Abstract
Cancer chemoprevention is a process of using either natural or synthetic compounds to reduce the risk of developing cancer. Observations that NF-E2-related factor 2 (Nrf2)-deficient mice lack response to some chemopreventive agents point to the important role of Nrf2 in chemoprevention. Nrf2 is a member of basic-leucine zipper transcription factor family and has been shown to regulate gene expression by binding to a response element, antioxidant responsive element. It is generally believed that activation of Nrf2 signaling is an adaptive response to the environmental and endogenous stresses. Under homeostatic conditions, Nrf2 is suppressed by association with Kelch-like ECH-associated protein 1 (Keap1), but is stimulated upon exposure to oxidative or electrophilic stress. Once activated, Nrf2 translocates into nuclei and upregulates a group of genes that act in concert to combat oxidative stress. Nrf2 is also shown to have protective function against inflammation, a pathological process that could contribute to carcinogenesis. In this review, we will discuss the current progress in the study of Nrf2 signaling, in particular, the mechanisms of Nrf2 activation by chemopreventive agents. We will also discuss some of the potential caveats of Nrf2 in cancer treatment and future opportunity and challenges on regulation of Nrf2-mediated antioxidant and antiinflammatory signaling in the context of cancer prevention. Antioxid. Redox Signal. 13, 1679–1698.
Introduction
Despite the tremendous progress in the early detection and treatment of cancer, overall mortality rates for most cancers of epithelial origin have not declined for the past three decades (49). Thus, much attention has been directed to cancer prevention in recent years. Research using animal models has offered better understanding of cancer development. Carcinogenesis can be viewed as a multistep process in which the genes controlling proliferation, differentiation, and apoptosis are transformed and altered under selective environmental pressures (14). Generally speaking, tumor development involves three distinct, yet closely linked, phases: initiation, promotion, and progression (13, 47). The initiation phase is a rapid and irreversible event that occurs when a normal cell is exposed to a carcinogenic event. Often, the unrepairable or misrepaired DNA damage happens in the initiation phase. Promotion and progression processes are relatively longer processes than the initiation stage, and are considered reversible. By using various animal cancer models (18, 23, 134), scientists found that all three cancer development stages can be intervened by treatment with natural or synthetic chemicals (Fig. 1). Epidemiological and population studies also established a close relationship between incidence of cancer and consumption of certain types of food (147, 169). The term “chemoprevention” was first coined by Michael Sporn in 1976, when he referred to the prevention of malignancy development by vitamin A and its synthetic analogs (141). Since then, chemoprevention has been adopted as one of the major tactics to modulate the process of carcinogenesis (49, 76). Many years of study have proven that this strategy is indeed effective in reducing the incidence of cancer in the well-defined high-risk groups (67, 76). Chemoprevention, hence by definition, is the use of pharmacologic or natural agents to inhibit the development of invasive cancer. The chemicals with a cancer preventive activity are referred to as chemopreventive agents. A chemopreventive agent can inhibit carcinogenesis either by blocking the DNA damage at initiation stage or by arresting or reversing the processes at promotion and progression stages (49, 76, 141). Nevertheless, an effective chemopreventive agent should be able to interfere with the early stages of carcinogenesis and to eliminate premalignant cells before they become malignant (49, 75, 140, 162).
FIG. 1.
Chemopreventive agents that block or suppress multistage carcinogenesis. Carcinogenesis is a multistep process. The initiation step is started by the transformation of the normal cell into a cancer cell (initiated cell). These cells undergo tumor promotion into preneoplastic cells, which progress to neoplastic cells. Chemopreventive agents can interfere with different steps of this process. Some agents inhibit metabolic activation of the procarcinogens to their ultimate electrophilic species, or their subsequent interaction with DNA. These agents therefore block tumor initiation (blocking agents). Alternatively, blocking agents can stimulate the detoxification of carcinogens, leading to their secretion from the body. Other agents suppress the later steps (promotion and progression) of multistage carcinogenesis (suppressing agents). Some agents can act as both blocking and suppressing agents.
Most of the chemical substances used in cancer chemoprevention studies are natural phytochemicals found in food (143). On the basis of the inhibition stages, Wattenberg has classified chemopreventive agents into two categories—blocking agents and suppressing agents (161). Blocking agents act by preventing carcinogens from reaching the target sites, from undergoing metabolic activation, or from subsequently interacting with crucial cellular macromolecules such as DNA, RNA, and proteins at initiation stages. Suppressing agents, on the other hand, inhibit the malignant transformation of initiated cells at either the promotion or the progression stage. Some agents may work on all three stages of carcinogenesis, and are hence classified into both categories (Fig. 1).
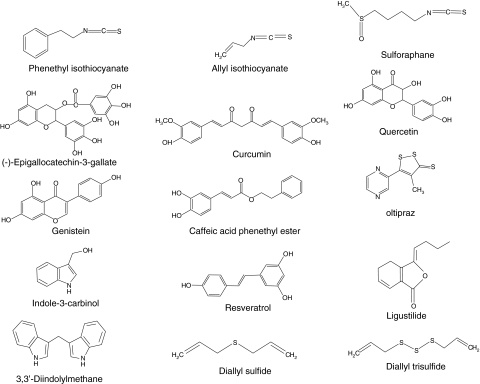
Many different animal models and cancer cell lines have been used to evaluate the chemopreventive values of phytochemicals, and have led to the discovery of new classes of chemopreventive agents, such as isothiocyanates from cruciferous vegetables, polyphenols from green and black tea, and flavonoids from soybeans (143) (Fig. 2). Progress has been also made in understanding the mode of action of newly identified chemopreventive agents. Exposure to the chemopreventive agents produces certain level of reactive oxygen species (ROS) or electrophiles, and causes mild oxidative/electrophilic stresses in cells (20, 84, 125). Such mild oxidative stresses are sufficient to initiate the signaling pathways that, in turn, can activate a variety of cellular events, such as induction of phase II detoxification enzymes and antioxidant enzymes, expression of tumor-suppressor genes, and inhibition of cell proliferation and angiogenesis (51). Although the signal transduction pathways in response to oxidative and/or electrophilic stress have been studied extensively for many years, our understanding in the cutting-edge area of redox signaling and cancer chemoprevention is still in its infancy. In this review, we will discuss the current progress in study of NF-E2-related factor 2 (Nrf2) signaling, in particular, the mechanisms of Nrf2 activation by chemopreventive agents. Future challenges on regulation of Nrf2-mediated antioxidant and antiinflammatory signaling in the context of cancer prevention will also be discussed.
FIG. 2.
Chemical structures of selected chemopreventive agents with ability to activate the NF-E2-related factor 2–antioxidant responsive element signaling pathway.
Kelch-Like ECH-Associated Protein 1-Nrf2-Antioxidant Responsive Element Signaling
To survive under a variety of environmental or intracellular stresses, eukaryotic cells have developed elaborate yet highly efficient cyto-protective machinery to protect themselves from oxidative or electrophilic challenges (57, 92). Proteins that comprise phase II detoxification and antioxidant enzymes provide an enzymatic line of defense against electrophiles and ROS. Among the family of phase II and antioxidant enzymes are glutathione S-transferase (GST), UDP-glucuronosyltransferase, heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), and glutamate cysteine ligase (gamma glutamylcysteine synthetase). Induction of phase II and antioxidant enzymes are coordinately regulated through a consensus cis-element at the 5′-flanking promoter region, named antioxidant responsive element (ARE) (33, 136) or electrophile response element (34) (for simplicity, we will use ARE from here on). ARE-mediated gene expression plays a central role in the cellular defense against cellular oxidative damage. Ample experimental evidence also supports the view that induction of ARE-mediated cytoprotective enzymes is a critical and sufficient mechanism to enable protection against carcinogenesis provoked by environmental and endogenous insults.
One of the key ARE-binding transcription factors is Nrf2 (108). Induction of cytoprotective enzymes in response to ROS, electrophiles, and chemopreventive agents is a cellular event that is highly dependent on Nrf2 protein. By activating Nrf2 signaling, chemopreventive agents are able to increase cellular detoxification and antioxidant enzymes, thereby enhancing removal of reactive carcinogens and blocking carcinogenesis. This hypothesis has been tested in many studies. For example, when investigating the chemoprotective effect of oltipraz in human subjects having a high incidence of liver cancer, Kensler's group found that oral administration of oltipraz significantly enhanced the urinary excretion of the phase II conjugation products of the carcinogen aflatoxin (77). Another study with sulforaphane (SFN), an isothiocyanate present abundantly in cruciferous vegetables, has shown that oral administration of SFN could effectively block benzo[a]pyrene-induced forestomach tumors in ICR mice and this protective effect of SFN was abrogated in the Nrf2 knockout (Nrf2 KO) mice (31), supporting a critical role of phase II detoxification and antioxidant enzymes in prevention of carcinogenesis by chemopreventive agents.
Given the different chemical nature of Nrf2 inducers (128), a mechanism of activation requiring the interaction of Nrf2 inducers with a structurally complementary receptor appears to be quite unlikely. However, many Nrf2 inducers and chemopreventive agents exhibit prooxidant and electrophilic property and may generate different levels of oxidative stress, depending on their doses. It would be reasonable to speculate that a mild oxidative stress generated by chemopreventive agents at appropriate concentrations is sufficient to activate Nrf2 signaling, but not strong enough to cause any adverse effect such as DNA damage or cells death. Activation of Nrf2 signaling further induces detoxification and antioxidant enzymes, thus protecting cells from damage by more active oxidants or electrophiles, such as carcinogens.
Nrf2 was initially discovered in a screen that utilized a tandem repeat of the consensus AP-1 and NF-E2-binding sequences identified in the 5′ locus control region of β-globin gene (108). Nrf2 belongs to basic-leucine zipper (bZip) transcription factor family and has six highly conserved domains called Nrf2-ECH homology (Neh) domains (Fig. 3A). The Neh1 contains a bZip DNA binding motif that enables Nrf2 to interact other bZip transcription factor and form a heterodimer on ARE (109). The Neh3 domain has been shown to function as a transactivation domain and might be involved in interaction with components of transcriptional apparatus (118). The Neh6 domain may contain a degron that contributes to Nrf2 degradation (107). Neh4 and Neh5 domains are found to cooperatively bind with the KIX and CH3 domains of coactivator CBP and to enhance Nrf2 transcription activity (74). The Neh2 domain located at the N-terminus mediates binding with Kelch-like ECH-associated protein 1 (Keap1), a cytosolic protein that inhibits Nrf2 signaling by promoting Nrf2 degradation through ubiquitin/proteasome pathway (73, 109). Similar to many other transcription factors, Nrf2 is subject to regulation by cellular localization. Under basal condition, majority of Nrf2 molecules are found to be in the cytoplasm. Upon exposure to the oxidative stresses, Nrf2 can quickly translocate into the nucleus (60, 123, 164) and form a heterodimer with small musculoaponeurotic fibrosarcoma (Maf ) protein (59), which then binds to ARE and enhances transcription of a group of genes that encode phase II detoxification and antioxidant enzymes (28, 121, 126). Many years of research establish that a signal transduction pathway consisting of Keap1-Nrf2-sMaf-ARE is a primary sensor of the signaling generated by electrophiles and chemopreventive agents, and is essential for elicitation of the antioxidant response.
