Abstract
Background and Purpose
Calcification has been associated with carotid plaque stability; however, an acceptable in vivo method to define plaques based on this component remains to be developed. The purpose of our study was to compare calcified and noncalcified volumes of carotid artery culprit symptomatic plaques with asymptomatic plaques using multidetector computed tomography.
Methods
We identified 102 patients with ≥50% stenosis using NASCET criteria by multidetector computed tomography angiography between January 2004 and May 2006, which included 35 symptomatic (stenosis: 82.0±11.9%) and 67 asymptomatic patients (stenosis: 79.4±10.8%). Total plaque volume, noncalcified plaque volume, calcified plaque volume, and calcified/total ratio were measured for the 102 index plaques causing stenosis.
Results
In a model including age, stenosis, and cardiovascular risk factors, calcified plaque volume/total plaque volume was significantly inversely associated with the occurrence of symptoms (P=0.012; odds ratio, 0.047; 95% CI, 0.004 to 0.511). There was a notable overlap in the calcified plaque volume/total plaque volume ratio between 0% and 45% for symptomatic and asymptomatic plaques. However, calcification >45% of the total plaque was very specific: 97.1% (34/35) for absence of symptoms (sensitivity: 28.4% 19/67). No significant association between total plaque volume, noncalcified plaque volume, or calcified plaque volume and symptomatology was found.
Conclusion
The proportion of carotid plaque calcification, rather than absolute volume, is associated with stability in patients with stenosis. Specifically, for a subset of patients, plaque calcification >45% of the total volume may represent a clinically useful cutoff. The carotid plaque calcium ratio, determined by multidetector computed tomography volume measurements, may help noninvasively risk stratify patients with asymptomatic stenosis.
Keywords: atherosclerosis, carotid disease, tomography
Stroke is the third leading cause of death in the United States, leading to >160 000 deaths annually.1 Moreover, it is a major cause of functional impairment, leaving 15% to 30% of survivors permanently disabled, and it is also a significant cost to the system, estimated at $53.6 billion (indirect and direct) in 2004.1 Despite advances in therapy, prevention by treatment of risk factors remains the cornerstone in the management of stroke.
Per the American Heart Association/American Stroke Association guidelines, asymptomatic carotid stenosis is a well-documented, modifiable risk factor for ischemic stroke.2 With ≈5% to 10% of people older than 65 years having stenoses >50%, it is a relatively common problem.3–5 However, management remains controversial. Given the results of the Asymptomatic Carotid Atherosclerosis Study and Medical Research Council Asymptomatic Surgery Trial, prophylactic carotid endarterectomy (CEA) should be considered for stenoses between 60% and 99%, provided that the performing surgeon has a low periprocedural complication rate.6,7 However, given the relatively low annual stroke risk for asymptomatic stenoses between 50% and 99%, estimated between 1% and 2%,2 and an overall risk for stroke and death after CEA approaching 4% in many areas,8 risk stratification of patients with stenosis is critical before considering CEA or endovascular intervention, a procedure also carrying serious associated complication rates.9
Recently, noninvasive imaging of plaque characteristics has been proposed as a method of identifying low- and high-risk patients.10 Specifically, calcification of carotid and coronary plaques has been found to represent a marker of plaque stability in ex vivo and in vivo studies.11–15 However, both unstable and stable plaques often contain varying amounts of calcium, and an acceptable in vivo method to define plaques based on this component remains to be developed. Multidetector computed tomography (MDCT) is a promising method in the evaluation of atherosclerotic plaque, given its high spatial resolution and superior capability in assessing calcium, which has correlated well with histopathology in the carotid circulation.16,17
We hypothesized that asymptomatic plaques could be differentiated from symptomatic plaques using MDCT volume measurements of the content of calcified and noncalcified plaque. The purpose of our study was to compare calcified and noncalcified volumes of the carotid artery plaque resulting in significant stenosis in symptomatic and asymptomatic patients using MDCT.
