Abstract
Background
The prevalence of diabetes in the world is projected to rise from 2.8% in the year 2000 to 4.4% in 2030, an increase suggesting an ongoing global epidemic of diabetes.
Objective
To examine time trends in fasting and 2-h glucose concentrations, prevalence and 10-year cumulative incidence of diabetes, and the role of education in these trends.
Design
Each year the Västerbotten Intervention Programme invites all 40, 50, and 60-year-old individuals to a health survey, which includes a cardiovascular risk factor screening and oral glucose tolerance test. The cross-sectional part of the study is based on health examinations conducted between 1990 and 2007 (n = 102,822). The prospective subset (panel dataset) of the study is based on individuals who have had two health examinations 10 years apart and were not defined as having diabetes at their first health examination (n = 23,546).
Results
Between 1990 and 2007, the mean population fasting glucose concentration increased 0.5 mmol/L. Comparing the prevalence in 1990–1995 with 2002–2007 demonstrated a significant 44% increase in men (p < 0.001) and a significant 17% increase in women (p<0.001). Socioeconomic status, here represented by education, clearly influenced both prevalence and incidence of diabetes and glucose concentration. In all time periods and in all age groups, individuals with low education were more likely to have or get diabetes. The 10-year risk of developing diabetes was four to five times higher in the oldest age group (50–60 years) compared with the youngest (30–40 years). A 30% reduction in the 10-year risk of developing diabetes was found in women (p<0.001) between 2000–2003 and 2004–2007.
Conclusions
Despite a clear increase in glucose concentrations and diabetes prevalence between 1990 and 2007, especially in men, there was a decline in the 10-year risk of developing diabetes in women between 2000–2003 and 2004–2007.
Keywords: diabetes, time trends, epidemiology, prevalence, cumulative incidence, education
The worldwide prevalence of diabetes is projected to rise from 2.8% in the year 2000 to 4.4% in 2030, increasing the number of individuals with diabetes from 171 to 366 million, and indicating the ongoing global epidemic of diabetes (1). An increase in the prevalence of diabetes has been demonstrated in many countries across the world. There could be many explanations for such a development. The most obvious would be a true increased incidence of diabetes. On the other hand, the prevalence could also increase if there was extended survival of diabetic patients, earlier onset of the disease, improved detection of diabetes, or aging of the underlying population (2). Despite these various possible explanations, some argue that there is a true diabetes epidemic, i.e. an increasing incidence of diabetes (2), while others question this (3, 4). Overall, there is a need for extended population-based diabetes registers that systematically examine the incidence of diabetes. In the last decade, an increasing prevalence of diabetes has been reported from the Scandinavian countries. In Denmark, 60-year-old adults were compared in 1974 and 1996. During this time period, the prevalence of diabetes increased from 7.8 to 12.3% in men and from 5.6 to 6.8% in women (5). In Norway, between 1984 and 1995, there was an increase in diabetes prevalence from 2.9 to 3.2% in men but not women (6). In the Swedish community of Laxå, where both prevalence and incidence of diabetes have been followed for many years, no increase in the incidence of diabetes was found between 1972 and 1988, despite a nearly 70% increase in prevalence (7). A Swedish national interview survey came to the same conclusion. There was no increase in the incidence of diabetes despite a 40% increase in prevalence between the periods 1980–1987 and 1996–2003 (8). Both the prevalence and incidence of type 2 diabetes, but not type 1 diabetes, have been inversely associated with socioeconomic status in earlier studies (9–11). Many of the risk factors considered to be important for developing type 2 diabetes, such as obesity, physical inactivity, and smoking, are also more common among underprivileged people (12). The primary aims of the present study were to examine time trends in fasting and 2-h glucose concentrations and the prevalence of diabetes during an 18-year observational period in the Swedish population of Västerbotten. A secondary aim was to examine how education, as a proxy for socioeconomic status, influences the prevalence of diabetes and glucose concentrations. Another secondary aim was to study the 10-year risk of developing diabetes, i.e. the 10-year cumulative incidence of diabetes, using a separate panel dataset.