FIG. 3.
Schematic representation of the conserved regions in Nrf2 and Keap1. (A) Conserved domains are referred to as Neh domains. The Neh2 domain contains the DLG and the ETGE motifs, which are Keap1 binding motifs. The Neh4 and Neh5 domains act cooperatively to bind the coactivator CREB-binding protein, thereby activating transcription. The Neh6 domain contains two highly conserved regions and acts as a linker domain. The Neh1 domain contains the CNC-bZIP region (CNC-bZIP), which promotes dimerization partners and confers DNA-binding specificity. (B) The Keap1 protein comprises five domains: the NTR; the BTB domain (present in actin-binding proteins and mediates Keap1 homodimerization, which is required for Nrf2 retention in the cytoplasm); the IVR (an especially cysteine-rich region); the DGR (which comprises six Kelch motifs that create multiple protein contact sites). The DGR domain of Keap1 is what combines with the Neh2 region of Nrf2, and the CTR. Functionally important cysteines are illustrated. BTB, broad complex, tramtrack, and bric a brac domain; CNC, Cap ’n’ Collar; CTR, C-terminal region; DGR, double glycine/Kelch repeat region; IVR, intervening region; Keap1, Kelch-like ECH-associated protein 1; Neh, Nrf2-ECH homology; Nrf2, NF-E2-related factor 2; NTR, N-terminal region.
Keap1
Keap1 plays a central role in the regulation of Nrf2 activity. Keap1 was isolated as a Nrf2-associating protein by using a yeast two-hybrid screening system (35). Knockout of Keap1 resulted in constitutive activation Nrf2 signaling (121, 156, 157). Keap1 is a 69-kDa cytosolic protein with high homology to Drosophila actin-binding protein Kelch (61). Primary sequence and alignment analysis reveals that human Keap1 consists of five domains: (i) the N-terminal region (a.a. 1–66), (ii) broad complex, tramtrack, and bric a brac domain (BTB, a.a. 67–178), (iii) an cysteine-rich intervening region (IVR, a.a. 179–321), (iv) double glycine/Kelch repeat region (DGR a.a. 322–608), and (v) C-terminal region (CTR, a.a. 609–625) (29) (Fig. 3B). Keap1 was observed to locate mainly in the cytoplasm, presumably by association with F-actin or myosin VIIa through the Kelch domain (61, 69, 70, 153). Accordingly, Keap1 is hypothesized to be a cytosolic anchor protein that sequesters Nrf2 in cytoplasm under the unstimulated condition. Site-directed mutagenesis of a conserved serine (S104A) within the Keap1 BTB domain revealed that Nrf2 is indeed sequestered in cytoplasm by Keap1 (182). A recent study using transgenic animal-originated cells demonstrated that direct interaction between the Neh2 domain of Nrf2 and Kelch repeat domain of Keap1 is essential for retaining of Nrf2 in the cytoplasm by Keap1 (70). Keap1 also contains a strong nuclear exporting signal (NES) sequence in the IVR domain, which may play a major role in determining the subcellular distribution of Keap1 (72, 142, 154). Keap1 is identified to be associated with Cullin-3, a scaffold protein responsible for formation of an ubiquitin ligase E3 complex (25, 36, 87, 177). It is now clear that Keap1 may not only dictate Nrf2 localization, but also actively targets Nrf2 to degradation.
Due to its high cysteine content, Keap1 was proposed to be the sought-after sensor of ARE inducers soon after its discovery as a suppressor of Nrf2 transcriptional activity (128). Convincing evidence has since accumulated that the cysteine residues in Keap1 indeed play an important role in sensing the presence of ARE inducers and oxidative stress. Several independent studies have demonstrated that the sulfhydryl groups of several Keap1 cysteine residues can be directly modified by oxidation, reduction, or alkylation. Among these cysteine residues, Cys151, 273, and 288 appear to be essential for regulating Nrf2 function (90, 102, 168, 176). Mutation of Keap1 Cys151 to Ser did not affect Nrf2 degradation by Keap1, but abolished response to electrophiles and oxidants (176). The role of Cys151 in repression of Nrf2 ubiquitination by electrophiles or oxidants has been verified in vivo (168). Mutation of either Cys273 or Cys288 residues prevented Keap1 from degrading Nrf2 protein, pointing to a critical role of these cysteine residues in Keap1-mediated Nrf2 ubiquitination (150, 176). A recent study confirmed that the Cys273 and Cys288 amino acids are essential for Nrf2 degradation by Keap1; however, mutation of these two cysteine residues does not affect interaction of Nrf2 and Keap1 (88). Further, both C273A and C288A mutants are able to complement each other in degradation of Nrf2 protein when coexpressed in cells (156). However, later, the same laboratory has reported that simultaneous expression of C273A and C288A mutant proteins in mice could not rescue Keap1 null mice from juvenile lethality and repress Nrf2 at all (168). Putting together, these studies demonstrate the significance of multiple Keap1 cysteine residues in sensing the presence of oxidants and electrophiles, although it is not clear how the modification of those cysteine residues alters Keap1 function.
Like Nrf2, Keap1 also was reported to be subject to ubiquitination, but its degradation was proteasome independent, which may play a role in activation of Nrf2 signaling by ARE inducers (178). However, different ARE inducers appear to have very different effects on Keap1 ubiquitination and stability. Zhang et al. first proposed that tert-butylhydroquinone (tBHQ)-induced ubiquitination of Keap1 results in increased degradation of Keap1 by a proteasome-independent mechanism. They observed that, unlike tBHQ, SFN treatment did not cause any Keap1 ubiquitination and degradation. They also noted that the increased Keap1 degradation occurred through a pathway independent of C151, which has been shown to be essential for regulation of Nrf2 degradation. Other groups also observed effects of ARE inducers on Keap1 ubiquitination and stability. For example, Hong et al. (48) noted that electrophilic adduction to Keap1 cysteine residues coincided with the increase of Keap1 ubiquitination at K298 and stabilization of Nrf2 protein in the cells exposed to N-iodoacetyl-N-biotinylhexylenediamine. Treatment of HepG2 cells with quercetin was found also to decrease endogenous Keap1 levels, although change in Keap1 ubiquitination was detected (145). These studies suggest that ubiquitination and subsequent degradation of Keap1 might be a mechanism of Nrf2 induction after exposure to certain ARE inducers. However, further studies are needed to clarify whether the different findings are consequence of using different cell lines or different ARE inducers. Studies are also needed to determine whether medication of Keap1 cysteine residues play the role in Keap1 ubiquitination.
The “hinge and latch” two-site binding model
Keap1 is characterized as a substrate adaptor protein for a cullin-3-based ubiquitin E3 ligase and is essential for ubiquitination of Nrf2 (25, 36, 87, 177). Keap1 has two functional domains: the Kelch/DGR domain, which binds Nrf2, and the BTB domain, which is responsible for homodimerization and interaction with the N-terminus of cullin-3 (25, 36, 87, 177). On the basis of sequence homology and mutation analyses (73, 89, 107), it was reported that Nrf2 has two Keap1 binding motifs in the Neh2 domain: an ETGE motif, which locates at the N-terminal region and is important for ubiquitination of Nrf2 (73, 107), and a DLG motif, which is essential for interacting with Keap1 (89) (Fig. 3A). The ETGE motif and the DLG motif mediate a cooperative binding to Keap1. Deletion of the ETGE motif significantly weakened Keap1 binding mediated by the DLG motif; therefore, the ETGE motif appears to play a permissive role for the DLG motif. Recently, isothermal calorimetry measurement also detected a two-phase binding between Keap1 and Neh2. The Keap1 binding affinity of the ETGE motif is almost two orders of magnitude stronger than the DLG motif (148). In addition, seven lysine residues of the Neh2 domain, which reside upstream of the ETGE motif, have been shown to be indispensable for Keap1-dependent poly-ubiquitination and degradation of Nrf2 (177).
A “hinge and latch” two-site binding model is proposed (149). Two Keap1 molecules form homodimer via their BTB domain (182). The high affinity ETGE motif functions as a hinge to pin down Neh2 to Keap1, while the low affinity DLG motif functions as a latch. Linking the hinge and the latch motif is a lysine-rich central α-helix. In this nine-turns α-helix, six of seven lysine residues are located on one side of the helical surface (148). Since these lysine residues are targets for Keap1-mediated ubiquitination by E3 ligase (177), these lysine residues are speculated to be biologically important for the regulation of Nrf2 stability. When the latch is in position, it helps to set the central α-helix in an adequate orientation to expose these lysine residues (177).
The two-site binding is relatively unstable. The low affinity latch binding may be more vulnerable to the conformation change of Keap1 dimer resulted from oxidative modification. It is proposed that the DLG motif may possess the signaling switch for the repressive regulation of Nrf2 via the Keap1-dependent ubiquitination and proteasomal degradation. The switch from two-site binding to one-site binding may explain the observed facts that the inhibition of Nrf2 degradation is not at the expense of Keap1/Nrf2 binding. Only recently, the X-ray crystallography structure of mouse Keap1 homodimer was reported. Three-dimensional reconstruction at 24-A resolution revealed two large spheres attached by short linker arms to the sides of a small forked-stem structure, resembling a cherry-bob. Each sphere has a tunnel corresponding to the central hole of the β-propeller domain, as determined by X-ray crystallography. The IVR domain appears to surround the core of the β-propeller domain. The unexpected proximity of IVR to the β-propeller domain suggests that any distortions generated during modification of reactive cysteine residues in the IVR domain may send a derepression signal to the β-propeller domain and thereby stabilize Nrf2. This study thus provides a structural basis for the two-site binding and hinge-latch model of stress sensing by the Nrf2-Keap1 system (120).
Nrf2
In human Nrf2 protein, one bipartite nuclear localization signal (NLS) was identified in the basic region, named bipartite nuclear localization signal at the basic region (bNLS) (62, 100). In addition, a monopartite NLS was characterized at the amino-terminus (NLSN) and another monopartite NLS was identified at the carboxyl-terminus (NLSC) of human and murine Nrf2 (146). One NES was characterized in the ZIP dimerization domain, named NESzip (62, 100). Recently, another NES was characterized in the Neh5 transactivation (TA) domain, named NESTA (103). In full-length wild-type (WT) Nrf2, the combined nuclear exporting activities of NESTA and NESzip appear to be able to counter balance the nuclear importing activities mediated by bNLS, NLSN, and NLSC motifs. We have shown that when expression of a green fluorescence protein (EGFP)-tagged Nrf2 was enhanced in HeLa cells, a heterogeneous distribution pattern of EGFP-Nrf2 was observed. Nearly 60% cells showed whole cell distribution, 30% cells showed nuclear distribution, and 12% cells showed cytosolic distribution (103). Mutation of single key leucine residue in the NES motif, such as L184 in the NESTA motif and L544 in the NESzip motif, was sufficient to abolish their nuclear exporting activity (100, 103). Mutation of either the L184 residue or the L544 residue in full-length Nrf2 was sufficient to convert the heterogeneous distribution of EGFP-Nrf2 to the homogeneous nuclear distribution pattern (103), suggesting that both the NESTA and NESzip motifs are indispensable for balancing the effect of bNLS, NLSN, and NLSC motif. We also found that the NESTA motif was redox sensitive (103), but not the bNLS motif and the NESzip motif (100). The redox sensitivity of the NLSN and NLSC motif has not been determined and was speculated to be redox insensitive, since no cysteine residue exists in these two NLS motifs (102).
