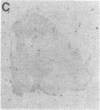
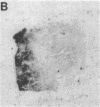
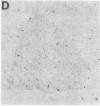
Abstract
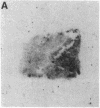
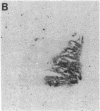
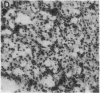
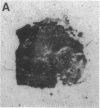
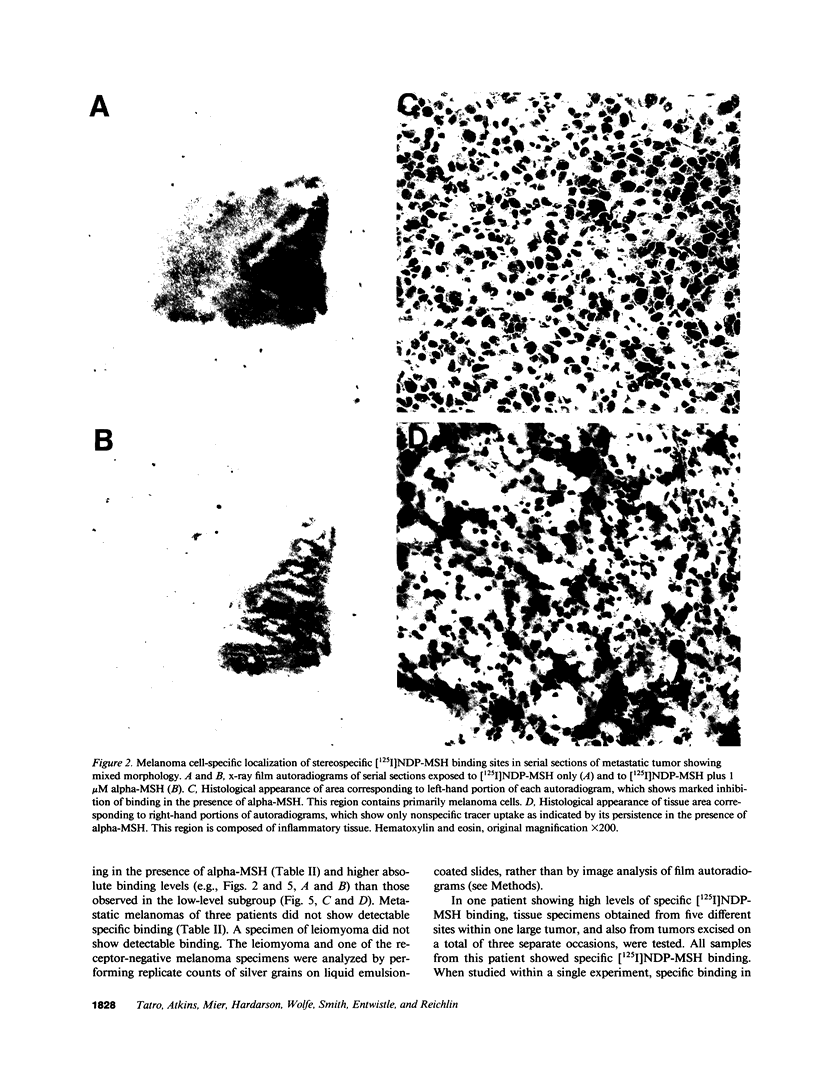
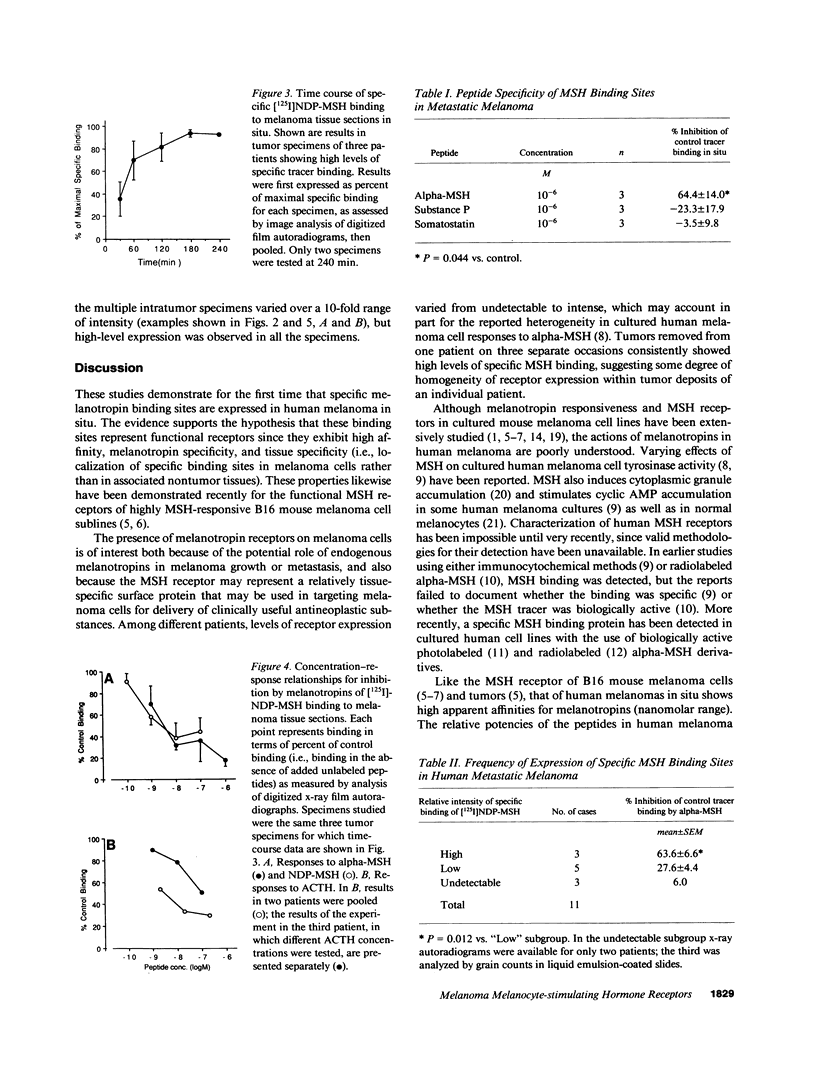
Although some cultured human melanoma cell lines are responsive to melanotropins (melanocyte-stimulating hormones [MSH]), the prevalence and tissue distribution of MSH receptors in melanoma are unknown. We report here the use of an in situ binding technique to demonstrate specific MSH receptors in surgical specimens of human melanoma. The distribution and binding properties of specific MSH binding sites were determined by autoradiography and image analysis after incubation of frozen tumor tissue sections with a biologically active, radiolabeled analogue of alpha-MSH, [125I]iodo-Nle4, D-Phe7-alpha-MSH ([125I]NDP-MSH). In melanoma specimens from 11 patients, 3 showed high levels of specific binding, 5 showed low levels, and in 3 patients specific binding of [125I]NDP-MSH was not detectable. Specific MSH binding sites were present in melanoma cells, but not in adjacent connective or inflammatory tissues. Melanotropins, including alpha-MSH, NDP-MSH, and ACTH, inhibited [125I]NDP-MSH binding in a concentration-dependent manner, whereas unrelated peptides (somatostatin and substance P) did not. The apparent affinity of alpha-MSH for this binding site was in the nanomolar range (EC50 = 2 X 10(-9) M for inhibition of [125I]NDP-MSH binding in situ), similar to that recently described for the murine melanoma receptor. In one patient, analysis of multiple intratumor samples and tumors excised on three separate occasions revealed high levels of specific MSH binding in all samples. These results suggest that endogenous melanotropins may modulate the activities of human melanoma cells in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel Malek Z. A., Hadley M. E., Bregman M. D., Meyskens F. L., Jr, Hruby V. J. Actions of melanotropins on mouse melanoma cell growth in vitro. J Natl Cancer Inst. 1986 May;76(5):857–863. [PubMed] [Google Scholar]
- Bennett D. C., Dexter T. J., Ormerod E. J., Hart I. R. Increased experimental metastatic capacity of a murine melanoma following induction of differentiation. Cancer Res. 1986 Jul;46(7):3239–3244. [PubMed] [Google Scholar]
- Cannon J. G., Tatro J. B., Reichlin S., Dinarello C. A. Alpha melanocyte stimulating hormone inhibits immunostimulatory and inflammatory actions of interleukin 1. J Immunol. 1986 Oct 1;137(7):2232–2236. [PubMed] [Google Scholar]