Study design and methods
Study population
Since 1985, there has been an ongoing community intervention programme on cardiovascular disease and diabetes in the province of Västerbotten in northern Sweden – the Västerbotten Intervention Programme (13). As part of this programme all men and women were invited to a health survey at the age of 30, 40, 50, and 60 years. As of 1996, 30-year-olds were no longer invited to the project. The participation rate between 1990 and 2007 varied from 48 to 67%, but has remained at 66–67% since 2005. To explore potential socioeconomic differences between study participants and non-participants, a record linkage was made between all those invited to the 1992 and 1993 health surveys (participants and non-participants) and the 1990 Population and Housing Census in Sweden. No obvious social differences (employment, education, total income) were found when the participants were compared with the non-participants (14).
Methods
All participants in the study received a questionnaire on social issues and education. After an overnight fast, all participants not having known diabetes and with fasting plasma glucose below 7.0 mmol/L, were offered a simplified oral glucose tolerance test (OGTT) according to WHO standard. This used a 75-g anhydric glucose load and measured plasma glucose after 2 h (15). All glucose concentrations were measured on capillary plasma using Reflotron bench-top analysers (Boehringer Mannheim GmbH, Mannheim, Germany) (16). Measuring 2-h glucose in capillary plasma instead of venous plasma yields higher glucose values (on average 1.1 mmol/L higher) (15). Before and during the OGTT, the subjects were instructed not to be physically active or to smoke. In this epidemiologic study, diabetes was defined as having on at least one occasion a fasting plasma glucose ≥7.0 mmol/L and/or a 2-h plasma glucose ≥12.2 mmol/L (capillary plasma) (17). In addition, participants who answered ‘yes’ to the question ‘Do you have diabetes?’ were also categorised into the diabetes group. Education was defined at three levels, where low education was completion of ≤9 years in school, medium education was between 10 and 12 years in school, and high education was ≥13 years in school, i.e. university/academic graduation. Since 30-year-old subjects only were invited to the health survey during the first years of the project, all 30-year-old subjects were excluded from the cross-sectional dataset. The inclusion criteria for the present study population were completion of a Västerbotten Intervention Programme health survey during the study period 1990–2007 and a non-missing answer on the education level question. From January 1990 to December 2007, 102,822 subjects (49,511 men and 53,311 women) fulfilled these criteria and formed the basis of the present study. Of these, a sample not having diabetes at the first health examination and completing two health examinations 10 years apart (n = 23,546 subjects) were used for the evaluation of the 10-year cumulative incidence of diabetes (panel study). The study was approved by the Research Ethics Committee at Umeå University and the data handling procedures were approved by the National Computer Data Inspection Board.
Statistical analysis
The Statistical Packages for Social Sciences (SPSS) version 14.0 was used. Linear regression was used for testing time trends in fasting and 2-h glucose. Logistic regression was used for risk analyses of prevalence and 10-year cumulative incidence of diabetes. Estimated prevalence and 10-year cumulative incidence, or 10-year risk of developing diabetes, in different subgroups of education level, time period of health examination and age at first health examination were calculated using the predicted probability function in the logistic regression procedure. A p-value less than 0.05 was chosen as the level of statistical significance.
Results
Table 1 shows the sample for the cross-sectional dataset in the Västerbotten Intervention Programme divided into different time periods, age groups, and education levels. Table 2 shows the sample size for the panel dataset, in which all subjects were examined twice, 10 years apart.
Table 1.
Sample of subjects participating in the Västerbotten Intervention Programme 1990–2007
| Sample size = 102,822 | |||||||
|---|---|---|---|---|---|---|---|
| Low education | Medium education | High education | |||||
| Age | Males | Females | Males | Females | Males | Females | |
| 1990–1995 | 40 | 919 | 871 | 2,967 | 2,946 | 1,218 | 1,887 |
| 50 | 1,803 | 1,900 | 2,055 | 2,259 | 934 | 1,224 | |
| 60 | 2,058 | 2,362 | 1,096 | 1,283 | 395 | 473 | |
| 40–60 | 4,780 | 5,133 | 6,118 | 6,488 | 2,547 | 3,584 | |
| 1996–2001 | 40 | 730 | 620 | 3,415 | 3,222 | 1,306 | 2,061 |
| 50 | 1,550 | 1,458 | 3,408 | 3,067 | 1,464 | 2,198 | |
| 60 | 2,314 | 2,442 | 1,793 | 1,995 | 703 | 898 | |
| 40–60 | 4,594 | 4,520 | 8,616 | 8,284 | 3,473 | 5,157 | |
| 2002–2007 | 40 | 431 | 333 | 4,066 | 3,463 | 1,712 | 2,600 |
| 50 | 870 | 830 | 3,947 | 3,420 | 1,699 | 2,600 | |
| 60 | 2,280 | 2,124 | 3,003 | 2,963 | 1,375 | 1,812 | |
| 40–60 | 3,581 | 3,287 | 11,016 | 9,846 | 4,786 | 7,012 | |
Note: Table is divided into sex, observational periods, age groups, and education categories.