Methods
Patient Selection
We searched the patient database at the University of Virginia Health System, an institution with Primary Stroke Center Certification from the Joint Commission on Accreditation of Healthcare Organizations, to identify all patients who underwent MDCT angiography of the carotid arteries between January 2004 and May 2006. Inclusion criteria were ≥50% extracranial carotid artery stenosis secondary to atherosclerosis disease on MDCT angiography. Patients were then classified as symptomatic, defined as ischemic neurological symptoms (stroke, transient ischemic attack, or amaurosis fugax) relevant to the index carotid lesion within 7 days of undergoing MDCT or as asymptomatic.
During the study period, 1076 patients underwent MDCT angiography of the carotid arteries; 873 patients were excluded secondary to lack of significant stenosis (≥50%). Seventy-two patients were excluded secondary to conflicting causes of neurological symptoms, eg, cardiac emboli or lacunar infarction. Twenty patients were excluded for previous CEA with restenosis and 9 for tandem lesions.
Stroke and transient ischemic attack were defined per previously published criteria.18 Amaurosis fugax was defined as acute onset of transient partial or complete monocular loss of vision. Symptoms were deemed relevant to the index lesion only after exclusion of other sources.19 Patients classified as asymptomatic had no history of symptoms, neither remote or at the time of examination, as determined with a detailed neurological examination by an experienced vascular surgeon. Clinical information regarding both groups, including the presence of coronary artery disease (angina, myocardial infarction, or coronary revascularization), diabetes mellitus, hypertension, remote or present history of smoking, and dyslipidemia, was recorded.
Institutional review board approval was obtained for this study; informed patient consent was not required. This study was compliant with the Health Insurance Portability and Accountability Act.
Scan Parameters
All patients underwent MDCT angiography using a 16-slice scanner (LightSpeed 16; GE Healthcare). Images were obtained from the aortic arch to the supraventricular white matter using helical acquisition with 6.25 mm/rotation and 1.25 × 1.25 mm collimation (120 kVp, 350 mA). A standard reconstruction kernel/algorithm was used. A total of 100 to 125 mL Omnipaque 300 was injected at a rate of 4.0 to 4.5 mL/s, with a delay determined by an automated bolus timing program. The image data were transferred to a computer workstation (Kodak PACS version 5.2.1; Kodak) for postprocessing.
Stenosis and Plaque Volume Determination
The degree of luminal stenosis was measured by a single experienced neuroradiologist, blinded to clinical data, based on analysis of axial slices, multiplanar reconstructions, maximum intensity projections, and 3-dimensional volume rendering reconstruction for optimal assessment, using North American Symptomatic Carotid Endarterectomy Trial Collaborators criteria.20
To determine total plaque volume (TV), calcified plaque volume (CV), noncalcified plaque volume (NCV), and calcified volume/total volume (CV/TV), contiguous 1.25-mm-thick cross-sectional images of the index carotid lesion causing stenosis were rendered. Wide fixed window settings were mainly used (700-HU window, 250-HU level), with several cases requiring individual changes in the level and windows for optimal visualization (range, 600 to 1000 HU window, 150 to 400 HU level) based on previous studies.21,22 For the length of plaque on the multiplanar reconstructed images, the lumen and the outer vessel wall were manually traced with a region of interest tool to determine the areas of each. Plaque area was calculated by subtracting the lumen area from the outer vessel wall cross-sectional area for each slice, and using Simpson's rule for additive method of volume determination, the sum of the plaque areas was multiplied by the slice increment to determine the TV of the whole plaque in mm3 (Figure 1).21 CV was determined by manually tracing the calcified plaque on each slice for the length of the plaque, with the sum of the areas of calcium multiplied by the slice increment to determine volume.22 NCV was calculated by subtracting CV from TV. Measurements were performed by a single blinded investigator, and reproducibility assessed by measurements in 50 consecutive patients by a second blinded investigator.
Figure 1.