- Farrar W. L., Kilian P. L., Ruff M. R., Hill J. M., Pert C. B. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987 Jul 15;139(2):459–463. [PubMed] [Google Scholar]
- Feng J. D., Dao T., Lipton J. M. Effects of preoptic microinjections of alpha-MSH on fever and normal temperature control in rabbits. Brain Res Bull. 1987 Apr;18(4):473–477. doi: 10.1016/0361-9230(87)90111-0. [DOI] [PubMed] [Google Scholar]
- Fuller B. B., Meyskens F. L., Jr Endocrine responsiveness in human melanocytes and melanoma cells in culture. J Natl Cancer Inst. 1981 May;66(5):799–802. [PubMed] [Google Scholar]
- Gerst J. E., Sole J., Mather J. P., Salomon Y. Regulation of adenylate cyclase by beta-melanotropin in the M2R melanoma cell line. Mol Cell Endocrinol. 1986 Jul;46(2):137–147. doi: 10.1016/0303-7207(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Ghanem G. E., Comunale G., Libert A., Vercammen-Grandjean A., Lejeune F. J. Evidence for alpha-melanocyte-stimulating hormone (alpha-MSH) receptors on human malignant melanoma cells. Int J Cancer. 1988 Feb 15;41(2):248–255. doi: 10.1002/ijc.2910410216. [DOI] [PubMed] [Google Scholar]
- Guerry D., 4th, Alexander M. A., Elder D. E., Herlyn M. F. Interferon-gamma regulates the T cell response to precursor nevi and biologically early melanoma. J Immunol. 1987 Jul 1;139(1):305–312. [PubMed] [Google Scholar]
- Halaban R., Lerner A. B. The dual effect of melanocyte-stimulating hormone (MSH) on the growth of cultured mouse melanoma cells. Exp Cell Res. 1977 Aug;108(1):111–117. doi: 10.1016/s0014-4827(77)80016-5. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Rao J., Wilson R. E. c-AMP-induced c-fos expression in cells of melanocyte origin. Biochem Biophys Res Commun. 1989 Mar 15;159(2):408–413. doi: 10.1016/0006-291x(89)90006-5. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982 Aug;2(8):1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn M., Clark W. H., Rodeck U., Mancianti M. L., Jambrosic J., Koprowski H. Biology of tumor progression in human melanocytes. Lab Invest. 1987 May;56(5):461–474. [PubMed] [Google Scholar]
- Herlyn M., Mancianti M. L., Jambrosic J., Bolen J. B., Koprowski H. Regulatory factors that determine growth and phenotype of normal human melanocytes. Exp Cell Res. 1988 Dec;179(2):322–331. doi: 10.1016/0014-4827(88)90271-6. [DOI] [PubMed] [Google Scholar]
- Kameyama K., Tanaka S., Ishida Y., Hearing V. J. Interferons modulate the expression of hormone receptors on the surface of murine melanoma cells. J Clin Invest. 1989 Jan;83(1):213–221. doi: 10.1172/JCI113861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman L. B., Dinarello C. A., Llansa N. D., Fidler I. J. Natural and recombinant human interleukin 1-beta is cytotoxic for human melanoma cells. J Immunol. 1986 Apr 15;136(8):3098–3102. [PubMed] [Google Scholar]