Table 2.
Sample of subjects participating in two health examinations, 10 years apart (panel study), in the Västerbotten Intervention Programme
| Sample size = 23,546 | ||||||
|---|---|---|---|---|---|---|
| Low education | Medium education | High education | ||||
| Age | Males | Females | Males | Females | Males | Females |
| 30 | 199 | 181 | 1,650 | 1,547 | 335 | 627 |
| 40 | 680 | 711 | 2,737 | 2,725 | 1,042 | 1,685 |
| 50 | 1,514 | 1,701 | 1,945 | 2,220 | 837 | 1,210 |
Note: Table is divided into sex, age at first examination, and education categories.
Trends in fasting and two-hour glucose
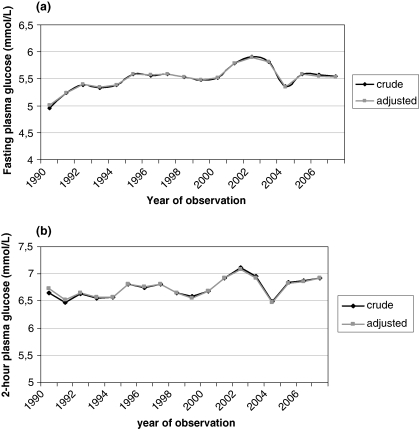
Fig. 1(a) shows the crude and age- and sex-adjusted mean fasting glucose concentrations during 18 years of health examinations (1990–2007). The difference between the crude and adjusted values was small. An increasing trend in fasting glucose was found (linear trend: p < 0.001) despite some fluctuations between years. The adjusted (age, sex) average change over the 18 years of observation was 0.52 mmol/L. Age-adjusted average change was 0.57 mmol/L (p < 0.001) in men and 0.47 mmol/L (p < 0.001) in women. The population trend in 2-h glucose concentrations, i.e. plasma glucose measurement 2 h after a 75-g OGTT, also increased but to a lesser degree (Fig. 1b). Adjusted (age, sex) average change in 2-h glucose during the study period was 0.35 mmol/L (linear trend: p<0.001). The average change was 0.51 mmol/L (p < 0.001) in men and 0.20 mmol/L (p < 0.001) in women.
Fig. 1.
(a) Fasting plasma glucose concentration (mmol/L) between 1990 and 2007 in the Västerbotten Intervention Programme. Crude and age- and sex-adjusted mean values are presented. (b) Post-challenge 2-h plasma glucose concentration (mmol/L) between 1990 and 2007 in the Västerbotten Intervention Programme. Crude and age- and sex-adjusted mean values are presented.