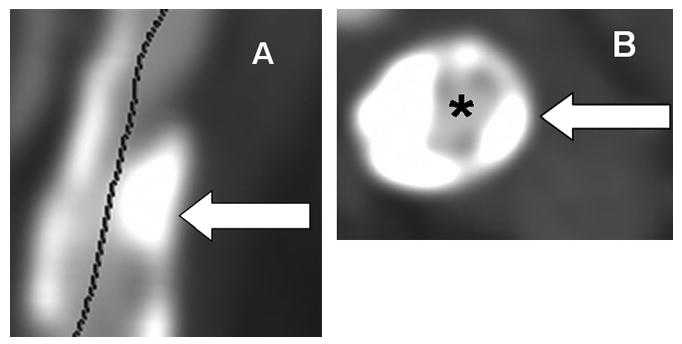
Patient with large calcified plaque involving internal carotid artery. A, Multiplanar reconstructed MDCT image showing large degree of calcified plaque (arrow) with narrowed lumen (line). B, Mulitplanar reconstructed MDCT images used for volume measurements demonstrating cross-section of internal carotid artery with corresponding lumen (asterisk) and calcified plaque (arrow).
Statistical Analysis
Simple logistic regression analysis was used to examine the relationship between the independent variables, TV, CV, NCV, and CV/TV, age, sex, percent stenosis, diabetes, hypertension, smoking, dyslipidemia, and the dichotomous dependent variable of symptomatology. In a model including cardiovascular risk factors, age, sex, and percent stenosis, backward stepwise logistic regression analysis (performed individually for each of the plaque characteristics) was then performed to select predictive variables for symptomatology by means of a log likelihood ratio test with an inclusion level of 0.05 and an exclusion level of 0.10. Receiver-operator curve analysis was used to determine a CV/TV threshold value that best discriminated patients without ischemic symptoms from those with symptoms. Interobserver agreement was assessed by the concordance correlation coefficient23 and visually with Bland-Altman plots.24 Unlike Pearson's correlation or R2 values, the concordance correlation coefficient is a function of both accuracy and precision. Medcalc software (version 8.2) and the SAS system (SAS Institute) were used for statistical analyses. P< 0.05 was regarded to indicate statistical significance.
Results
Patient Population
A total of 102 patients were included in the study, with 67 asymptomatic patients and 35 symptomatic patients (17 stroke, 13 transient ischemic attack, 5 amaurosis fugax). Patient demographics are summarized in Table 1. Hypertension was significantly greater in the symptomatic than asymptomatic patients, and age significantly greater in the asymptomatic patients. Referral for MDCT for all symptomatic patients was for primary evaluation of neurological symptoms; for the asymptomatic patients, 62 were referred for evaluation of stenosis after ultrasound as an alternative to digital subtraction angiography (referral for ultrasound: 45 physical examination findings [bruit], 10 precardiac or general surgery clearance, 7 routine screening), and 5 discovered during precoronary artery bypass grafting work-up for co-morbidities using MDCT.
TABLE 1. Clinical Characteristics of Study Population.
| Asymptomatic Patients, N (%) | Symptomatic Patients, N (%) | P | |
|---|---|---|---|
| Age, y | 70.9±10.0 | 65.3±13.3 | 0.02* |
| Female | 28 (42) | 12 (34) | ns |
| % Stenosis | 79.4±10.8 | 82.0±11.9 | ns |
| Hypertension | 43 (66) | 32 (91) | 0.01* |
| Diabetes | 16 (25) | 12 (34) | ns |
| Smoking | 32 (49) | 24 (69) | ns |
| Dyslipidemia | 40 (62) | 23 (66) | ns |
| Coronary artery disease | 32 (49) | 11 (31) | ns |
P<0.05.
ns indicates no significance.
Plaque Volume Measurements
The 102 plaques causing stenosis in the 102 patients were analyzed. No exclusions were deemed necessary based on image quality by either observer. The means and standard deviations for TV, CV, NCV, and CV/TV are shown in Table 2.
TABLE 2. Summary of Quantitative Plaque Characteristics.
| Asymptomatic Patients | Symptomatic Patients | |
|---|---|---|
| Total plaque, mm3 | 449±398 | 586±520 |
| Noncalcified plaque, mm3 | 334±333 | 510±486 |
| Calcified plaque, mm3 | 114±118 | 76±69 |
| Calcified/total plaque ratio | 0.31±0.26 | 0.17±0.16 |
The results of simple logistic regression analysis are described in Table 3. On simple logistic regression, there was a statistically significant negative association between CV/TV and age with symptomatology, and a statistically significant positive association with hypertension. Other variables did not demonstrate a significant association with the presence of symptoms.