- Legros F., Coel J., Doyen A., Hanson P., Van Tieghem N., Vercammen-Grandjean A., Fruhling J., Lejeune F. J. alpha-Melanocyte-stimulating hormone binding and biological activity in a human melanoma cell line. Cancer Res. 1981 Apr;41(4):1539–1544. [PubMed] [Google Scholar]
- Murphy J. R., Bishai W., Borowski M., Miyanohara A., Boyd J., Nagle S. Genetic construction, expression, and melanoma-selective cytotoxicity of a diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion protein. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8258–8262. doi: 10.1073/pnas.83.21.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard B. S. Identification of a synthetic nonapeptide sequence that inhibits motility in culture of a melanoma subclone that possesses a high metastatic potential. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9015–9019. doi: 10.1073/pnas.84.24.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek J. M. Factors regulating growth and pigmentation of melanoma cells. J Invest Dermatol. 1976 Apr;66(4):201–209. doi: 10.1111/1523-1747.ep12482134. [DOI] [PubMed] [Google Scholar]
- Preston S. F., Volpi M., Pearson C. M., Berlin R. D. Regulation of cell shape in the Cloudman melanoma cell line. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5247–5251. doi: 10.1073/pnas.84.15.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson M., Posen S., Mason R. S. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988 Dec;91(6):593–598. doi: 10.1111/1523-1747.ep12477126. [DOI] [PubMed] [Google Scholar]
- Rheins L. A., Cotleur A. L., Kleier R. S., Hoppenjans W. B., Saunder D. N., Nordlund J. J. Alpha-melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice. J Invest Dermatol. 1989 Oct;93(4):511–517. doi: 10.1111/1523-1747.ep12284064. [DOI] [PubMed] [Google Scholar]
- Sauder D. N. Biologic properties of epidermal cell thymocyte-activating factor (ETAF). J Invest Dermatol. 1985 Jul;85(1 Suppl):176s–179s. doi: 10.1111/1523-1747.ep12276378. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R., Koestler T. P., Corwin S. P., Buscarino C., Doll J., Lester B., Greig R. G., Poste G. Experimental metastasis correlates with cyclic AMP accumulation in B16 melanoma clones. Nature. 1984 Apr 5;308(5959):544–547. doi: 10.1038/308544a0. [DOI] [PubMed] [Google Scholar]
- Siegrist W., Oestreicher M., Stutz S., Girard J., Eberle A. N. Radioreceptor assay for alpha-MSH using mouse B16 melanoma cells+. J Recept Res. 1988;8(1-4):323–343. doi: 10.3109/10799898809048996. [DOI] [PubMed] [Google Scholar]