Fasting glucose, two-hour glucose and education
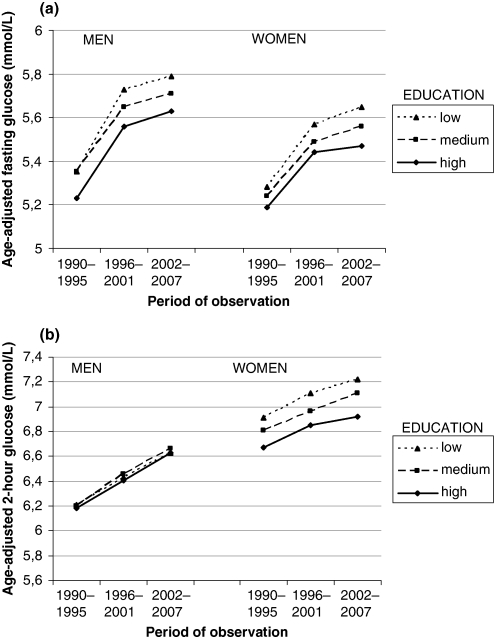
In Fig. 2(a), the mean fasting glucose concentrations are shown for three 6-year time periods and by three education levels. For both men and women, there was a trend of increasing fasting glucose over time at all education levels (p < 0.001 in all comparisons). Throughout the study period, individuals in the lowest educational category had the highest age-adjusted mean fasting glucose values and those in the highest educational category had the lowest. In general, men had higher fasting glucose than women (p < 0.001). Fig. 2(b) demonstrates the association between 2-h glucose concentrations at different time periods and education levels in men and women. In men, age-adjusted mean 2-h glucose was similar irrespective of education. In women, 2-h glucose concentrations were higher than in men (p < 0.001) and for women there was a clear separation in mean 2-h glucose between different education levels (p < 0.001 for all comparisons). Women with low education had the highest 2-h glucose concentrations and those with high education had the lowest. The interaction between the time period and education, i.e. the interaction term (time period×education), for both fasting and 2-h glucose was significant for women (p < 0.05) but not for men. The effect size of the significant interaction in women was small.
Fig. 2.
(a) Mean age-adjusted fasting plasma glucose concentrations (mmol/L) during three 6-year observational periods in the Västerbotten Intervention Programme. Data presented by educational strata. (b) Mean age-adjusted 2-h plasma glucose concentrations (mmol/L) during three 6-year observational periods in the Västerbotten Intervention Programme. Data presented by educational strata.
Prevalence of diabetes
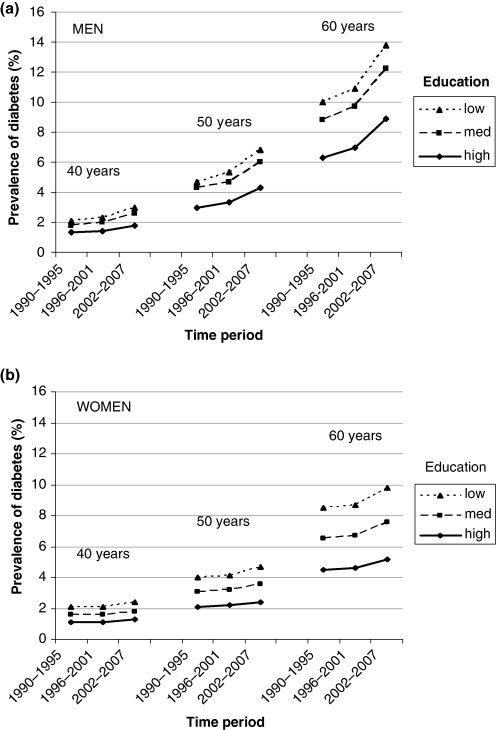
A logistic regression was used to estimate the prevalence of diabetes in men (n = 47,163) and women (n = 50,624). In Fig. 3(a, b), the prevalence of diabetes in men and women are presented at three ages (40, 50, and 60 years), in three time periods (1990–1995, 1996–2001, and 2002–2007), and at three education levels (low, medium, and high). In men, the risk of having diabetes was lowest (1.3%) among 40-year-olds with high education during 1990–1995 and highest (13.8%) among 60-year-olds with low education during 2002–2007. The corresponding figures for women were 1.1 and 9.8%, respectively. Diabetes was twice as common among 50-year-olds as among 40-year-olds (OR for men 2.4, OR for women 2.0) and four to five times as common in 60-year-olds compared with 40-year-olds (OR for men 5.2, OR for women 4.4). The prevalence of diabetes in this 40 to 60-year-old sample of the population in the period 1990–1995 was 4.7% in men and 3.7% in women. The prevalence in the period of 2002–2007 was 6.8% in men and 4.3% in women. Between the two time periods, the overall age- and education-adjusted prevalence increased 44% in men (OR 1.44 [95% CI 1.30–1.60]) and 17% in women (OR 1.17 [95% CI 1.04–1.32]). No significant increase was found between 1990–1995 and 1996–2001. In all time periods, diabetes prevalence was highest among those with low education and lowest among those with high education.