TABLE 3. Simple Logistic Regression Analysis Between Clinical Characteristics and Plaque Components With Symptoms.
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Age | 0.96 | 0.92–0.99 | 0.02* |
| Sex | 1.46 | 0.63–3.41 | ns |
| % Stenosis | 1.02 | 0.98–1.02 | ns |
| Hypertension | 5.58 | 1.54–20.2 | 0.009* |
| Diabetes | 1.46 | 0.58–3.62 | ns |
| Smoking | 2.25 | 0.95–5.30 | ns |
| Dyslipidemia | 1.22 | 0.52–2.85 | ns |
| Total plaque | 1.00 | 0.99–1.01 | ns |
| Noncalcifed plaque | 1.00 | 0.99–1.01 | ns |
| Calcified plaque | 0.99 | 0.99–1.00 | ns |
| Calcified/total | 0.05 | 0.01–0.48 | 0.01* |
P<0.05.
ns indicates no significance.
With backward stepwise logistic regression using CV/TV, cardiovascular risk factors, age, sex, and percent stenosis as independent variables, CV/TV demonstrated a significant negative association with symptoms (odds ratio, 0.04; 95% CI, 0.004 to 0.51; P=0.01), and hypertension showed a significant positive association (odds ratio, 6.4; 95% CI, 1.7 to 24.7; P=0.006). Other variables did not contribute significantly to the presence of symptoms.
No significant association between TV, NCV, or CV and symptoms was found on simple or stepwise logistic regression.
Threshold Determination
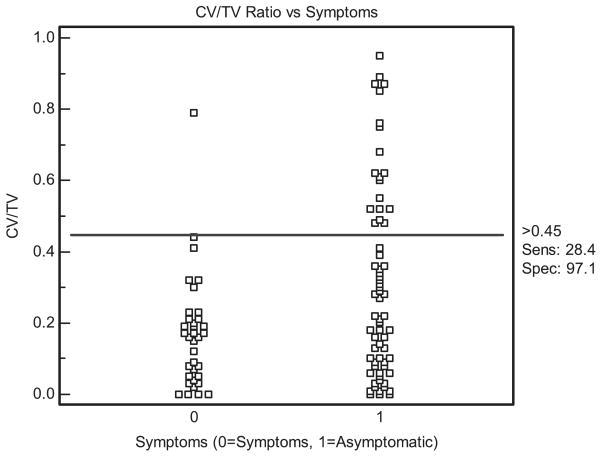
A plot of CV/TV against symptoms is shown in Figure 2. There is a large overlap in the CV/TV for symptomatic and asymptomatic plaques between 0% and 45%. However, with plaques with >45% calcification of the total plaque, which constitute 19.6% (20/102) of the total plaques and 28.4% (19/67) of the asymptomatic plaques, there is a high specificity of 97.1% (34/35) with a concomitant limited sensitivity of 28.4% (19/67) for absence of symptoms.
Figure 2.
Dot diagram demonstrating distribution of CV/TV ratio in symptomatic and asymptomatic patients. A ratio >45% is seen in 19 asymptomatic patients and only 1 symptomatic patient, leading to high specificity but limited sensitivity.
