applications for clinical and research purposes; however, measurement of these variables has limited feasibility (3). While AAR measurement is possible following technetium perfusion tracer injection during coronary occlusion (21), this approach has been difficult to implement in both clinical practice and large multicenter studies. MRI may now enable AAR delineation and estimation of salvage. First, recent technical advances have overcome problems due to signal drop-off (4) and cardiorespiratory motion (22). Second, AAR measurements by T2 weighted MRI has been validated in reperfused (7) and non-reperfused (23) MI. Third, since MVO and intra-myocardial hemorrhage may cause AAR to be underestimated by MRI (24), our approach to image analyses was designed to minimize this problem. Fourth, our study was prospectively performed in a broad range of patients with acute MI. Previous studies have had several exclusion criteria (17), such as symptoms > 24 h from PCI, history of prior MI, signs of clinical instability (9), or persistent TIMI flow at angiography. In order to enhance the applicability of our findings to clinical practice, we included all MI patients who would consent to MRI regardless of presentation type or success of reperfusion. Our results indicate that T2 weighted MRI enables AAR estimation even in patients with a second MI.
In line with our first hypothesis, we found that AAR estimated by T2 weighted MRI was a predictor of the APPROACH Lesion Score, which is an anatomical and prognostically validated measure of the extent of myocardial jeopardy. Additionally, AAR was a multivariable predictor of infarct size.
Correlations and limits of agreement between T2-weighted MRI and the angiographic estimates of AAR leave questions regarding which answer is correct. The Bland Altman analysis suggests either T2-weighted MRI overestimates AAR or that the APPROACH Lesion Score AAR underestimates AAR or some combination of these two factors. However, the relationships between IS derived by MRI with AAR derived by MRI, the APPROACH Lesion Score, the DUKE Jeopardy Score and the ST elevation jeopardy score (Figure 2) provide an independent metric to help resolve these possibilities. As infarct size is physiologically a subset of the AAR, the expected relationship between AAR and infarct size involves all points at or below the line of identity. Of all metrics analyzed, T2-weighted MRI had the fewest underestimations of AAR (1 underestimate), APPROACH was almost as good on this metric (5 underestimates) while the Duke Jeopardy Score and the ST Elevation Score underestimated AAR in 10 and 19 cases, respectively. While these data cannot unequivocally resolve which method provides the best measurement of AAR, all analyses indicate that T2-weighted MRI provides a good measure of AAR.
Consistent with previous studies in which salvage has been measured by nuclear perfusion imaging (25) and also with what might be expected from a biological perspective, coronary flow grades at baseline and post-procedure were predictors of salvage derived by MRI. Furthermore, given the pathophysiologic importance of no-reflow (26), which is also a determinant of IS (27), we also found that AAR and IS were larger in patients with MVO compared to in those with no evidence of microvascular injury. These results were also observed in analyses restricted to STEMI patients.
We confirmed the recent observations by Ortiz-Pérez et al (17) who demonstrated that IS estimated by MRI closely matched angiographic estimates of jeopardy in a group of patients undergoing primary angioplasty for a first MI. Our results extend this analysis since AAR derived by T2 weighted MRI represents all of the ischemic territory, including viable and infarcted myocardium, permitting estimation of myocardial salvage. Our results extend those of Carlsson et al (25) and O'Regan et al (28) who used black blood T2 weighted MRI to estimate myocardial salvage in MI patients. This method may be less applicable to clinical practice since it has lower diagnostic accuracy than bright blood T2-weighted SSFP (4).
Confirming our second hypothesis, T2 weighted MRI enabled delineation of the acute infarct territory in patients with prior MI. This is consistent with the observations of Abdel-Aty et al (9) who found that T2 weighted black blood MRI combined with delayed enhancement permitted discrimination of acute versus chronic MI with a specificity of 96% in 57 infarct zones when evaluated by 2 blinded observers. Prior MI was also a predictor of myocardial salvage. One explanation for this result may be due to a pre-conditioning effect from chronic myocardial ischemia, or enhanced coronary flow grades related to collateral artery supply (29). Several other observations merit comment. We found that coronary flow grade at initial angiography, represented by the sum of the TIMI and Rentrop collateral flow grades, was a negative multivariable predictor of IS, which is consistent with previous observations (17;21). Our study extends these earlier findings since coronary flow grades pre- and post-procedure were also predictors of myocardial salvage derived by MRI. Our results add to the role of MRI in post-infarct imaging, where the utility of MRI to discriminate acute from chronic MI (9), and depict adverse characteristics, such as LV remodeling (30), has already been established. To our knowledge, T2-weighted SSFP methods are being developed by several MRI vendors.
We also found that there was a fairly wide scatter for AAR estimates using the ECG or DUKE angiographic risk score compared to by the APPROACH lesion score or contrast-enhanced MRI (Figure 3). While all of these variables correlated with AAR estimated by T2-weighted MRI, the magnitude of the differences in AAR estimates between the variables (agreement) varied and appeared best for contrast-enhanced MRI and least good for the ECG.
Conclusions
Our findings indicate MRI can delineate the ischemic AAR and quantify myocardial salvage in MI patients, including those with prior MI. Our results are relevant to clinical practice since MRI is the only method which can provide an AAR estimate without using radiation. Since angiographic estimates of AAR are either time-consuming and not normally used clinically, our findings open the door to measurement of myocardial salvage following acute MI not only for clinical research purposes but also for routine clinical practice. Future studies are required in larger patient groups to further evaluate these observations.
