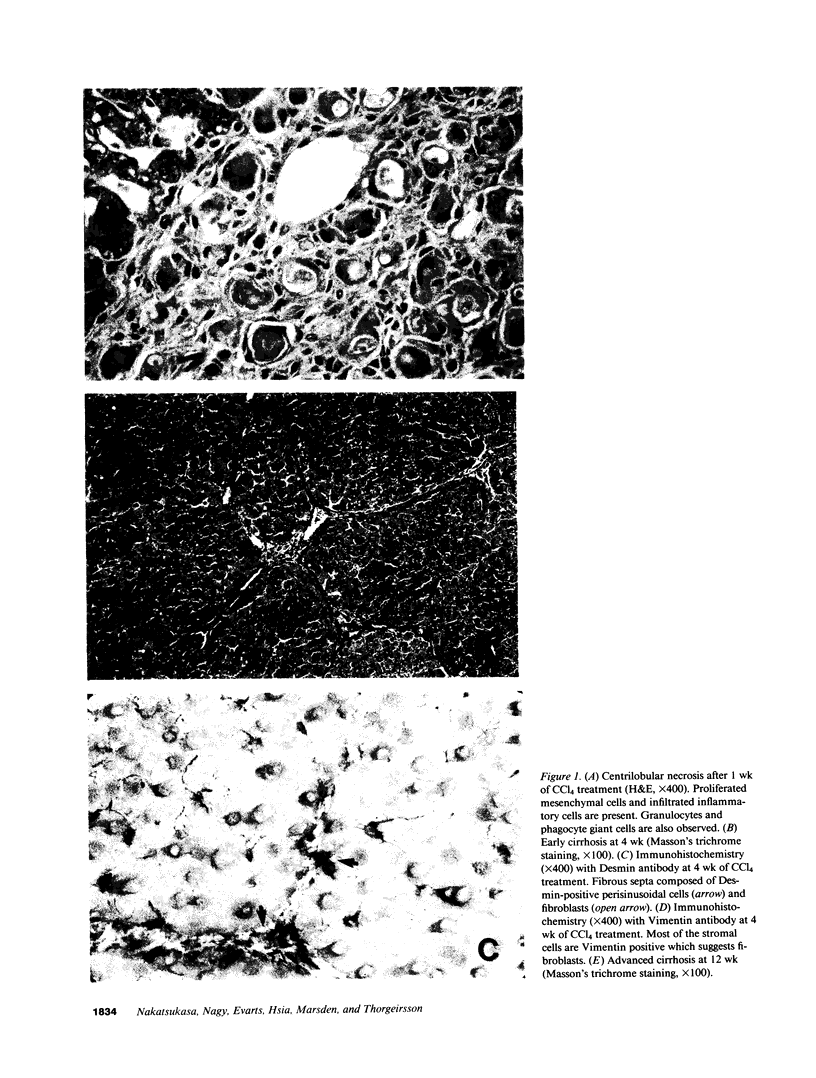
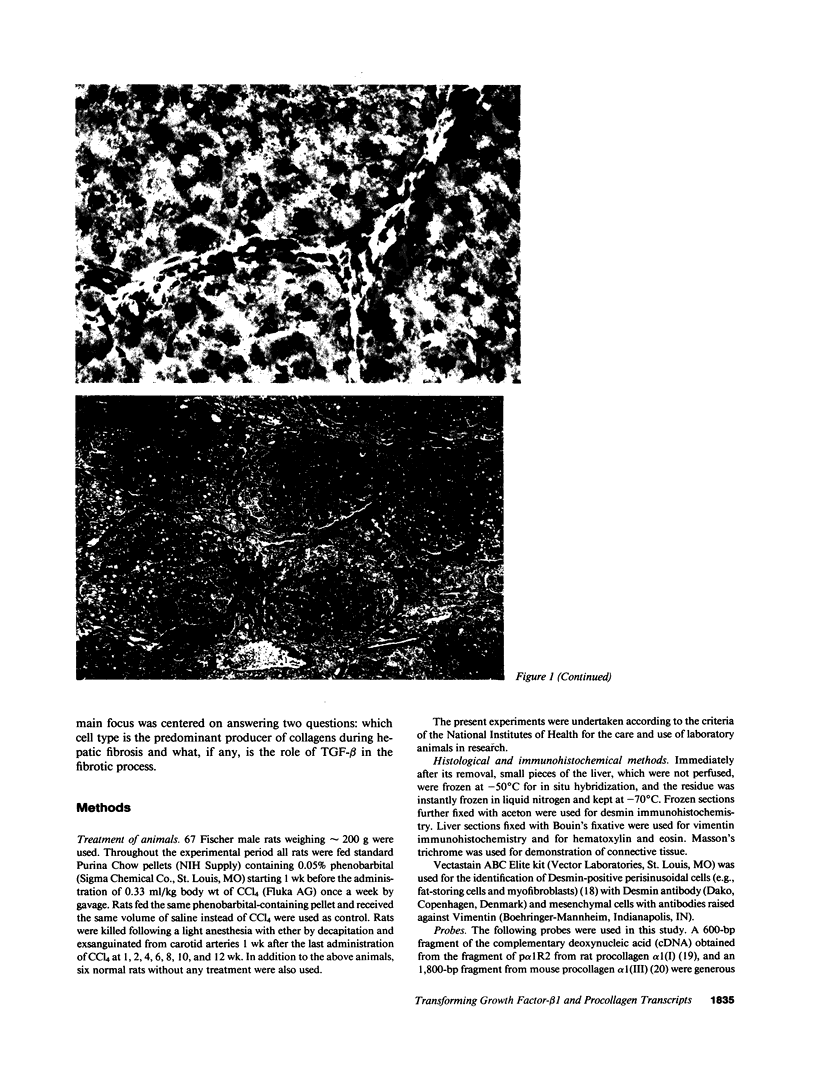
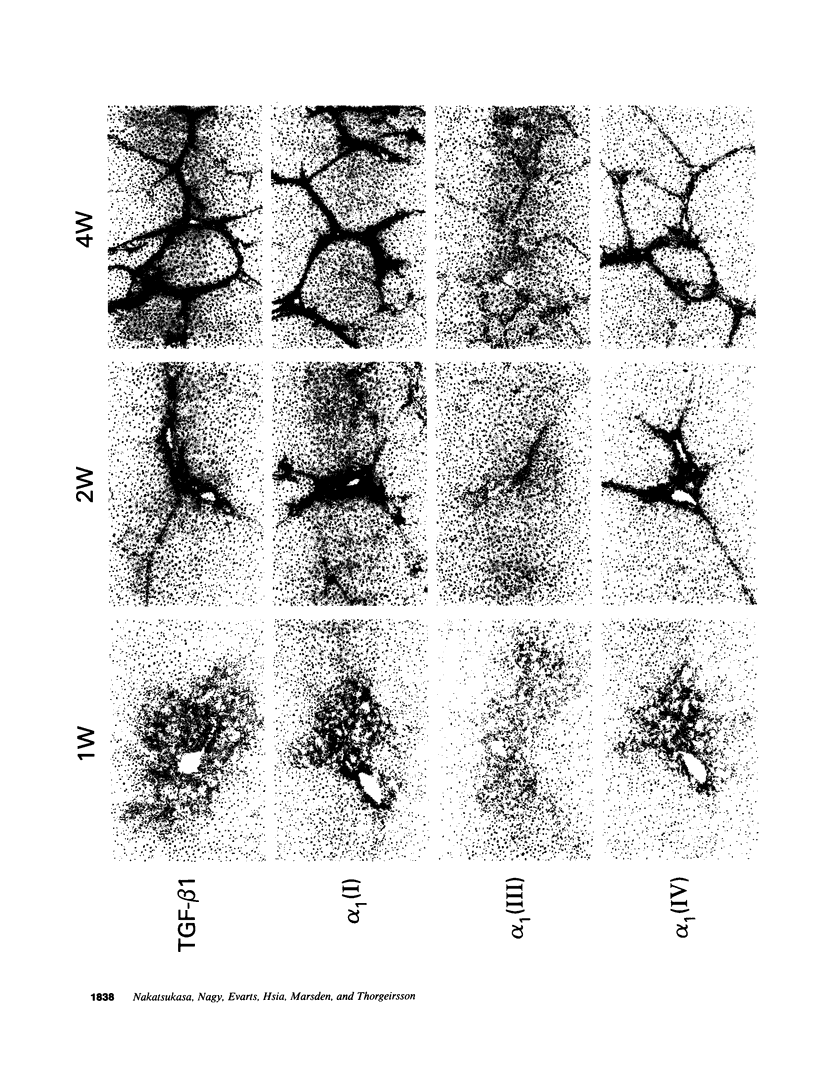
Abstract
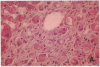
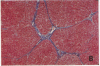
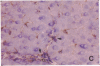
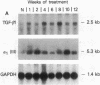
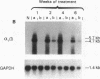
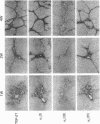
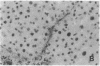
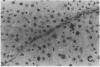
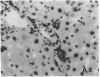
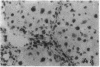
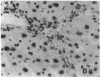
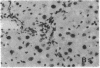
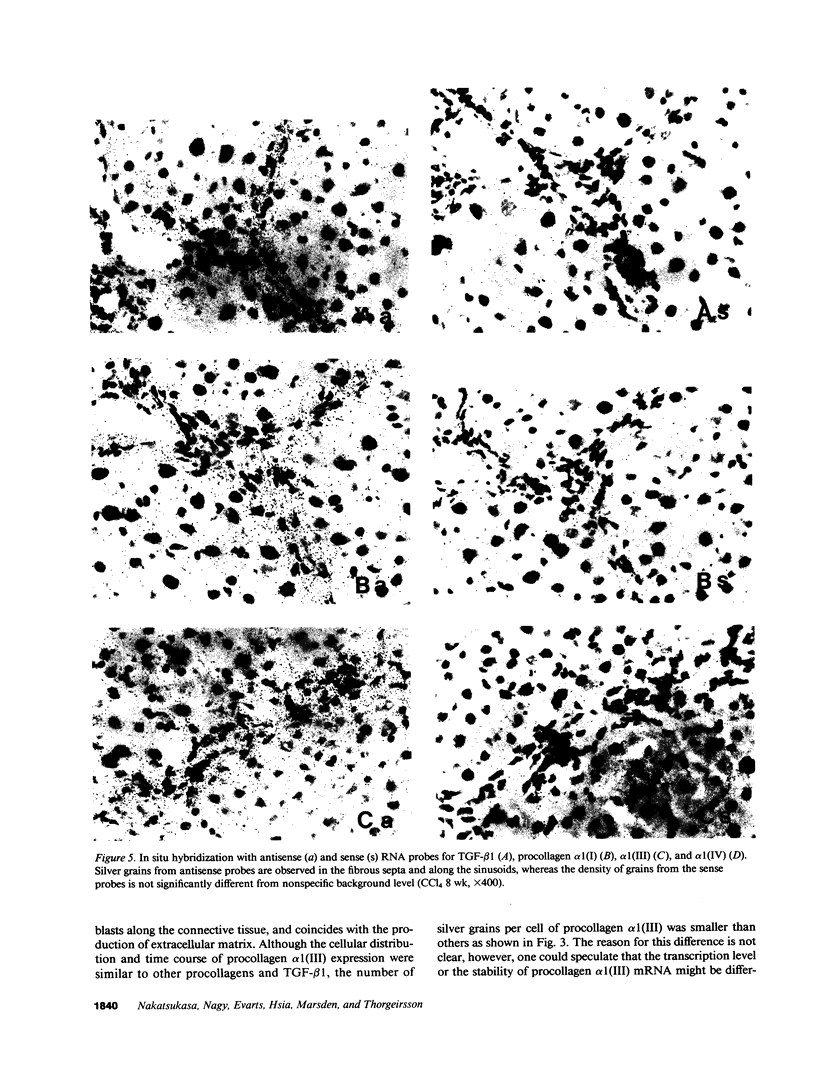
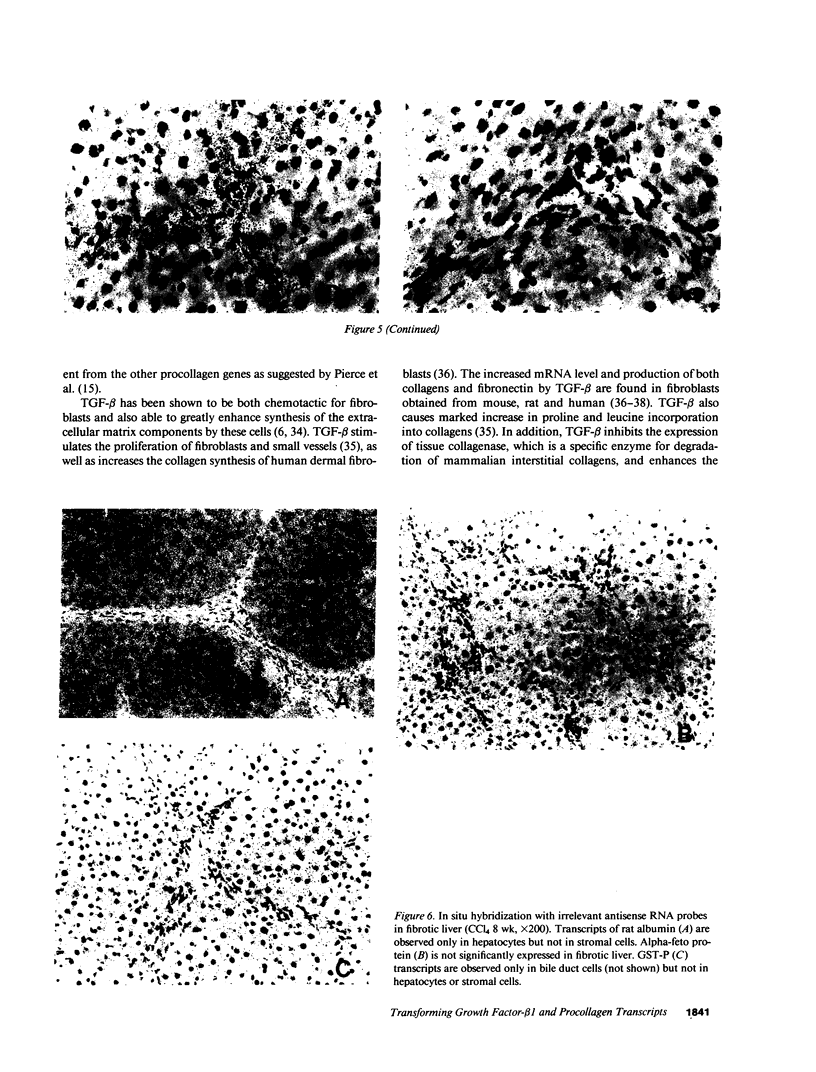
The cellular distribution and temporal expression of transcripts from transforming growth factor-beta 1 (TGF-beta 1) and procollagen alpha 1(I), alpha 1(III), and alpha 1(IV) genes were studied in carbon tetrachloride (CCl4)-induced rat liver fibrosis by using in situ hybridization technique. During the fibrotic process, TGF-beta 1 and procollagen genes were similarly and predominantly expressed in Desmin-positive perisinusoidal cells (e.g., fat-storing cells and myofibroblasts) and fibroblasts and their expression continued to be higher than those observed in control rats. These transcripts were also observed in inflammatory cells mainly granulocytes and macrophage-like cells at the early stages of liver fibrosis. The production of