Fig. 3.
(a) Prevalence of diabetes in men in the Västerbotten Intervention Programme between 1990 and 2007 by age and educational strata. (b) Prevalence of diabetes in women in the Västerbotten Intervention Programme between 1990 and 2007 by age and educational strata.
Ten-year risk of developing diabetes
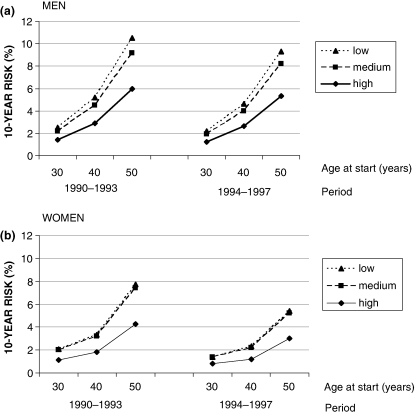
Cumulative incidence of diabetes (%), i.e. the 10-year risk of developing diabetes, was estimated using the panel dataset composed of people who were 30, 40, and 50 years old at their first health examination and were invited for a re-examination 10 years later. The 10-year risk of developing diabetes was estimated after excluding all individuals who had diabetes at their first health examination. The risk of developing diabetes was calculated by age at first health examination, level of education, and time period (Fig. 4a, b). Among the 10,939 men, after adjusting for education and time period, there was a 2-fold increase in the 10-year risk of developing diabetes for a 40-year-old man compared with a 30-year-old man; there was a greater than 4-fold risk for a 50-year-old man compared with a 30-year-old man. The corresponding risks for women were 1.6 and 4.0, respectively.
Fig. 4.
(a) 10-year risk of developing diabetes in men in the Västerbotten Intervention Programme. The risk is presented as a function of age at the start of the 10-year period and education. Two consecutive time periods (1990–1993 and 1994–1997) are compared. (b) 10-year risk of developing diabetes in women in the Västerbotten Intervention Programme. The risk is presented as a function of age at the start of the 10-year period and education. Two consecutive time periods (1990–1993 and 1994–1997) are compared.
There was a strong association between education and risk of developing diabetes. After adjusting for age and time period, there was an 83% increased risk for diabetes in men with low education compared with high education. The corresponding risk for women was 87%. In all age groups and time periods, the difference in risk was larger between high education compared with medium education in men and women than between medium education compared with low education groups.
The 10-year risk of developing diabetes was also compared between adjacent time periods. The study population was divided into two groups containing individuals who underwent their first health examination between 1990 and 1993 with a 10-year follow-up between 2000 and 2003 (n = 10,874), and individuals who had their first examination between 1994 and 1997 with follow up between 2004 and 2007 (n = 12,594). Women aged 50 with low education examined between 1990 and 1993 had a 7.7% 10-year risk of developing diabetes compared with a 5.4% risk in women with low education examined between 1994 and 1997. The 10-year risk for 50-year-old women with high education examined in 1990–1993 was 4.3% compared with 3% among those examined 1994–1997. In men, the corresponding figures were 10.5 and 9.3% among those with low education, and 6 and 5.3% among those with high education. A 20% lower risk of developing diabetes (OR 0.8 [95% CI 0.7–0.9]), was found during 2004–2007 compared with 2000–2003, after adjustment for age, sex, and education. However, the decline in risk between the two time periods was stronger among women, in the order of 30% (OR 0.7 [95% CI 0.6–0.8]). The decline among men was 10% (OR 0.9 [95% CI 0.8–1.0]).
Discussion
The prevalence of diabetes in northern Sweden is about the same as in many other Western populations (18) and, as shown in this paper, is on the rise, especially in men. Comparing the time period 2002–2007 with 1990–1995, diabetes prevalence increased by a significant 44% in men and 17% in women. Our findings are corroborated by a simultaneous increase in the mean fasting plasma glucose concentration of 0.52 mmol/L in the population from 1990 to 2007. The study has also demonstrated that socioeconomic status, represented here by education, influences both the prevalence of diabetes and the 10-year risk of developing diabetes. In all time periods and in all age groups, a higher proportion of people with low education had diabetes or developed diabetes than among people with high education. Individuals with medium levels of education had prevalence and 10-year risk for diabetes in between those with low or high education.