As discussed above, Nrf2 contains a redox-sensitive NES motif; it is, therefore, reasonable to assume that Nrf2 by itself can sense and transducer redox signaling. We speculate that, under homeostatic conditions, both the NLS and the NES counteract each other and reach a dynamic balance; as a result, Nrf2 exhibits whole cell distribution. The presence of Nrf2 in nuclei may account for the basal or constitutive Nrf2 activities. When challenged with oxidative stress, the redox-sensitive NESTA is disabled, whereas the redox-insensitive NLS remains functional (15, 100), leading to predominant nuclear distribution of Nrf2. Nrf2 consists of a constitutively active NESzip bNLS-NLSN-NLSC tandem and a conditional NESTA motif. The NESTA motif may function as a redox-sensitive switch to determine Nrf2 localization under different redox conditions.
A redox-signaling model of the antioxidant response
Accumulating evidence showed that Keap1-mediated Nrf2 ubiquitination is highly sensitive to redox status, although Keap1/Nrf2 dissociation is relatively insensitive. Nrf2 nuclear translocation also has high redox sensitivity. Accordingly, we propose a redox-signaling model for activation of Nrf2 signaling by oxidative stress (Fig. 4). There are two pools of Nrf2 proteins in cells: the free-floating Nrf2 (fNrf2) and the Keap1-binding Nrf2 (kNrf2). Under homeostatic condition, kNrf2 are constantly degraded through ubiquitin/proteasome pathway, and cells will maintain a relatively small size of fNrf2 pool when the equilibrium is reached between Nrf2 synthesis and its degradation. However, upon exposure to oxidative stress, the Keap1-mediated redox-sensitive Nrf2 ubiquitination and degradation is impeded, and the pool of fNrf2 proteins begins to expand (129, 175, 178). The fNrf2 proteins can further response to the redox signals and translocate into the nucleus. On the basis of this model, the size of fNrf2 pool is believed to be a key determinant of the magnitude of antioxidant response (170). If cells have more fNrf2, same amount of ROS may elicit more Nrf2 influx into the nucleus and consequently induce a stronger antioxidant response. Thus, it would be more reasonable to hypothesize that both Keap1 and Nrf2 are functioning as redox sensors in response to oxidative stress.
FIG. 4.
Hypothetic model of Nrf2-mediated redox signaling. There are two pools of Nrf2 proteins, fNrf2 and kNrf2. Under homeostatic conditions (A), kNrf2 binds to Keap1 dimer via a high affinity ETGE (hinge) motif and a low affinity DLG (latch) motif. The two-site binding exposes the Ub-acceptor site(s) in Nrf2. Ubiquitinated Nrf2 proteins are destined to proteasomal degradation. There appears to be an equilibrium between protein synthesis and degradation. As a result, there is only a small pool of fNrf2, contributing to basal activation. When stimulated by oxidative stress or upstream kinases (B), the conformation change of the Keap1 dimer, probably via intermolecular disulfide bond formation, disrupts the two-site binding. As a result, the Ub-acceptor site(s) is/are not easily accessible. The ubiquitination and the proteasomal degradation of Nrf2 are impeded and Keap1 is saturated by kNrf2. Nrf2 protein synthesis, however, is elevated. As a consequence, the pool of fNrf2 expands and it can transmit redox signals to cell nucleus via gradient nuclear translocation. fNrf2, free-floating Nrf2; Keap1, Kelch-like ECH-associated protein 1; kNrf2, Keap1-binding Nrf2; Nrf2, NF-E2-related factor 2; Ub, ubiquitination.
This model may explain the chemoprotective effect of phase II enzyme inducers. Treatment with a low dosage of phase II enzyme inducers can impede Keap1-mediated ubiquitination of Nrf2 and expand the fNrf2 pool. As such, the phase II inducers exert a priming effect to tune up the overall redox sensitivity of the cell. Once the cell confronts oxidative stress, it can readily initiate an effective antioxidant response to neutralize the oxidative toxicity and restore redox homeostasis.
Regulation of Nrf2 signaling by other pathways
Other signaling pathways have been reported to influence Keap1-Nrf2-sMaf-ARE signaling, among which are several kinases, such as protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K), and PKR-like endoplasmic reticulum kinase (PERK) (24, 53, 99, 170). Activation of the extracellular signal-regulated protein kinase 2 (ERK2) and c-Jun NH(2)-terminal kinase-1 (JNK1) pathway appeared to enhance Nrf2 signaling (6, 137, 167, 170, 171, 180, 181). In contrast, the activation of the p38 MAPK pathway appeared to inhibit Nrf2 signaling (79, 137, 172, 181). Most recently, it was found that phosphorylation of Nrf2 by p38 increases Keap1/Nrf2 binding (79). This finding suggests that p38 may inhibit Nrf2 signaling by enhancing Keap1 sequestering of Nrf2 in cytoplasm. By using a cell-free system, Huang et al. have demonstrated that PKC can directly phosphorylate Nrf2 at Serine 40 (52), thereby promoting its dissociation from Keap1 (16, 53, 119). PI3K is also reported to play a role in Nrf2 activation (68, 69, 99, 113). In response to oxidative stress, PI3K signaling is activated and results in depolymerization of actin microfilaments, which somehow facilitates Nrf2 nuclear translocation (69). PERK has been shown to phosphorylate Nrf2 and enhance its nuclear translocation by disrupting Keap1 binding (24).
Several coactivators, like p160 and CBP/p300, are shown to interact with the Nrf2–Maf–ARE complex and enhance Nrf2 transcriptional activity (74, 179). A recent study shows that activation of ERK and JNK pathways promotes recruitment of coactivator to the transcription initiation complex and upregulates Nrf2 transcriptional activity (137).
Nrf2 and Chemoprevention
Cancer has been reported to be a leading cause of death in the United States (64). As discussed earlier, carcinogenesis is a multistep process, thus providing numerous opportunities for cancer prevention. At this time, prevention could be very costly, but it could be the best approach toward cancer managements. It is estimated that it would cost clinical prevention in the United States from under $US2,000 to over $US6,000,000 per life-year saved (54). Even so, American society would appear to be willing to pay for such costly cancer prevention programs since the disease inflicts great pains and the burden for treatment is overwhelming. The good news is, cancer is a preventable disease. After going through the review of previous section on Keap-Nrf2-ARE signaling, it is timely to enter into the topic on regulation of Nrf2 signaling for cancer chemoprevention.
Current studies have shown that some chemopreventive compounds are not only effective in animal models, but also promising in the on-going clinical trials (50). In general, chemopreventive compounds may work via multifaceted molecular pathways (4, 46, 66), such as by suppressing inflammation, cell proliferation, invasion, and angiogenesis of tumor cells (5), and by enhancing the antioxidant response (102, 174). A growing body of evidence justifies that targeting the Nrf2 pathway is a viable approach in cancer prevention (82, 83, 94, 112).
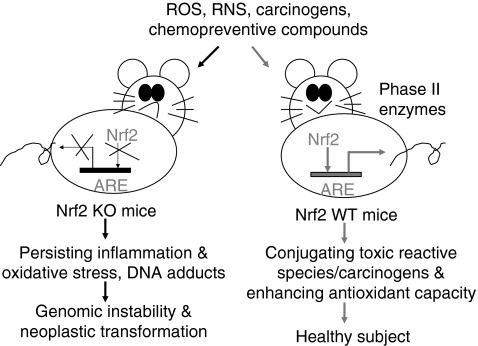
The first in vivo evidence showing a critical role of Nrf2 in induction of phase II detoxification enzymes was published in 1997 (58). By using Nrf2-deficient (Nrf2 KO) mice, Ito et al. found that the magnitude of GST and NQO1 induction was significantly lower in the Nrf2-deficient mice than that in Nrf2 heterozygous mice. Such a critical role of Nrf2 in activation of phase II antioxidant genes has been further confirmed in many studies using Nrf2-deficient mice (Table 1). An immediate application of this finding in cancer prevention is to test the anticarcinogenic activity of chemopreventive compounds in Nrf2 KO mice. Figure 5 has shown how the Nrf2 pathway protects mice from carcinogen-induced neoplastic transformation. As Nrf2 is disrupted, induction of cytoprotective phase II enzymes is impaired. When Nrf2 KO mice are exposed to carcinogens, the uncontrollable oxidative stress generated by carcinogens would damage DNA and induce persistent inflammation, which, in turn, increases genomic instability and enhance neoplastic transformation. In comparison with Nrf2 KO mice, the WT mice maintain normal induction of phase II detoxification enzymes, and hence limit the cytotoxic effect of carcinogen-generated oxidative stress. Many chemopreventive compounds have been shown to induce phase II detoxification enzymes through a mechanism dependent on Keap1-Nrf2-ARE signaling (Fig. 2). Thus, it has been hypothesized that chemopreventive agents may act as oxidants or electrophiles to modify several key cysteine residues of Keap1, thereby preventing Nrf2 degradation; meanwhile, chemopreventive-agent-generated redox signal may directly act on Nrf2 molecules and enhance their nuclear localization. Given the important role of Nrf2 in protection against carcinogenesis, attempts have been made to develop more effective chemopreventive agents by targeting Nrf2 pathway.
Table 1.