extracellular matrix along small blood vessels and fibrous septa coincided with the expression of these genes. Expression of TGF-beta 1 and procollagen genes were not detected in hepatocytes throughout the experiment. No significant differences in cellular distribution or time course of gene expression among procollagen alpha 1(I), alpha 1(III), and alpha 1(IV) were observed. Desmin-positive perisinusoidal cells and fibroblasts appeared to play the principal role in synthesis of collagens in CCl4-induced hepatic fibrosis. The simultaneous expression of TGF-beta 1 and procollagen genes in mesenchymal cells, including Desmin-positive perisinusoidal cells, during hepatic fibrosis suggests the possibility that TGF-beta 1 may have an important role in the production of fibrosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Pihlajaniemi T., Myers J. C., Kivirikko K. I., Savolainen E. R. Gene expression of type I, III and IV collagens in hepatic fibrosis induced by dimethylnitrosamine in the rat. Biochem J. 1987 May 15;244(1):75–79. doi: 10.1042/bj2440075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechtsen R., Wewer U. M., Thorgeirsson S. S. De novo deposition of laminin-positive basement membrane in vitro by normal hepatocytes and during hepatocarcinogenesis. Hepatology. 1988 May-Jun;8(3):538–546. doi: 10.1002/hep.1840080318. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi F. B., Biagini G., Ballardini G., Cenacchi G., Faccani A., Pisi E., Laschi R., Liotta L., Garbisa S. Basement membrane production by hepatocytes in chronic liver disease. Hepatology. 1984 Nov-Dec;4(6):1167–1172. doi: 10.1002/hep.1840040612. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojkier M., Lyche K. D., Filip M. Increased production of collagen in vivo by hepatocytes and nonparenchymal cells in rats with carbon tetrachloride-induced hepatic fibrosis. Hepatology. 1988 Jul-Aug;8(4):808–814. doi: 10.1002/hep.1840080419. [DOI] [PubMed] [Google Scholar]
- Clement B., Grimaud J. A., Campion J. P., Deugnier Y., Guillouzo A. Cell types involved in collagen and fibronectin production in normal and fibrotic human liver. Hepatology. 1986 Mar-Apr;6(2):225–234. doi: 10.1002/hep.1840060212. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Guzelian P. S., Gay R., Gay S. Collagen formation by the hepatocyte in primary monolayer culture and in vivo. Science. 1983 Mar 18;219(4590):1343–1345. doi: 10.1126/science.6828863. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. In situ hybridization studies on expression of albumin and alpha-fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987 Oct 15;47(20):5469–5475. [PubMed] [Google Scholar]
- Fine A., Goldstein R. H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987 Mar 15;262(8):3897–3902. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Kent G., Gay S., Inouye T., Bahu R., Minick O. T., Popper H. Vitamin A-containing lipocytes and formation of type III collagen in liver injury. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3719–3722. doi: 10.1073/pnas.73.10.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau G., Yamada Y., de Crombrugghe B. Coordinate regulation of the levels of type III and type I collagen mRNA in most but not all mouse fibroblasts. J Biol Chem. 1985 Jan 10;260(1):531–536. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Kim K. Y., Riecken E. O., Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990 Jan;98(1):175–184. doi: 10.1016/0016-5085(90)91307-r. [DOI] [PubMed] [Google Scholar]
- Oberbäumer I., Laurent M., Schwarz U., Sakurai Y., Yamada Y., Vogeli G., Voss T., Siebold B., Glanville R. W., Kühn K. Amino acid sequence of the non-collagenous globular domain (NC1) of the alpha 1(IV) chain of basement membrane collagen as derived from complementary DNA. Eur J Biochem. 1985 Mar 1;147(2):217–224. doi: 10.1111/j.1432-1033.1985.tb08739.x. [DOI] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R. A., Glaug M. R., Greco R. S., Mackenzie J. W., Boyd C. D., Deak S. B. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987 Feb 5;262(4):1652–1658. [PubMed] [Google Scholar]
- Raghow R., Gossage D., Seyer J. M., Kang A. H. Transcriptional regulation of type I collagen genes in cultured fibroblasts by a factor isolated from thioacetamide-induced fibrotic rat liver. J Biol Chem. 1984 Oct 25;259(20):12718–12723. [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Thompson N. L., Heine U., Flanders C., Sporn M. B. Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer. 1988 Jun;57(6):594–600. doi: 10.1038/bjc.1988.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkind M., Pérez-Tamayo R. Liver fibrosis. Int Rev Connect Tissue Res. 1983;10:333–393. doi: 10.1016/b978-0-12-363710-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Ruebner B. H. Collagen formation and cirrhosis. Semin Liver Dis. 1986 Aug;6(3):212–220. doi: 10.1055/s-2008-1040604. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Takahara T., Kojima T., Miyabayashi C., Inoue K., Sasaki H., Muragaki Y., Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988 Oct;59(4):509–521. [PubMed] [Google Scholar]
- Tsutsumi M., Takada A., Takase S. Characterization of desmin-positive rat liver sinusoidal cells. Hepatology. 1987 Mar-Apr;7(2):277–284. doi: 10.1002/hep.1840070212. [DOI] [PubMed] [Google Scholar]
- Varga J., Rosenbloom J., Jimenez S. A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987 Nov 1;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss B., Rauterberg J., Pott G., Brehmer U., Allam S., Lehmann R., von Bassewitz D. B. Nonparenchymal cells cultivated from explants of fibrotic liver resemble endothelial and smooth muscle cells from blood vessel walls. Hepatology. 1982 Jan-Feb;2(1):19–28. doi: 10.1002/hep.1840020105. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]