Furthermore, there was a very pronounced age-specific effect. The oldest age group in the 10-year panel observation study (50–60 years) had a 4- to 5-fold increased risk of developing diabetes compared with the youngest age group (30–40 years). Somewhat surprisingly, we found a 20% reduction in the 10-year risk of developing diabetes between 2000–2003 and 2004–2007, despite a marked increase in the prevalence of diabetes. Our findings are similar to a study from Laxå, Sweden, where dissociation between incidence and prevalence of diabetes was also seen. In that study, no progression in incidence was seen while prevalence increased (7). Similar findings were also reported from a Swedish national diabetes interview survey (8). These studies show that dissociation between time trends in prevalence and incidence of diabetes is not uncommon. As discussed in the introduction, there may be a number of reasons for this (2). In Sweden during the study period (1990–2007), more active screening, especially opportunistic screening, for diabetes was taking place. One would expect that intensified screening, at least initially, would lead to an increase in the incidence of diabetes. On the other hand, there are indications of a slowed weight gain in the population of Västerbotten after the millennium shift, especially among well-educated women. For more details concerning the time trends in body weight, physical activity, and other cardiometabolic risk factors within VIP, we refer to ongoing work in other planned papers from the VIP.
Today, diabetes is considered by many to belong to the group of cardiovascular diseases (CVD) and active prevention of risk factors for CVD among diabetes patients prolongs life (19, 20). In such circumstances, an increased prevalence of diabetes can easily go hand in hand with a lack of increase or even a decline in the incidence of diabetes. A weakness of this panel study is that, currently, we can only examine the 10-year cumulative incidence of diabetes within a narrow time frame, i.e. 2000–2003 compared with 2004–2007. It would be more interesting to examine the 10-year cumulative incidence of diabetes in two time periods that were further apart. The power of this study did not allow for such a comparison at present, but this will be an important task for the future. At best, the decline in the 10-year risk of getting diabetes may be interpreted as a turning point that in the future will smooth out the increasing trend of diabetes prevalence. At worst, the decline is merely a fluctuation in the long-term progression of diabetes development. Our results support earlier findings of an inverse relationship between socioeconomic status and the prevalence (9, 10) and incidence of type 2 diabetes (11), as well as between socioeconomic status and important risk factors for diabetes such as obesity (12).
Modern research has shown that an individual's lifestyle strongly influences the risk of developing diabetes (21), and that much of the disease can be prevented by lifestyle changes (22, 23). In many parts of the world, a widening social gap in health is in progress. Consequently, the major future challenge for health care must be to find lifestyle interventions that also work for underprivileged groups in society (24). Another major challenge is to determine how to change society as a whole in order to facilitate the choice of a health-promoting lifestyle.
The strength of this study is its combination of large size and long duration, which is a consequence of the fact that the study is based on a clinical intervention programme incorporated within the primary health care organisation. Another strength, also a consequence of this, is the understanding that the results from the study have been attained during conditions of clinical practice, i.e. the translation from research to clinic has already taken place. However, this closeness to clinical practice also introduces some important limitations to the study. One being, the large annual variation between certain years in participation rate, and another being the low overall participation rate, at least in comparison with more strictly randomised clinical trials. An obvious explanation for this is the knowledge from the VIP that some of the primary health care units during certain years, due to work overload, were not able to offer all eligible subjects their health examination. In other words, during these years the participation rate could differ due to structural problems within the programme (subjects were not invited) and to drop out on behalf of the subjects themselves.
In conclusion, the present study demonstrated increasing glucose concentrations (fasting and 2 h) and an increasing prevalence of diabetes, especially among men, between 1990 and 2007. After 2000, a decreasing cumulative incidence of diabetes was observed, especially among women. The study has also shown a strong inverse association between education and type 2 diabetes.