Interobserver Agreement
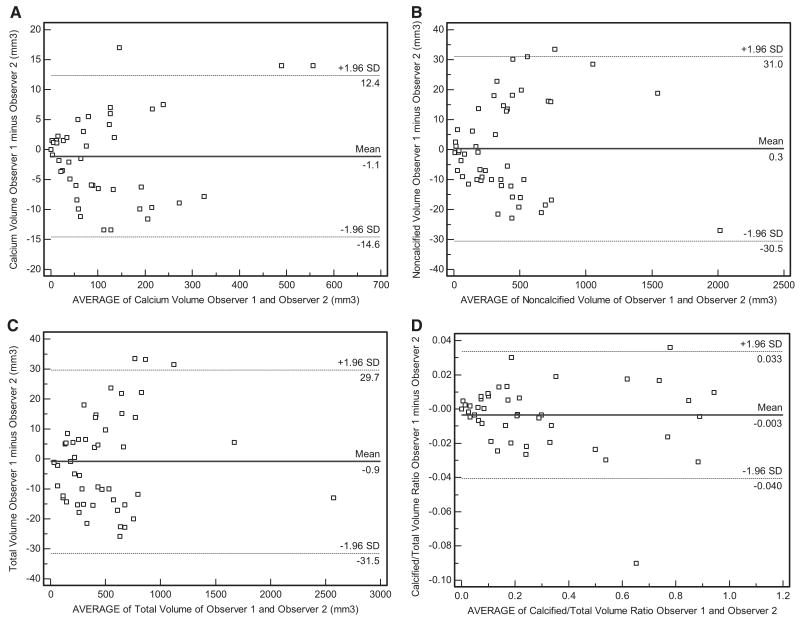
The estimated concordance correlation coefficients, representing agreement between observers, are 0.998, 0.999, 0.999, and 0.998 for CV, NCV, TV, and CV/TV, respectively. These estimates are close to 1 (best possible value) and indicate excellent agreement. Bland-Altman plots also demonstrate good interobserver agreement for CV, NCV, TV, and CV/TV in 50 consecutive patients. For calcified plaque, mean interobserver difference was 1.1 mm3 and 95% limits of agreement were ± 13.5 mm3 (Figure 3a). For noncalcified plaque, mean interobserver difference was 0.3 mm3 and 95% limits of agreement were ± 30.7 mm3 (Figure 3b). For total plaque, mean interobserver difference was 0.9 mm3 and 95% limits of agreement were ± 30.6 mm3 (Figure 3c). For CV/TV, mean interobserver difference was 0.003 and 95% limits of agreement were 0.037 (Figure 3d).
Figure 3.
A, Bland-Altman plot demonstrating good agreement between observer 1 and observer 2 for calcified plaque volumes, with mean interobserver difference and 95% limits of agreement demonstrated. B, Bland-Altman plot demonstrating good agreement between observer 1 and observer 2 for noncalcified plaque volumes, with mean interobserver difference and 95% limits of agreement demonstrated. C, Bland-Altman plot demonstrating good agreement between observer 1 and observer 2 for total plaque volumes, with mean interobserver difference and 95% limits of agreement demonstrated. D, Bland-Altman plot demonstrating good agreement between observer 1 and observer 2 for the calcified/total plaque volume ratios with mean interobserver difference and 95% limits of agreement demonstrated.
Discussion
In the present study, we used MDCT to compare noncalcified and calcified plaque volumes of plaques causing carotid artery stenoses in symptomatic and asymptomatic patients; we found an inverse relationship between the proportion of calcium in a plaque and symptoms, even with a model including age, sex, percent stenosis, and cardiovascular risk factors. Of clinical significance, there is a great deal of overlap between stable and unstable plaques between 0% and 45% calcification of the total plaque volume, but in a distinct subset of patients, heavily calcified plaques (>45% calcified) demonstrate a strong predilection toward stability, as defined by lack of associated symptoms. Moreover, there was no significant relationship between the absolute volumes of total plaque, calcified plaque, and noncalcified plaque with symptoms, suggesting the ratio of contents may play a more important role than the amount of plaque contents.
The relationship between calcium and in vivo carotid plaque stability has been previously studied by our group.11 In a study population of 31 patients with 36 plaques, using attenuation measurements in small regions of interest with MDCT, calcified plaques were less likely to be associated with symptoms than noncalcified plaques. However, the use of attenuation measurements, Hounsfield units, to characterize plaques can be markedly limited by volume averaging and by the selection of regions of interest. In addition, this method does not quantify the proportion or amount of plaque contents but rather just reflects the density of the plaque, which is troublesome given the heterogeneity of many plaques. The use of MDCT volume measurements allows for the quantitative determination of the proportion of calcified plaque relative to the total plaque, which appears to be more significant than absolute volume of calcium. This methodology also demonstrates good interobserver agreement for both calcified and noncalcified plaque volumes.