Dependency of Carcinogenesis and Chemoprevention on Nrf2-Knockout In Vivo Models
| Carcinogen/chemopreventive compounds | Model | Phenotype/outcome | Reference |
|---|---|---|---|
| Phenolic antioxidant butylated hydroxyanisole | Heterozygous/homozygous Nrf2 KO mice | The magnitude of the induction of GST and NQO1 genes was significantly lower in the homozygous Nrf2 KO than in the Nrf2 heterozygous mouse. | 58 |
| BaP/oltipraz, a substituted 1,2-dithiole-3-thione (4-methyl-5-[2-pyrazinyl]-1,2-dithiole-3-thione) | Nrf2 WT vs. Nrf2 KO mice | Constitutive hepatic and gastric activities of GST and NQO1 ↓ 50%–80% in Nrf2 KO vs. Nrf2 WT mice | 132, 133 |
| Nrf2 KO mice had a significantly ↑ burden of gastric neoplasia after treatment with BaP than did WT mice. | |||
| Oltipraz significantly ↓ multiplicity of gastric neoplasia in WT mice by 55%, but had no effect on tumor burden in Nrf2 KO mice. | |||
| ↑ BaP-DNA adduct in the forestomach of Nrf2 KO mice | |||
| D3T/oltipraz | Nrf2 WT vs. Nrf2 KO mice | Hepatic activities of GST and NQO1 ↑ by D3T in WT mice but not in Nrf2 KO mice | 93, 95 |
| NQO1 in liver, forestomach, and kidney ↑ by oltipraz significantly in WT mice but not in Nrf2 KO mice | |||
| Basal activity of hepatic, forestomach, and kidney NQO1 in Nrf2 KO mice was 70% ↓ than in WT mice. | |||
| BaP/SFN | Nrf2 WT vs. Nrf2 KO mice | ↑ significantly BaP-evoked forestomach tumors in Nrf2 KO mice, which was blocked by SFN significantly in WT mice. This protection resulted from ↑ phase II enzymes. | 31 |
| Diesel exhaust | Heterozygous/homozygous Nrf2 KO mice | ↑ DNA adducts formation in the lungs, severe hyperplasia, and accumulation of the oxidative DNA adduct 8-hydroxydeoxyguanosine in the bronchial epidermis in Nrf2 KO mice | 8 |
| BaP | Transgenic mice harboring gpt with heterozygous/homozygous Nrf2 KO mice (nrf2+/−::gpt and nrf2−/−::gpt mice) | ↑ spontaneous mutation of the lung gpt gene in Nrf2 KO mice | 7 |
| ↑ the lung mutation frequency 3.1- and 6.1-fold in nrf2+/− and nrf2−/− mice, respectively, compared with BaP-untreated nrf2+/− mice, showing that nrf2−/− mice are more susceptible to genotoxic carcinogens | |||
| BBN/oltipraz | Nrf2 WT and Nrf2 KO mice | ↑ BBN-induced bladder carcinogenesis in Nrf2 KO mice, higher incidence of bladder carcinoma and invasive carcinoma | 56 |
| Oltipraz ↓ incidence of bladder carcinoma by BBN in WT but little effects in KO | |||
| Oltipraz ↑ BBN glucuronidation and decreased the urinary concentration of a carcinogen of BBN and counteract the BBN suppression of UGT1A in the bladder in WT | |||
| DMBA/TPA/SFN | Nrf2 WT vs. Nrf2 KO mice | ↑ skin tumorigenesis in Nrf2 KO mice than WT | 166 |
| Nrf2 and HO-1 ↓ in skin tumors | |||
| SFN significantly inhibits skin tumorigenesis in WT but not in KO. | |||
| DMBA/TPA/tBHQ | Nrf2 WT and transgenic mice with overexpression of dominant-negative Nrf2 mutant in the epidermis under the control of the K14-dnNrf2 | ↑ onset, incidence, and multiplicity of DMBA/TPA induced skin papillomas in K14-dnNrf2 mice | 9 |
| DSS | Nrf2 WT vs. Nrf2 KO mice | Nrf2 KO mice had significantly shorter colon than Nrf2 WT mice (p < 0.05). | 80 |
| ↑ DSS-induced colitis, ↓ HO-1, NQO1, UGT1A1, GSTM1, ↑ proinflammatory mediators/cytokines: COX-2, iNOS, iL-1b, iL-6 | |||
| AOM-DSS | Nrf2 WT vs. Nrf2 KO mice | ↑ colonic tumor vs. 53% Nrf2 WT (p < 0.05) in Nrf2 KO, ↑ COX-2, 5-LOX, PGE2, LTB4, inflamed colonic mucosa (nitrotyrosine expression) | 81 |
| ↑ NQO1, UGT1A1 in WT mice | |||
| AOM/DSS | Nrf2 WT and Nrf2 KO mice | DSS treatment significantly increased ACF, inflammation, and mucosal damage in Nrf2 KO mice, but not WT (p < 0.05). | 122 |
| IQ | Nrf2 WT and Nrf2 KO mice | ↑ incidence and multiplicity of hepatocellular adenoma and carcinoma in Nrf2 KO, ↓ UGT, and GST activities as compared to WT | 85 |
ACF, aberrant crypt foci; AOM, azoxymethane; BaP, benzo[a]pyrene; BBN, N-nitrosobutyl(4-hydroxybutyl)amine; D3T, 3H-1,2-dithiole-3-thione; DMBA, 7,12-Dimethylbenz(a)anthracene; DSS, dextran sulfate sodium; gpt, guanine phosphoribosyltransferase; GST, glutathione S-transferase; HO-1, heme oxygenase-1; IQ, 2-amino-3-methylimidazo[4,5-f]quinoline; K14, keratin 14; Nrf2, NF-E2-related factor 2; KO, knockout; NQO1, NAD(P)H:quinone oxidoreductase 1; SFN, sulforaphane; tBHQ, tert-butylhydroquinone; TPA, 12-O-tetradecanoylphorbol-13-acetate; UGT, UDP-glucuronosyltransferase; WT, wild type.
FIG. 5.
Schematic presentation showing that Nrf2-disrupted mice (Nrf2 KO) have a higher susceptibility to carcinogenesis. The critical role of Nrf2 in protecting mice from neoplastic transformation when subject to oxidative stress and carcinogens is intact in Nrf2 WT mice having functional Nrf2-ARE signaling, by enhancing expression of detoxifying metabolizing enzymes and maintaining oxidative stress homeostasis by producing antioxidative stress enzymes. Application of chemopreventive compounds in Nrf2 WT mice can further enhance expression of phase II detoxifying and antioxidant enzymes by regulating the Nrf2-ARE signaling. ARE, antioxidant responsive element; Nrf2, NF-E2-related factor 2; Nrf2 KO, Nrf2 knockout; Nrf2 WT mice, wild-type mice with intact Nrf2 function.
Nrf2 and Antiinflammation–Nrf2 KO-Dextran Sulfate Sodium and Azoxymethane-Dextran Sulfate Sodium Colon Model
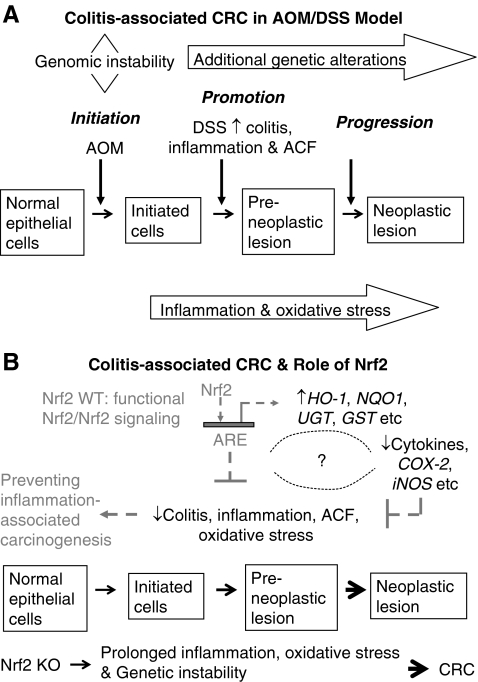
Current studies have linked chronic inflammation to cancer development (105). Colorectal cancer (CRC) is one of the classic examples that demonstrate a close relationship between chronic inflammation and carcinogenesis (106) (Fig. 6A). Treatment of animals with dextran sulfate sodium (DSS) has been shown to induce colitis, a chronic inflammatory disease, whereas treatment of animals with both azoxymethane (AOM) and DSS induces CRC. Using Nrf2 KO mice, we found that disruption of Nrf2 gene renders animals more susceptible to DSS-induced colitis and to AOM-DSS-induced colon carcinogenesis (80, 81). Similar results were obtained by Kensler's group when investigating the role of Nrf2 in prevention of inflammation-associated aberrant crypt foci (ACF) formation (122). They noted that Nrf2 KO mice developed significantly higher incidence of colonic tumor than WT mice. Further, AOM-DSS-treated Nrf2 KO mice had more severe colitis and higher incidence of prolapsed rectum and bleeding than WT mice. Therefore, Nrf2 pathway appears to mediate a strong antiinflammatory response, besides induction of detoxification and antioxidant enzymes as described above.
FIG. 6.
A simplified illustration shows the carcinogenesis in human CRC and the role of Nrf2 in antiinflammation and oxidative stress. (A) Pathways that connect inflammation and CRC in AOM/DSS animal model, a chemical-carcinogen-induced CRC model. (B) Role of Nrf2 in protecting inflammation-associated cancer, showing a potential strategy for prevention of inflammation-associated cancers. When the Nrf2 signaling pathway is activated, activation of expression of phase II antioxidant and detoxifying enzymes such as HO-1, NQO1, UGT, and GST alike in Nrf2 WT animals can reduce the oxidative stress, and it is postulated that Nrf2 could also regulate proinflammatory genes and cytokines such as iNOS, COX-2, and iL-6 alike, and together, they protect the animal from developing inflammation-associated cancer, such as CRC (broken arrows). In contrast, Nrf2 KO animals do not possess functional Nrf2 and Nrf2 signaling pathway; after prolonged inflammatory and oxidative stress caused by AOM/DSS, they have increased incidence, multiplicity, and size of all colorectal tumors, including adenoma and adenocarcinoma as compared with WT animals (solid arrows). ACF, aberrant crypt foci; AOM, azoxymethane; CRC, colorectal cancer; DSS, dextran sulfate sodium; GST, glutathione S-transferase; HO-1, heme oxygenase-1; Nrf2, NF-E2-related factor 2; Nrf2 KO, Nrf2 knockout; Nrf2 WT mice, wild-type mice with intact Nrf2 function; NQO1, NAD(P)H:quinone oxidoreductase 1; UGT, UDP-glucuronosyltransferase.
Regulation of Nrf2-Target Genes in Transgenic Adenocarcinoma Mouse Prostate Mice Prostate Cancer Model
Prostate cancer as a major health concern
Prostate cancer (PCa) remains the top leading cancer types responsible for 25% of new cancer cases with an estimated 192,280, and is second leading cause of cancer death in men, estimated 27,360 deaths in the United States in 2009 (64). Presently, metastatic PCa is not curable and is associated with a mean survival of 2–3 years. Development of PCa is often characterized by a relatively long latent period from the precursor lesions, called prostatic intraepithelial neoplasia (PIN), to the invasive carcinoma and metastasis that is typically associated with age and elderly men (1, 26, 86, 91, 96, 115–117, 124, 165). Therefore, to decrease the incidence of PCa, or to delay the neoplastic development, or to slow the progression of PCa to late stage aggressive diseases achieved through either dietary or chemical intervention would be logical and would be tremendous clinical benefit to hundreds of thousands of men (10). Further, chemoprevention of PCa will be more cost effective than therapeutics designed to treat/cure the disease.
The transgenic adenocarcinoma mouse prostate mouse model for prostate neoplasia
Development of effective chemopreventive compounds against these malignancies is important and requires conclusive evidence from animal models that emulate human cancers. The transgenic adenocarcinoma mouse prostate (TRAMP) mouse is an autochthonous transgenic animal model of PCa that recapitulates the whole spectrum of human prostate tumorigenesis from the earliest PIN lesions to androgen-independent disease (71).