Acknowledgements
This study was supported by the Västerbotten County Council. We would like to thank all of the specially trained nurses within the Västerbotten Intervention Programme for their devoted work conducting the health examinations.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Colagiuri S, Borch-Johnsen K, Glümer C, Vistisen D. There really is an epidemic of type 2 diabetes. Diabetologia. 2005;48:1459–63. doi: 10.1007/s00125-005-1843-y. [DOI] [PubMed] [Google Scholar]
- 3.Wareham NJ, Forouhi NG. Is there really an epidemic of diabetes? Diabetologia. 2005;48:1454–5. doi: 10.1007/s00125-005-1845-9. [DOI] [PubMed] [Google Scholar]
- 4.Green A, Støvring H, Andersen M, Beck-Nielsen H. The epidemic of type 2 diabetes is a statistical artefact. Diabetologia. 2005;48:1456–8. doi: 10.1007/s00125-005-1841-0. [DOI] [PubMed] [Google Scholar]
- 5.Drivsholm T, Ibsen H, Schroll M, Davidsen M, Borch-Johnsen K. Increasing prevalence of diabetes mellitus and impaired glucose tolerance among 60-year-old Danes. Diabet Med. 2001;18:126–32. doi: 10.1046/j.1464-5491.2001.00417.x. [DOI] [PubMed] [Google Scholar]
- 6.Midthjell K, Krüger Ø, Holmen J, Tverdal A, Claudi T, Bjørndal A, et al. Rapid changes in the prevalence of obesity and known diabetes in an adult Norwegian population. Diabetes Care. 1999;22:1813–20. doi: 10.2337/diacare.22.11.1813. [DOI] [PubMed] [Google Scholar]
- 7.Jansson SPO, Andersson DKG, Svärdsudd K. Prevalence and incidence rate of diabetes mellitus in a Swedish community during 30 years of follow-up. Diabetologia. 2007;50:703–10. doi: 10.1007/s00125-007-0593-4. [DOI] [PubMed] [Google Scholar]
- 8.Eliasson M, Boström G. Chapter 5.2: major public health problems – diabetes. Scand J Publ Health. 2006;34:59–68. doi: 10.1080/14034950600676883. [DOI] [PubMed] [Google Scholar]
- 9.Connolly V, Unwin N, Sherriff P, Bilous R, Kelly W. Diabetes prevalence and socioeconomic status: a population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J Epidemiol Commun Health. 2000;54:173–7. doi: 10.1136/jech.54.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JMM, Newton RW, Ruta DA, MacDonald TM, Morris AD. Socio-economic status, obesity and the prevalence of type 1 and type 2 diabetes mellitus. Diabet Med. 2000;17:478–80. [PubMed] [Google Scholar]
- 11.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. 2004;164:1873–80. doi: 10.1001/archinte.164.17.1873. [DOI] [PubMed] [Google Scholar]
- 12.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53:891–5. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 13.Norberg M, Wall S, Boman K, Weinehall L. The Västerbotten Intervention Programme: background, design and implications. Global Health Action. 2010;5:4643. doi: 10.3402/gha.v3i0.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinehall L, Hallgren C-G, Westman G, Janlert U, Wall S. Reduction of selection bias in primary prevention of cardiovascular disease through involvement of primary health care. Scand J Prim Health Care. 1998;16:171–6. doi: 10.1080/028134398750003133. [DOI] [PubMed] [Google Scholar]
- 15.WHO Expert Committee on diabetes mellitus. Geneva: WHO; 1985. Technical report series 727. [Google Scholar]
- 16.von Schenk H, Treichl L, Tilling B, Olsson AG. Laboratory and field evaluation of three desk top instruments for blood cholesterol and triglyceride assay. Clin Chem. 1987;33:1230–2. [PubMed] [Google Scholar]
- 17.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs FDR. Type-2 diabetes mellitus related cardiovascular risk: new options for interventions to reduce risk and treatment goals. Atherosclerosis Suppl. 2006;7:29–32. doi: 10.1016/j.atherosclerosissup.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. Eur Heart J. 2009;30:834–9. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 22.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Lanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 23.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkman LF. Social epidemiology: social determinants of health in the United States: are we losing ground? Annu Rev Public Health. 2009;30:27–41. doi: 10.1146/annurev.publhealth.031308.100310. [DOI] [PubMed] [Google Scholar]