The stabilizing role of calcium has been demonstrated in ex vivo carotid artery studies and within the coronary arteries. Hunt et al collected endarterectomy specimens from 142 patients with stenosis, and found that patients with the presence of calcium within plaques had fewer symptoms of transient ischemic attack and stroke.12 Importantly, they also specifically found large calcific granules (heavily calcified plaques) to be less associated with symptoms. Shaalan et al studied 48 CEA specimens using computed tomography and found asymptomatic plaques to be more calcified than symptomatic plaques.13 In the coronary circulation, plaques in patients with stable angina were found to be more calcified than those in patients with unstable angina or myocardial infarction using intravascular ultrasound.15
The mechanism by which calcium confers stability to plaques has both a mechanical and functional basis. Using large-strain finite element analysis on postmortem coronary arteries, Huang et al found that calcification does not significantly impact the biomechanical stress on the fibrous cap, unlike lipid pools which increase the stress.14 They also found that large amounts of calcification, via bearing mechanical load, may confer plaque stability. The overlap in calcium ratios of stable and unstable plaques with lower degrees of calcium and stability of heavily calcified plaques also has an explanation in the differences in failure stresses of the different plaques.25 Failure stress, leading to rupture, often occurs at interfaces between materials of different stiffness. Given the increased stiffness of calcium relative to noncalcified plaque, as the calcified content increases up to a point, there are increased interfaces between the plaque contents leading to possible increased risk of rupture. However, at a point where plaques become heavily calcified, there is coalescence of the plaque, which decreases the interface area between the stiffer and more distensible plaque, which may lead to decreased risk of rupture. Functionally, there is an inverse relationship between the degree of carotid plaque calcification and fibrous cap inflammation, as determined by macrophage infiltration in CEA specimens, suggesting the association of stability with calcium.13
The implications of the current study have potential applications to the management of asymptomatic carotid stenosis. Given the serious risks of surgical and endovascular intervention, a noninvasive method of determining patients who are at decreased risk and may be amenable to medical therapy is critical. The degree of calcification within the plaque causing stenosis may play an important marker for patients who could potentially be treated less aggressively. In our series, calcification of >45% of the plaque had a strong association with absence of symptoms. Although this threshold is limited by a low sensitivity of ≈30%, given the absolute high number of patients with asymptomatic stenosis, there is prospective clinical pertinence to the utilization of the plaque content ratio. If this threshold is validated in larger, longitudinal series, there could be potential avoidance of the morbidity and mortality associated with CEA/endovascular intervention for many patients and possibly savings to cost to the health system. In addition, if the results of the study could be reproduced in the coronary vasculature, there is potential for the risk stratification of patients with coronary artery disease as well, especially relevant given the likely increase in discovery of many asymptomatic stenoses on MDCT, as it becomes increasingly used.
There are several limitations to our study. First, MDCT angiography was used to determine the percent stenosis, not catheter-based angiography, which is considered the gold standard. Although a recent meta-analysis found computed tomography to be accurate for the detection of disease from 70% to 99%, the accuracy for moderate stenosis, 50% to 69%, is less established.26 However, the Brain Attack Coalition has recently assigned computed tomographic angiography a grade 1A recommendation for evaluating the vasculature of the neck based on evidence-based medicine assessment criteria.27 In addition, our study used 16-slice MDCT, which likely has greater accuracy in quantifying disease, even in the presence of heavy calcification, than those included in the meta-analysis, which were mainly performed with single-slice computed tomography.28,29 Another limitation is the exclusion of silent ischemia in the asymptomatic patients. Although each of the patients underwent a detailed neurological examination, they did not undergo imaging given their asymptomatic state, making exclusion of silent infarction difficult. In addition, the lack of inclusion of patients with lacunar infarction and carotid stenosis, who also may benefit from CEA, represents a shortcoming of the study.20
Another potential limitation is the degree of individual variability in the adjustment of window settings and its possible effects on volume measurements, which was not assessed in the present study. Further research in this area is necessary to identify the optimal values. The presence of contrast obscuring the detection of calcium could also present a limitation of our study, given that a noncontrast examination was not performed, as is blooming from calcium on MDCT leading to an overestimation of calcified plaque volumes. However, MDCT angiography has been found to be excellent in the detection and quantification of calcium despite the presence of contrast and the potential effects of blooming.21,30,31 Finally, a limitation to the use of MDCT angiography in the evaluation of carotid plaque morphology is the amount of radiation exposure, estimated at 3.1 mSv per scan, and the utilization of potentially nephrotoxic contrast agent.