The TRAMP model was originally developed in Norman Greenberg's laboratory using the minimal prostate specific rat probasin promoter to drive expression of the simian virus 40 (SV40) early region tumor antigen-coding region (T, t; large T and small t antigens) specifically in prostatic epithelium (40). The tumor (T) antigens have the ability to induce transformation in vivo (19) and have been used successfully in transgenic mice to induce a transformed state in a variety of systems, including pancreas (45), mammary gland (152), and others [reviewed in Adams and Cory (2)]. The large T antigen acts as an oncoprotein through interaction with and inhibition of retinoblastoma (Rb) and p53 tumor-suppressor gene products, whereas the small t antigen interacts with a protein phosphatase (40). At first glance, it appears that this artificially expressed SV40 trangene oncogene may not be an appropriate model for human PCa; however, given that the loss of WT p53 and Rb has been implicated in the development and progression of PCa (17, 135, 139, 160), this would seem logical. The TRAMP mice express the T antigen oncoprotein by 8 weeks of age and develop distinct pathology in the epithelium of the dorsolateral prostate by 10 weeks of age. TRAMP mice are first observed to develop mild epithelial hyperplasia between 8 and 12 weeks of age. Lymph node metastases can be detected between 18 and 24 weeks of age, and pulmonary metastases were frequently identified by 24 weeks of age. By 28 weeks of age, 100% of the TRAMP mice harbor metastatic PCa in the lymph nodes or lungs (39).
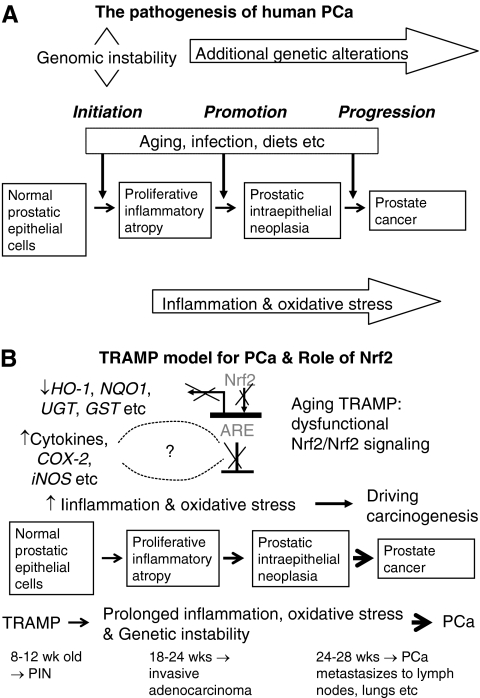
Therefore, the TRAMP model may provide a consistent source of primary and metastatic tumors for histopathobiological and molecular analysis to further define the earliest molecular events involved in the genesis, progression, and metastasis of PCa (38). Further, as seen in human PCa, TRAMP mice develop androgen-independent PCa after castration (38). Overall, the TRAMP model exhibits many similarities to human PCa, including epithelial origin, progression from the PIN stage to adenocarcinoma, and metastasis by a transgene that is hormonally regulated by androgen (38–41), so that it serves as a suitable model (114) (Fig. 7A). PCa, just like CRC, has a critical component of inflammation in the prostate carcinogenesis as well (116, 124). Specifically, for example, immunotherapy consisting of irradiated tumor cell vaccine and anti-CTLA-4 antibodies has been shown to markedly decrease the incidence of prostate tumors in TRAMP mice (55). CTLA-4 is a T cell surface antigen that plays an important role in attenuating T cell activity, and mice treated with anti-CTLA-4 antibodies were found to display a robust immune response to a variety of antigens (98). The same group has continued this work and found that indeed TRAMP mice allowed the functional identification of immunogenic prostate tumor antigens with relevance for human immunotherapy (32). Further studies with the TRAMP model by Gupta et al. have demonstrated a remarkable reduction in the rate of prostate tumorigenesis and metastasis formation between TRAMP mice fed a control diet and those fed a diet supplemented with the COX-2 inhibitor celecoxib (100% vs. 25% and 65% vs. 0%, respectively) (42). These findings suggest a critical role of proinflammatory enzyme COX-2 in PCa development and progression in the TRAMP model (Fig. 7B).
FIG. 7.
A simplified illustration shows the pathogenesis in human PCa and the role of Nrf2 in TRAMP PCa model. (A) Normal prostatic epithelial cells may be subjected to the chronic inflammation and oxidative stress in addition to other factors such as aging and diets. For more detailed molecular pathogenesis of PCa, please refer to Nelson et al. (117). As found in CRC, inflammation is believed to be one of the key events before the formation of PCa, and proliferative inflammatory atrophy is a precursor to PIN and PCa. (B) The TRAMP mouse is an autochthonous transgenic animal model of PCa that recapitulates the whole spectrum of human prostate tumorigenesis from the earliest PIN lesions to androgen-independent disease (71). Without chemical or hormonal treatment, 100% of male TRAMP develops PCa and progress from PIN to histological cancer to carcinoma metastasis to lymph nodes, lungs, and occasionally bones sequentially over 12–28 weeks (39, 40). A schematic illustration of the putative role of inflammation and oxidative stress and their effect on Nrf2 and related phase II detoxifying and antioxidant enzymes in prostate carcinogenesis in TRAMP mice. Effective chemopreventive compounds such as COX-2 inhibitors and gamma tocopherol-enriched mixed tocopherols significantly downregulated proinflammatory genes and upregulated expression of Nrf2 and its related detoxifying and antioxidant enzymes, respectively, preventing PCa carcinogenesis in TRAMP mice, especially when they are given early before the formation of PIN. CRC, colorectal cancer; Nrf2, NF-E2-related factor 2; PCa, prostate cancer; PIN, prostatic intraepithelial neoplasia; TRAMP, transgenic adenocarcinoma mouse prostate.
Nrf2 and GSTs are downregulated in human and TRAMP PCa and regulation of Nrf2-target genes in TRAMP mice by chemopreventive compounds
Importantly, it has been found that from a meta-analysis on 10 human PCa gene expression studies (35), Nrf2 and members of GSTmu are extensively decreased statistically. Using the TRAMP transgene and Rb and Nrf2 KO mouse models, the same group has also demonstrated that the loss of Nrf2 initiates a detrimental cascade of reduced GST expression, elevated ROS levels, and ultimately DNA damage associated with tumorigenesis. Our laboratory has also found that tumor progression in TRAMP occurred with a significant suppression of antioxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase, HO-1, and phase II detoxification enzymes, supporting the relevant use of TRAMP in examining Nrf2-related pathways in human PCa (12). Curcumin and phenethyl isothiocyanate alone or in combination significantly decreased the incidence of PCa tumor formation (11), by downregulating Akt signaling pathway, which later was also found in the TRAMP mice treated with broccoli sprout, a rich source of SFN (78). Again, the important role of Nrf2 pathway for effectiveness of SFN in preventing PCa is confirmed in TRAMP mice by inducing expression of Nrf2 and HO-1, along with other apoptotic proteins and suppressing Akt-dependent pathways in the prostate of TRAMP mice. Increasing evidence points out that the Akt-dependent signaling regulatory proteins are critical regulators of prostate tumorigenesis in vivo (21, 37, 151). Our latest data present the first experimental evidence that inhibition of Akt signaling pathway might be an important cellular mechanism for prevention of prostate tumor growth in TRAMP mice by broccoli sprout. The data also suggest that both the activation of Nrf2 pathway and the inhibition of Akt are required for effective prevention of PCa growth in TRAMP mice (78). Our current understanding of the interplays between Nrf2 and other signaling pathways is incomplete; research is undergoing to shed more light on this aspect.
TRAMP model has been used extensively to evaluate agents against PCa by various chemopreventive compounds, including difluoromethylornithine (43), R-flurbiprofen (163), toremifene (130), green tea (3, 44), genistein (158), and non-steroidal antiinflammatory drugs, celecoxib and exisulind (42, 114), silibinin (131), phenethyl isothiocyanate and curcumin (11), and methyl selenium (159). Most recently, we found that gamma tocopherol-enriched mixed tocopherols suppressed PIN and tumor development in the TRAMP mice, upregulated expression of Nrf2, and induced phase II detoxification and antioxidant enzymes (12). Again, these findings substantiate the important role of Nrf2 pathway in cancer prevention, at least, in TRAMP mice, by chemopreventive agents (Fig. 7B).
Potential epigenomic regulation of Nrf2 in TRAMP mice
The currently accepted paradigm of the regulation of Nrf2 signaling appears to be mainly achieved via posttranslational mechanism, as discussed previously. Briefly, Nrf2 is functionally suppressed by Keap1 protein, which binds to and sequesters Nrf2 in the cytoplasm leading to the degradation of Nrf2, and thus prevents Nrf2 to translocate into nucleus to activate its targeted genes (102). When stimulated by oxidative stress or upstream kinases, the ubiquitination and the proteosomal degradation of Nrf2 are impeded and Nrf2 is being released from Keap1, translocates into the nucleus, can dimerize with Maf proteins, binds to ARE, and transcriptionally activates the Nrf2-targeted genes. To date, it is not clear as how expression of Nrf2 in human PCa or in TRAMP mouse tumor is suppressed. However, our most recent studies indicate that expression of Nrf2 is epigenetically silenced by DNA hypermethylation in TRAMP prostate tumor (173), which warrants further studies.
Interplay Between Inflammation and Nrf2 Pathways
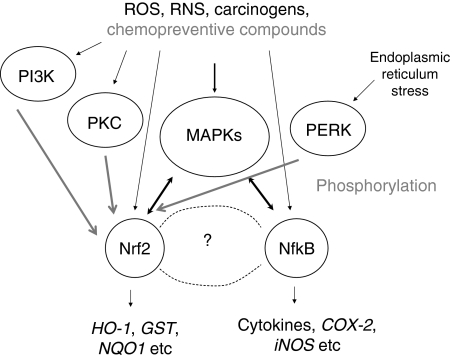
Dysfunctional Nrf2 signaling/suppression of Nrf2 in aging TRAMP mice (Fig. 7B) appears to be similar to the Nrf2 KO mice (Fig. 5) and causes a persisting chronic inflammation (Fig. 6B) with increased oxidative stress, and eventually could contribute to and driving carcinogenesis if other factors are present. Interplay between Nrf2 signaling pathway and inflammatory pathway has recently be reviewed (82, 101). Increasing evidence has shown that Nrf2 could play an important role in defense against oxidative stress possibly by activation of cellular antioxidant machinery as well as suppression of proinflammatory signaling pathways. In addition, in vivo and in vitro data suggest that many dietary chemopreventive compounds can differentially regulate Nrf2-mediated antioxidant/antiinflammatory signaling pathways as the first line defense or induce apoptosis once the cells have been damaged (101), and many other pathways can also regulate Nrf2, including a wide variety of kinase signaling pathways such as MAPKs, PI3K, PI3K, and PERK as discussed earlier in regulation of Nrf2 signaling by other pathways section (65, 138). Many signaling pathways are involved in the cytokines and inflammatory response, such as nuclear factor-kappa-B (NF-κB) (30, 127) and MAPKs (155) pathways. Many chemopreventive compounds that act through Nrf2 pathway are also having antiinflammatory activities (82, 83, 101). As an initial study, we explored the relationship between Nrf2 and NF-κB, a key transcriptional factor involved in inflammatory response (111). Our bioinformatic analysis of the promoter regions of Nrf2 and NF-κB genes reveals that 75% of the members in the regulatory network are MAPKs, which appears to be consistent with the current role of MAPKs in modulation of both Keap1-Nrf2-ARE (63, 137, 144, 170, 175) and NF-κB signaling pathways (27, 110). Collectively, we are presenting a simplified model for the potential concerted modulation of Nrf2 and NF-κB in inflammation and carcinogenesis via upstream MAPKs pathway (Fig. 8).