Acknowledgements
We would like to thank Pamela Vincent for her help with data acquisition and Dr John McClure, Statistician.
Sources of Funding
This research was funded by the Division of Intramural Research of the National Institutes of Health. Dr Berry was supported by a University of Glasgow Lord Kelvin Adam Smith Fellowship (2005 – 2009) and is currently supported by a Senior Fellowship from the Scottish Funding Council.
Footnotes
Subject Codes: [4] Acute myocardial infarction; [30] CT and MRI; [124] Cardiovascular imaging agents/Techniques
Disclosures
None.
References
- 1.Califf RM, Phillips HR, Hindman MC, Mark DB, Lee KL, Behar VS, Johnson RA, Pryor DB, Rosati RA, Wagner GS, Harrell FE. Prognostic value of a coronary-artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–1063. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 2.Graham MM, Faris PD, Ghali WA, Galbraith PD, Norris CM, Badry JT, Mitchell LB, Curtis MJ, Knudtson ML. Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J. 2001;142:254–261. doi: 10.1067/mhj.2001.116481. [DOI] [PubMed] [Google Scholar]
- 3.Pennell DJ. Myocardial salvage - Retrospection, resolution, and radio waves. Circulation. 2006;113:1821–1823. doi: 10.1161/CIRCULATIONAHA.105.618942. [DOI] [PubMed] [Google Scholar]
- 4.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JJ, Peterson TM, Slutsky RA. Regional myocardial blood flow, edema formation, and magnetic relaxation times during acute myocardial ischemia in the canine. Invest Radiol. 1985;20:465–471. doi: 10.1097/00004424-198508000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys. 1984;11:425–428. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- 7.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging - Histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Dorado D, Oliveras J, Gili J, Sanz E, Perezvilla F, Barrabes J, Carreras MJ, Solares J, Soler-Soler J. Analysis of myocardial edema by magnetic-resonance-imaging early after coronary-artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich MG, Abdel-Aty H, Taylor AJ, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Krushner FG, Lamas GA. ACC-AHA guidelines for the management of patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 14.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:298–308. doi: 10.1002/jmri.20496. [DOI] [PubMed] [Google Scholar]
- 16.Hsu LY, Ingkanisorn WP, Kellman P, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part II: Clinical application of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:309–314. doi: 10.1002/jmri.20495. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz-Perez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007;28:1750–1758. doi: 10.1093/eurheartj/ehm212. [DOI] [PubMed] [Google Scholar]
- 18.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 19.The TIMI Study Group Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med. 1989;320:618–627. doi: 10.1056/NEJM198903093201002. [DOI] [PubMed] [Google Scholar]
- 20.Birnbaum Y, Maynard C, Wolfe S, Mager A, Strasberg B, Rechavia E, Gates K, Wagner GS. Terminal QRS distortion on admission is better than ST-segment measurements in predicting final infarct size and assessing the potential effect of thrombolytic therapy in anterior wall acute myocardial infarction. Am J Cardiol. 1999;84:530–534. doi: 10.1016/s0002-9149(99)00372-0. [DOI] [PubMed] [Google Scholar]
- 21.Christian TF, Gibbons RJ, Clements IP, Berger PB, Selvester RH, Wagner GS. Estimates of myocardium at risk and collateral flow in acute myocardial-infarction using electrocardiographic indexes with comparison to radionuclide and angiographic measures. J Am Coll Cardiol. 1995;26:388–393. doi: 10.1016/0735-1097(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 22.Kellman P, Chefd'hotel C, Lorenz CH, Mancini C, Arai AE, McVeigh ER. Fully automatic, retrospective enhancement of real-time acquired cardiac cine MR images using image based navigators and respiratory motion corrected averaging. Magn Reson Med. 2008;59:771–778. doi: 10.1002/mrm.21509. [DOI] [PubMed] [Google Scholar]
- 23.Tilak GS, Hsu LY, Hoyt RF, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7–15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 24.O'Regan DP, Ahmed A, Karunanithy N, Neuwirth C, Tan Y, Durighel G, Hajnal JV, Nadra I, Corbett SJ, Cook SA. Reperfusion hemorrhage following acute myocardial infarction: assessment with T2* mapping and effect on measuring the area at risk. Radiology. 2009;250:916–922. doi: 10.1148/radiol.2503081154. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson M, Ubachs JFA, Hedström E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance. Quantitative assessment during follow-up and validation with single-photon emission computed tomography. J Am Coll Cardiol Imaging. 2009;2:569–576. doi: 10.1016/j.jcmg.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Reffelmann T, Kloner RA. The no-reflow phenomenon: A basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol. 2006;101:359–372. doi: 10.1007/s00395-006-0615-2. [DOI] [PubMed] [Google Scholar]
- 27.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Regan DP, Ahmed R, Neuwirth C, Tan Y, Durighel G, Hajnal JV, Nadra I, Corbett SJ, Cook SA. Cardiac MRI of myocardial salvage at the peri-infarct border zones after primary coronary intervention. . Am J Physiol Heart Circ. 2009;297:H340–H346. doi: 10.1152/ajpheart.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry C, Balachandran KP, L'Allier PL, Lesperance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heart disease. Eur Heart J. 2007;28:278–291. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- 30.Fieno DS, Hillenbrand HB, Rehwald WG, Harris KR, Decker RS, Parker MA, Klocke FJ, Kim RJ, Judd RM. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol. 2004;43:2124–2131. doi: 10.1016/j.jacc.2004.01.043. [DOI] [PubMed] [Google Scholar]