- Siegrist W., Solca F., Stutz S., Giuffrè L., Carrel S., Girard J., Eberle A. N. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989 Nov 15;49(22):6352–6358. [PubMed] [Google Scholar]
- Smith A. I., Funder J. W. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988 Feb;9(1):159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- Solca F., Siegrist W., Drozdz R., Girard J., Eberle A. N. The receptor for alpha-melanotropin of mouse and human melanoma cells. Application of a potent alpha-melanotropin photoaffinity label. J Biol Chem. 1989 Aug 25;264(24):14277–14281. [PubMed] [Google Scholar]
- Swope V. B., Abdel-Malek Z. A., Sauder D. N., Nordlund J. J. A new role for epidermal cell-derived thymocyte activating factor/IL-1 as an antagonist for distinct epidermal cell function. J Immunol. 1989 Mar 15;142(6):1943–1949. [PubMed] [Google Scholar]
- Tatro J. B., Entwistle M. L., Lester B. R., Reichlin S. Melanotropin receptors of murine melanoma characterized in cultured cells and demonstrated in experimental tumors in situ. Cancer Res. 1990 Feb 15;50(4):1237–1242. [PubMed] [Google Scholar]
- Tatro J. B., Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987 Nov;121(5):1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- Thody A. J., Fisher C., Kendal-Taylor P., Jones M. T., Price J., Abraham R. R. The measurement and characterisation by high pressure liquid chromatography of immunoreactive alpha-melanocyte stimulating hormone in human plasma. Acta Endocrinol (Copenh) 1985 Nov;110(3):313–318. doi: 10.1530/acta.0.1100313. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Kuhar M. J., Niehoff D. L., Palacios J. M. Benzodiazepine receptors are coupled to a subpopulation of gamma-aminobutyric acid (GABA) receptors: evidence from a quantitative autoradiographic study. J Pharmacol Exp Ther. 1981 Sep;218(3):797–804. [PubMed] [Google Scholar]
- Varga J. M., Asato N., Lande S., Lerner A. B. Melanotropin-daunomycin conjugate shows receptor-mediated cytotoxicity in cultured murine melanoma cells. Nature. 1977 May 5;267(5606):56–58. doi: 10.1038/267056a0. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Dipasquale A., Pawelek J., McGuire J. S., Lerner A. B. Regulation of melanocyte stimulating hormone action at the receptor level: discontinuous binding of hormone to synchronized mouse melanoma cells during the cell cycle. Proc Natl Acad Sci U S A. 1974 May;71(5):1590–1593. doi: 10.1073/pnas.71.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Pawelek J., Sansone M., Morowitz J. Response of mouse melanoma cells to melanocyte stimulating hormone. Nature. 1974 Mar 22;248(446):351–354. doi: 10.1038/248351a0. [DOI] [PubMed] [Google Scholar]