FIG. 8.
A simplified scheme illustrating the interplay between Nrf2 and NF-κB leading to carcinogenesis/chemoprevention. Stimuli from ROS, RNS, and chemopreventive compounds could directly interact with members of upstream kinase signaling pathways such as PI3K, PKC, MAPK, and PERK, especially the MAPKs family, as well as interacting with Nrf2 and NF-κB pathways concurrently, the potential cross-talk between Nrf2 and Nf-κB is denoted by double-head arrows. Such multiple interactions allow chemopreventive compounds to exert their multiple beneficial cancer preventive properties. MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-kappa-B; Nrf2, NF-E2-related factor 2; PERK, PKR-like endoplasmic reticulum kinase; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; ROS, reactive oxygen species; RNS, reactive nitrogen species.
Conclusion and Future Perspective
Many oncogenic transcription factors can be targeted for preventing cancer from forming (22), and Nrf2 is one of the very promising target as evidenced from the findings of increased Nrf2-dependent susceptibility in Nrf2 KO mice to various chemical-carcinogen-induced cancers and the effectiveness of chemopreventive compounds to inhibit carcinogenesis in the counterparts Nrf2 WT mice (with intact Nrf2 function) (Table 1). In addition, the role of Nrf2 in carcinogenesis of PCa in TRAMP mice appears to be implicated with the downregulation of expression of Nrf2 and its target genes (12), which can be explained in part via epigenetic mechanism (173). Epigenetic is also modulating the CRC carcinogenesis (104). A variety of antioxidants and antiinflammatory drugs, which are likely to be capable of attenuating procarcinogenic genomic damage from ROS and reactive nitrogen species, are also under current development for PCa prevention (10, 115).
On the other hand, Nrf2-regulated high expression of phase II/antioxidant enzymes during chemotherapy might be one of the major causes of ineffectiveness of chemotherapeutic agents, and such dark side of Nrf2 has been reviewed recently by Zhang's group (97). As we progress with future studies, optimization of the timing of when to give chemopreventive compounds to patients in lieu of the potential dark side of Nrf2 in cancer chemoresistance would need to be considered, in particular with many advanced tumors that are resistance to radiation and chemotherapeutic drugs. Nevertheless, the studies using Nrf2 KO models have clearly shown the protective role of Nrf2 in cancer prevention particularly during the earlier phases of initiation of carcinogenesis. The dark side of Nrf2 would need further investigation, and may depend on the animal models and the compounds tested. It is believed that basal Nrf2 is indeed a carcinogenesis protective transcriptional factor under normal physiological conditions when it is functioning properly. However, the high levels of Nrf2 as well as many other drug resistance genes, tumor oncogenes, and growth factors found in malignant tumors will need further integration and investigation in terms of translating to patients.
Abbreviations Used
- ACF
aberrant crypt foci
- AOM
azoxymethane
- ARE
antioxidant responsive element
- BaP
benzo[a]pyrene
- BBN
N-nitrosobutyl(4-hydroxybutyl)amine
- bNLS
bipartite nuclear localization signal at the basic region
- BTB
broad complex, tramtrack, and bric a brac
- bZip
basic-leucine zipper
- CNC
Cap ’n’ Collar
- CRC
colorectal cancer
- CTR
C-terminal region
- D3T
3H-1,2-dithiole-3-thione
- DGR
double glycine/Kelch repeat region
- DLG
(formulated as) L-X-X-Q-D-X-D-L-G
- DMBA
7,12-dimethylbenz(a)anthracene
- DSS
dextran sulfate sodium
- EGFP
green fluorescence protein
- ERK2
extracellular signal-regulated protein kinase 2
- ETGE
(formulated as) D/N-X-E-T/-S-G-E
- fNrf2
free-floating Nrf2
- gpt
guanine phosphoribosyltransferase
- GST
glutathione S-transferase
- HO-1
heme oxygenase-1
- IQ
2-amino-3-methylimidazo[4,5-f]quinoline
- IVR
intervening region
- JNK1
c-Jun NH(2)-terminal kinase-1
- Keap1
Kelch-like ECH-associated protein 1
- kNrf2
Keap1-binding Nrf2
- Maf
musculoaponeurotic fibrosarcoma
- MAPKs
mitogen-activated protein kinases
- Neh
Nrf2-ECH homology
- NES
nuclear exporting signal
- NESTA
nuclear exporting signal at transactivation (TA) domain
- NESzip
nuclear exporting signal in the ZIP dimerization domain
- NF-κB
nuclear factor-kappa-B
- NLS
nuclear localization signal
- NLSC
monopartite NLS at the carboxyl-terminus
- NLSN
monopartite NLS at the amino-terminus
- NQO1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
NF-E2-related factor 2
- Nrf2 KO
Nrf2 knockout
- Nrf2 WT
wild-type mice with intact Nrf2 function
- NTR
N-terminal region
- PCa
prostate cancer
- PERK
PKR-like endoplasmic reticulum kinase
- PI3K
phosphatidylinositol 3-kinase
- PIN
prostatic intraepithelial neoplasia
- PKC
protein kinase C
- Rb
retinoblastoma
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SFN
sulforaphane
- SV40
simian virus 40
- tBHQ
tert-butylhydroquinone
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TRAMP
transgenic adenocarcinoma mouse prostate
- Ub
ubiquitination
- UGT
UDP-glucuronosyltransferase
Acknowledgments
This work was supported in part by Grant no.30801410 from the Natural Science Foundation of China (R.H.), NIH R01-CA094828 (A.N.T.K.), and NIH R01-AG023497 (R.Y.). The authors thank all the members of Dr. Tony Kong's lab for their help in the discussion and preparation of this article.
References
- 1.Abate-Shen C. Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM. Cory S. Transgenic models of tumor development. Science. 1991;254:1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- 3.Adhami VM. Siddiqui IA. Ahmad N. Gupta S. Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB. Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB. Van Kuiken ME. Iyer LH. Harikumar KB. Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam J. Wicks C. Stewart D. Gong P. Touchard C. Otterbein S. Choi AM. Burow ME. Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 7.Aoki Y. Hashimoto AH. Amanuma K. Matsumoto M. Hiyoshi K. Takano H. Masumura K. Itoh K. Nohmi T. Yamamoto M. Enhanced spontaneous and benzo(a)pyrene-induced mutations in the lung of Nrf2-deficient gpt delta mice. Cancer Res. 2007;67:5643–5648. doi: 10.1158/0008-5472.CAN-06-3355. [DOI] [PubMed] [Google Scholar]
- 8.Aoki Y. Sato H. Nishimura N. Takahashi S. Itoh K. Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 9.auf dem Keller U. Huber M. Beyer TA. Kumin A. Siemes C. Braun S. Bugnon P. Mitropoulos V. Johnson DA. Johnson JA. Hohl D. Werner S. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol Cell Biol. 2006;26:3773–3784. doi: 10.1128/MCB.26.10.3773-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardia A. Platz EA. Yegnasubramanian S. De Marzo AM. Nelson WG. Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol. 2009;9:419–426. doi: 10.1016/j.coph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barve A. Khor TO. Hao X. Keum YS. Yang CS. Reddy B. Kong AN. Murine prostate cancer inhibition by dietary phytochemicals—curcumin and phenyethylisothiocyanate. Pharm Res. 2008;25:2181–2189. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barve A. Khor TO. Nair S. Reuhl K. Suh N. Reddy B. Newmark H. Kong AN. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenblum I. Armuth V. Two independent aspects of tumor promotion. Biochim Biophys Acta. 1981;651:51–63. doi: 10.1016/0304-419x(81)90005-6. [DOI] [PubMed] [Google Scholar]
- 14.Bertram JS. The molecular biology of cancer. Mol Aspects Med. 2000;21:167–223. doi: 10.1016/s0098-2997(00)00007-8. [DOI] [PubMed] [Google Scholar]
- 15.Bloom D. Dhakshinamoorthy S. Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2002;21:2191–2200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- 16.Bloom DA. Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 17.Bookstein R. Rio P. Madreperla SA. Hong F. Allred C. Grizzle WE. Lee WH. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci U S A. 1990;87:7762–7766. doi: 10.1073/pnas.87.19.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosland MC. Use of animal models in defining efficacy of chemoprevention agents against prostate cancer. Eur Urol. 1999;35:459–463. doi: 10.1159/000019879. [DOI] [PubMed] [Google Scholar]
- 19.Brinster RL. Chen HY. Messing A. van Dyke T. Levine AJ. Palmiter RD. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C. Shen G. Hebbar V. Hu R. Owuor ED. Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 21.Chen ML. Xu PZ. Peng XD. Chen WS. Guzman G. Yang X. Di Cristofano A. Pandolfi PP. Hay N. The deficiency of Akt1 is sufficient to suppress tumor development in Pten +/− mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colburn NH. Kensler TW. Targeting transcription factors for cancer prevention—the case of Nrf2. Cancer Prev Res (Phila Pa) 2008;1:153–155. doi: 10.1158/1940-6207.CAPR-08-0025. [DOI] [PubMed] [Google Scholar]
- 23.Corpet DE. Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 24.Cullinan SB. Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 25.Cullinan SB. Gordan JD. Jin J. Harper JW. Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Marzo AM. Meeker AK. Zha S. Luo J. Nakayama M. Platz EA. Isaacs WB. Nelson WG. Human prostate cancer precursors and pathobiology. Urology. 2003;62:55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 27.de Sousa RR. Queiroz KC. Souza AC. Gurgueira SA. Augusto AC. Miranda MA. Peppelenbosch MP. Ferreira CV. Aoyama H. Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin. J Enzyme Inhib Med Chem. 2007;22:439–444. doi: 10.1080/14756360601162063. [DOI] [PubMed] [Google Scholar]
- 28.Dhakshinamoorthy S. Porter AG. Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J Biol Chem. 2004;279:20096–20107. doi: 10.1074/jbc.M312492200. [DOI] [PubMed] [Google Scholar]
- 29.Dinkova-Kostova AT. Holtzclaw WD. Cole RN. Itoh K. Wakabayashi N. Katoh Y. Yamamoto M. Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixit V. Mak TW. NF-kappaB signaling. Many roads lead to madrid. Cell. 2002;111:615–619. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 31.Fahey JW. Haristoy X. Dolan PM. Kensler TW. Scholtus I. Stephenson KK. Talalay P. Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fasso M. Waitz R. Hou Y. Rim T. Greenberg NM. Shastri N. Fong L. Allison JP. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci U S A. 2008;105:3509–3514. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favreau LV. Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 34.Friling RS. Bensimon A. Tichauer Y. Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frohlich DA. McCabe MT. Arnold RS. Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa M. Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao H. Ouyang X. Banach-Petrosky WA. Gerald WL. Shen MM. Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103:14477–14482. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingrich JR. Barrios RJ. Kattan MW. Nahm HS. Finegold MJ. Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–4691. [PubMed] [Google Scholar]
- 39.Gingrich JR. Barrios RJ. Morton RA. Boyce BF. DeMayo FJ. Finegold MJ. Angelopoulou R. Rosen JM. Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 40.Greenberg NM. DeMayo F. Finegold MJ. Medina D. Tilley WD. Aspinall JO. Cunha GR. Donjacour AA. Matusik RJ. Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg NM. DeMayo FJ. Sheppard PC. Barrios R. Lebovitz R. Finegold M. Angelopoulou R. Dodd JG. Duckworth ML. Rosen JM, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S. Adhami VM. Subbarayan M. MacLennan GT. Lewin JS. Hafeli UO. Fu P. Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–3343. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S. Ahmad N. Marengo SR. MacLennan GT. Greenberg NM. Mukhtar H. Chemoprevention of prostate carcinogenesis by alpha-difluoromethylornithine in TRAMP mice. Cancer Res. 2000;60:5125–5133. [PubMed] [Google Scholar]
- 44.Gupta S. Hastak K. Ahmad N. Lewin JS. Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanahan D. Oncogenes in transgenic mice. Nature. 1984;312:503–504. doi: 10.1038/312503a0. [DOI] [PubMed] [Google Scholar]
- 46.Hatcher H. Planalp R. Cho J. Torti FM. Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heidelberger C. Freeman AE. Pienta RJ. Sivak A. Bertram JS. Casto BC. Dunkel VC. Francis MW. Kakunaga T. Little JB. Schechtman LM. Cell transformation by chemical agents—a review and analysis of the literature. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983;114:283–385. doi: 10.1016/0165-1110(83)90036-2. [DOI] [PubMed] [Google Scholar]
- 48.Hong F. Sekhar KR. Freeman ML. Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J Biol Chem. 2005;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- 49.Hong WK. Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 50.Hsu CH. Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 51.Hu R. Kong AN. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutrition. 2004;20:83–88. doi: 10.1016/j.nut.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Huang HC. Nguyen T. Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang HC. Nguyen T. Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 54.Hunt TL. Luce BR. Page MJ. Pokrzywinski R. Willingness to pay for cancer prevention. Pharmacoeconomics. 2009;27:299–312. doi: 10.2165/00019053-200927040-00003. [DOI] [PubMed] [Google Scholar]
- 55.Hurwitz AA. Foster BA. Kwon ED. Truong T. Choi EM. Greenberg NM. Burg MB. Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 56.Iida K. Itoh K. Kumagai Y. Oyasu R. Hattori K. Kawai K. Shimazui T. Akaza H. Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 57.Ishii T. Itoh K. Sato H. Bannai S. Oxidative stress-inducible proteins in macrophages. Free Radic Res. 1999;31:351–355. doi: 10.1080/10715769900300921. [DOI] [PubMed] [Google Scholar]
- 58.Itoh K. Chiba T. Takahashi S. Ishii T. Igarashi K. Katoh Y. Oyake T. Hayashi N. Satoh K. Hatayama I. Yamamoto M. Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 59.Itoh K. Igarashi K. Hayashi N. Nishizawa M. Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itoh K. Tong KI. Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 61.Itoh K. Wakabayashi N. Katoh Y. Ishii T. Igarashi K. Engel JD. Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain AK. Bloom DA. Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 63.Jakubikova J. Sedlak J. Mithen R. Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Jemal A. Siegel R. Ward E. Hao Y. Xu J. Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 65.Jeong WS. Jun M. Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 66.Juge N. Mithen RF. Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakizoe T. Chemoprevention of cancer—focusing on clinical trials. Jpn J Clin Oncol. 2003;33:421–442. doi: 10.1093/jjco/hyg090. [DOI] [PubMed] [Google Scholar]
- 68.Kang KW. Choi SH. Kim SG. Peroxynitrite activates NF-E2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: the role of nitric oxide synthase in rat glutathione S-transferase A2 induction. Nitric Oxide. 2002;7:244–253. doi: 10.1016/s1089-8603(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 69.Kang KW. Lee SJ. Park JW. Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 70.Kang MI. Kobayashi A. Wakabayashi N. Kim SG. Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan-Lefko PJ. Chen TM. Ittmann MM. Barrios RJ. Ayala GE. Huss WJ. Maddison LA. Foster BA. Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 72.Karapetian RN. Evstafieva AG. Abaeva IS. Chichkova NV. Filonov GS. Rubtsov YP. Sukhacheva EA. Melnikov SV. Schneider U. Wanker EE. Vartapetian AB. Nuclear oncoprotein prothymosin alpha is a partner of Keap1: implications for expression of oxidative stress-protecting genes. Mol Cell Biol. 2005;25:1089–1099. doi: 10.1128/MCB.25.3.1089-1099.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katoh Y. Iida K. Kang MI. Kobayashi A. Mizukami M. Tong KI. McMahon M. Hayes JD. Itoh K. Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433:342–350. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Katoh Y. Itoh K. Yoshida E. Miyagishi M. Fukamizu A. Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 75.Kelloff GJ. Crowell JA. Steele VE. Lubet RA. Boone CW. Malone WA. Hawk ET. Lieberman R. Lawrence JA. Kopelovich L. Ali I. Viner JL. Sigman CC. Progress in cancer chemoprevention. Ann N Y Acad Sci. 1999;889:1–13. doi: 10.1111/j.1749-6632.1999.tb08718.x. [DOI] [PubMed] [Google Scholar]
- 76.Kelloff GJ. Lieberman R. Steele VE. Boone CW. Lubet RA. Kopelovich L. Malone WA. Crowell JA. Higley HR. Sigman CC. Agents, biomarkers, and cohorts for chemopreventive agent development in prostate cancer. Urology. 2001;57:46–51. doi: 10.1016/s0090-4295(00)00940-7. [DOI] [PubMed] [Google Scholar]
- 77.Kensler TW. Curphey TJ. Maxiutenko Y. Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17:3–22. doi: 10.1515/dmdi.2000.17.1-4.3. [DOI] [PubMed] [Google Scholar]
- 78.Keum YS. Khor TO. Lin W. Shen G. Kwon KH. Barve A. Li W. Kong AN. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 79.Keum YS. Yu S. Chang PP. Yuan X. Kim JH. Xu C. Han J. Agarwal A. Kong AN. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 80.Khor TO. Huang MT. Kwon KH. Chan JY. Reddy BS. Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 81.Khor TO. Huang MT. Prawan A. Liu Y. Hao X. Yu S. Cheung WK. Chan JY. Reddy BS. Yang CS. Kong AN. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila Pa) 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khor TO. Yu S. Kong AN. Dietary cancer chemopreventive agents—targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 83.Kim J. Cha YN. Surh YJ. A protective role of nuclear erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2009 Sep 30; doi: 10.1016/j.mrfmmm.2009.09.007. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 84.Kim SG. Cho MK. Choi SH. Kim HJ. Kwak MK. Kim ND. Molecular basis for hepatic detoxifying enzyme induction by 2-(allylthio)pyrazine in rats in comparison with oltipraz: effects on prooxidant production and DNA degradation. Drug Metab Dispos. 1999;27:667–673. [PubMed] [Google Scholar]
- 85.Kitamura Y. Umemura T. Kanki K. Kodama Y. Kitamoto S. Saito K. Itoh K. Yamamoto M. Masegi T. Nishikawa A. Hirose M. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazo[4,5-f ]quinoline. Cancer Sci. 2007;98:19–24. doi: 10.1111/j.1349-7006.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein EA. Thompson IM. Update on chemoprevention of prostate cancer. Curr Opin Urol. 2004;14:143–149. doi: 10.1097/00042307-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi A. Kang MI. Okawa H. Ohtsuji M. Zenke Y. Chiba T. Igarashi K. Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobayashi A. Kang MI. Watai Y. Tong KI. Shibata T. Uchida K. Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kobayashi M. Itoh K. Suzuki T. Osanai H. Nishikawa K. Katoh Y. Takagi Y. Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi M. Li L. Iwamoto N. Nakajima-Takagi Y. Kaneko H. Nakayama Y. Eguchi M. Wada Y. Kumagai Y. Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kucuk O. Chemoprevention of prostate cancer. Cancer Metastasis Rev. 2002;21:111–124. doi: 10.1023/a:1020809806121. [DOI] [PubMed] [Google Scholar]
- 92.Kwak MK. Egner PA. Dolan PM. Ramos-Gomez M. Groopman JD. Itoh K. Yamamoto M. Kensler TW. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res. 2001;480–481:305–315. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 93.Kwak MK. Itoh K. Yamamoto M. Sutter TR. Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- 94.Kwak MK. Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kwak MK. Ramos-Gomez M. Wakabayashi N. Kensler TW. Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes. Methods Enzymol. 2004;382:414–423. doi: 10.1016/S0076-6879(04)82022-6. [DOI] [PubMed] [Google Scholar]
- 96.Landis SH. Murray T. Bolden S. Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. 1. [DOI] [PubMed] [Google Scholar]
- 97.Lau A. Villeneuve NF. Sun Z. Wong PK. Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leach DR. Krummel MF. Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 99.Lee JM. Hanson JM. Chu WA. Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001;276:20011–20016. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- 100.Li W. Jain MR. Chen C. Yue X. Hebbar V. Zhou R. Kong AN. Nrf2 Possesses a redox-insensitive nuclear export signal overlapping with the leucine zipper motif. J Biol Chem. 2005;280:28430–28438. doi: 10.1074/jbc.M410601200. [DOI] [PubMed] [Google Scholar]
- 101.Li W. Khor TO. Xu C. Shen G. Jeong WS. Yu S. Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li W. Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li W. Yu SW. Kong AN. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J Biol Chem. 2006;281:27251–27263. doi: 10.1074/jbc.M602746200. [DOI] [PubMed] [Google Scholar]
- 104.Lipkin M. Reddy B. Newmark H. Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–586. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 105.Mantovani A. Allavena P. Sica A. Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 106.McConnell BB. Yang VW. The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep. 2009;5:69–74. doi: 10.1007/s11888-009-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McMahon M. Thomas N. Itoh K. Yamamoto M. Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 108.Moi P. Chan K. Asunis I. Cao A. Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Motohashi H. Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Murakami A. Song M. Ohigashi H. Phenethyl isothiocyanate suppresses receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis by blocking activation of ERK1/2 and p38 MAPK in RAW264.7 macrophages. Biofactors. 2007;30:1–11. doi: 10.1002/biof.5520300101. [DOI] [PubMed] [Google Scholar]
- 111.Nair S. Doh ST. Chan JY. Kong AN. Cai L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99:2070–2082. doi: 10.1038/sj.bjc.6604703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nair S. Li W. Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007;28:459–472. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 113.Nakaso K. Yano H. Fukuhara Y. Takeshima T. Wada-Isoe K. Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 114.Narayanan BA. Narayanan NK. Pittman B. Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004;10:7727–7737. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- 115.Nelson WG. Agents in development for prostate cancer prevention. Expert Opin Investig Drugs. 2004;13:1541–1554. doi: 10.1517/13543784.13.12.1541. [DOI] [PubMed] [Google Scholar]
- 116.Nelson WG. De Marzo AM. DeWeese TL. Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–S11. doi: 10.1097/01.ju.0000142058.99614.ff. discussion S-2. [DOI] [PubMed] [Google Scholar]
- 117.Nelson WG. De Marzo AM. Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 118.Nioi P. Nguyen T. Sherratt PJ. Pickett CB. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol Cell Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Numazawa S. Ishikawa M. Yoshida A. Tanaka S. Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 120.Ogura T. Tong KI. Mio K. Maruyama Y. Kurokawa H. Sato C. Yamamoto M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc Natl Acad Sci U S A. 2010;107:2842–2847. doi: 10.1073/pnas.0914036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okawa H. Motohashi H. Kobayashi A. Aburatani H. Kensler TW. Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 122.Osburn WO. Karim B. Dolan PM. Liu G. Yamamoto M. Huso DL. Kensler TW. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–1891. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 123.Osburn WO. Wakabayashi N. Misra V. Nilles T. Biswal S. Trush MA. Kensler TW. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454:7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palapattu GS. Sutcliffe S. Bastian PJ. Platz EA. De Marzo AM. Isaacs WB. Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 125.Paolini M. Perocco P. Canistro D. Valgimigli L. Pedulli GF. Iori R. Croce CD. Cantelli-Forti G. Legator MS. Abdel-Rahman SZ. Induction of cytochrome P450, generation of oxidative stress and in vitro cell-transforming and DNA-damaging activities by glucoraphanin, the bioprecursor of the chemopreventive agent sulforaphane found in broccoli. Carcinogenesis. 2004;25:61–67. doi: 10.1093/carcin/bgg174. [DOI] [PubMed] [Google Scholar]
- 126.Park EY. Kim SG. NO signaling in ARE-mediated gene expression. Methods Enzymol. 2005;396:341–349. doi: 10.1016/S0076-6879(05)96028-X. [DOI] [PubMed] [Google Scholar]
- 127.Pomerantz JL. Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 128.Prestera T. Holtzclaw WD. Zhang Y. Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Purdom-Dickinson S. Sheveleva E. Sun H. Chen QM. Translational control of Nrf2 protein in activation of antioxidant response by oxidants. Mol Pharmacol. 2007;72:1074–1081. doi: 10.1124/mol.107.035360. [DOI] [PubMed] [Google Scholar]
- 130.Raghow S. Hooshdaran MZ. Katiyar S. Steiner MS. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res. 2002;62:1370–1376. [PubMed] [Google Scholar]
- 131.Raina K. Rajamanickam S. Singh RP. Deep G. Chittezhath M. Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramos-Gomez M. Dolan PM. Itoh K. Yamamoto M. Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 133.Ramos-Gomez M. Kwak MK. Dolan PM. Itoh K. Yamamoto M. Talalay P. Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reddy BS. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- 135.Rubin SJ. Hallahan DE. Ashman CR. Brachman DG. Beckett MA. Virudachalam S. Yandell DW. Weichselbaum RR. Two prostate carcinoma cell lines demonstrate abnormalities in tumor suppressor genes. J Surg Oncol. 1991;46:31–36. doi: 10.1002/jso.2930460108. [DOI] [PubMed] [Google Scholar]
- 136.Rushmore TH. Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 137.Shen G. Hebbar V. Nair S. Xu C. Li W. Lin W. Keum YS. Han J. Gallo MA. Kong AN. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 138.Shen G. Jeong WS. Hu R. Kong AN. Regulation of Nrf2, NF-kappaB, and AP-1 signaling pathways by chemopreventive agents. Antioxid Redox Signal. 2005;7:1648–1663. doi: 10.1089/ars.2005.7.1648. [DOI] [PubMed] [Google Scholar]
- 139.Singh A. Jones RF. Friedman H. Hathir S. Soos G. Zabo A. Haas GP. Expression of p53 and pRb in bladder and prostate cancers of patients having both cancers. Anticancer Res. 1999;19:5415–5417. [PubMed] [Google Scholar]
- 140.Smith TJ. Hong JY. Wang ZY. Yang CS. How can carcinogenesis be inhibited? Ann N Y Acad Sci. 1995;768:82–90. doi: 10.1111/j.1749-6632.1995.tb12112.x. [DOI] [PubMed] [Google Scholar]
- 141.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- 142.Sun Z. Chin YE. Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 144.Svehlikova V. Wang S. Jakubikova J. Williamson G. Mithen R. Bao Y. Interactions between sulforaphane and apigenin in the induction of UGT1A1 and GSTA1 in CaCo-2 cells. Carcinogenesis. 2004;25:1629–1637. doi: 10.1093/carcin/bgh169. [DOI] [PubMed] [Google Scholar]
- 145.Tanigawa S. Fujii M. Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 146.Theodore M. Kawai Y. Yang J. Kleshchenko Y. Reddy SP. Villalta F. Arinze IJ. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J Biol Chem. 2008;283:8984–8994. doi: 10.1074/jbc.M709040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tominaga S. Kuroishi T. An ecological study on diet/nutrition and cancer in Japan. Int J Cancer Suppl. 1997;10:2–6. doi: 10.1002/(sici)1097-0215(1997)10+<2::aid-ijc2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 148.Tong KI. Katoh Y. Kusunoki H. Itoh K. Tanaka T. Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tong KI. Kobayashi A. Katsuoka F. Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 150.Tong KI. Padmanabhan B. Kobayashi A. Shang C. Hirotsu Y. Yokoyama S. Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Trotman LC. Alimonti A. Scaglioni PP. Koutcher JA. Cordon-Cardo C. Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tzeng YJ. Guhl E. Graessmann M. Graessmann A. Breast cancer formation in transgenic animals induced by the whey acidic protein SV40 T antigen (WAP-SV-T) hybrid gene. Oncogene. 1993;8:1965–1971. [PubMed] [Google Scholar]
- 153.Velichkova M. Guttman J. Warren C. Eng L. Kline K. Vogl AW. Hasson T. A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton. 2002;51:147–164. doi: 10.1002/cm.10025. [DOI] [PubMed] [Google Scholar]
- 154.Velichkova M. Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol. 2005;25:4501–4513. doi: 10.1128/MCB.25.11.4501-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wada T. Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 156.Wakabayashi N. Dinkova-Kostova AT. Holtzclaw WD. Kang MI. Kobayashi A. Yamamoto M. Kensler TW. Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wakabayashi N. Itoh K. Wakabayashi J. Motohashi H. Noda S. Takahashi S. Imakado S. Kotsuji T. Otsuka F. Roop DR. Harada T. Engel JD. Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 158.Wang J. Eltoum IE. Lamartiniere CA. Genistein alters growth factor signaling in transgenic prostate model (TRAMP) Mol Cell Endocrinol. 2004;219:171–180. doi: 10.1016/j.mce.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 159.Wang L. Bonorden MJ. Li GX. Lee HJ. Hu H. Zhang Y. Liao JD. Cleary MP. Lu J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila Pa) 2009;2:484–495. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X. Deng H. Basu I. Zhu L. Induction of androgen receptor-dependent apoptosis in prostate cancer cells by the retinoblastoma protein. Cancer Res. 2004;64:1377–1385. doi: 10.1158/0008-5472.can-03-2428. [DOI] [PubMed] [Google Scholar]
- 161.Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 162.Wattenberg LW. What are the critical attributes for cancer chemopreventive agents? Ann N Y Acad Sci. 1995;768:73–81. doi: 10.1111/j.1749-6632.1995.tb12111.x. [DOI] [PubMed] [Google Scholar]
- 163.Wechter WJ. Leipold DD. Murray ED., Jr. Quiggle D. McCracken JD. Barrios RS. Greenberg NM. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000;60:2203–2208. [PubMed] [Google Scholar]
- 164.Wilson LA. Gemin A. Espiritu R. Singh G. ets-1 is transcriptionally up-regulated by H2O2 via an antioxidant response element. FASEB J. 2005;19:2085–2087. doi: 10.1096/fj.05-4401fje. [DOI] [PubMed] [Google Scholar]
- 165.Wingo PA. Cardinez CJ. Landis SH. Greenlee RT. Ries LA. Anderson RN. Thun MJ. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 166.Xu C. Huang MT. Shen G. Yuan X. Lin W. Khor TO. Conney AH. Kong AN. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 167.Xu C. Yuan X. Pan Z. Shen G. Kim JH. Yu S. Khor TO. Li W. Ma J. Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 168.Yamamoto T. Suzuki T. Kobayashi A. Wakabayashi J. Maher J. Motohashi H. Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang CX. Kuroishi T. Huang XE. Inoue M. Tajima K. Correlation between food consumption and colorectal cancer: an ecological analysis in Japan. Asian Pac J Cancer Prev. 2002;3:77–83. [PubMed] [Google Scholar]
- 170.Yu R. Chen C. Mo YY. Hebbar V. Owuor ED. Tan TH. Kong AN. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J Biol Chem. 2000;275:39907–39913. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- 171.Yu R. Lei W. Mandlekar S. Weber MJ. Der CJ. Wu J. Kong AN. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- 172.Yu R. Mandlekar S. Lei W. Fahl WE. Tan TH. Kong AN. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J Biol Chem. 2000;275:2322–2327. doi: 10.1074/jbc.275.4.2322. [DOI] [PubMed] [Google Scholar]
- 173.Yu S. Khor TO. Cheung KL. Li W. Wu TY. Ying H. Foster BA. Kan YW. Kong AN. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One. 2010;5:e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu S. Kong AN. Targeting carcinogen metabolism by dietary cancer preventive compounds. Curr Cancer Drug Targets. 2007;7:416–424. doi: 10.2174/156800907781386669. [DOI] [PubMed] [Google Scholar]
- 175.Yuan X. Xu C. Pan Z. Keum YS. Kim JH. Shen G. Yu S. Oo KT. Ma J. Kong AN. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol Carcinog. 2006;45:841–850. doi: 10.1002/mc.20234. [DOI] [PubMed] [Google Scholar]
- 176.Zhang DD. Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang DD. Lo SC. Cross JV. Templeton DJ. Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang DD. Lo SC. Sun Z. Habib GM. Lieberman MW. Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem. 2005;280:30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- 179.Zhu M. Fahl WE. Functional characterization of transcription regulators that interact with the electrophile response element. Biochem Biophys Res Commun. 2001;289:212–219. doi: 10.1006/bbrc.2001.5944. [DOI] [PubMed] [Google Scholar]
- 180.Zipper LM. Mulcahy RT. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci. 2003;73:124–134. doi: 10.1093/toxsci/kfg083. [DOI] [PubMed] [Google Scholar]
- 181.Zipper LM. Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]
- 182.Zipper LM. Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]