In conclusion, using MDCT volume measurements of calcified and noncalcified plaque volumes, the proportion of plaque calcification can be determined noninvasively, which appears to represent a marker of stability. This finding may have important implications for the risk stratification of asymptomatic carotid disease.
Footnotes
Disclosures: C.M.K. is on the speaker's bureau for GE Healthcare. No other authors report conflicts.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics—2004 Update. Dallas Tex: American Heart Association; 2003. [Google Scholar]
- 2.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL, American Heart Association. Amercican Stroke Association Stroke Council Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 3.O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, Bommer W, Price TR, Gardin JM, Savage PJ. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 4.Fine-Edelstein JS, Wolf PA, O'Leary DH, Poehlman H, Belanger AJ, Kase CS, D'Agostino RB. Precursors of extracranial carotid atherosclerosis in the Framingham Study. Neurology. 1994;44:1046–1050. doi: 10.1212/wnl.44.6.1046. [DOI] [PubMed] [Google Scholar]
- 5.Hillen T, Nieczaj R, Munzberg H, Schaub R, Borchelt M, Steinhagen-Thiessen E. Carotid atherosclerosis, vascular risk profile and mortality in a population-based sample of functionally healthy elderly subjects: the Berlin ageing study. J Intern Med. 2000;247:679–688. doi: 10.1046/j.1365-2796.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 6.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 7.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D, MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 8.Kresowik TF, Bratzler DW, Kresowik RA, Hendel ME, Grund SL, Brown KR, Nilasena DS. Multistate improvement in process and outcomes of carotid endarterectomy. J Vasc Surg. 2004;39:372–380. doi: 10.1016/j.jvs.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 10.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 11.Nandalur KR, Baskurt E, Hagspiel KD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR Am J Roentgenol. 2005;184:295–298. doi: 10.2214/ajr.184.1.01840295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B, Cusack A, Mohler ER., 3rd Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 13.Shaalan WE, Cheng H, Gewertz B, McKinsey JF, Schwartz LB, Katz D, Cao D, Desai T, Glagov S, Bassiouny HS. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J Vasc Surg. 2004;40:262–269. doi: 10.1016/j.jvs.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol. 2001;21:1618–1622. doi: 10.1161/hq0901.095554. [DOI] [PubMed] [Google Scholar]
- 16.de Weert TT, Ouhlous M, Meijering E, Zondervan PE, Hendriks JM, van Sambeek MR, Dippel DW, van der Lugt A. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol. 2006;26:2366–2372. doi: 10.1161/01.ATV.0000240518.90124.57. [DOI] [PubMed] [Google Scholar]
- 17.de Weert TT, Ouhlous M, Zondervan PE, Hendriks JM, Dippel DW, van Sambeek MR, van der Lugt A. In vitro characterization of atherosclerotic carotid plaque with multidetector computed tomography and histopathological correlation. Eur Radiol. 2005;15:1906–1914. doi: 10.1007/s00330-005-2712-2. [DOI] [PubMed] [Google Scholar]
- 18.Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, Sherman DG, TIA Working Group Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347:1713–1716. doi: 10.1056/NEJMsb020987. [DOI] [PubMed] [Google Scholar]
- 19.Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, Barnett HJ. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North Am Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000;342:1693–1700. doi: 10.1056/NEJM200006083422302. [DOI] [PubMed] [Google Scholar]
- 20.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, Pohle K, Baum U, Anders K, Jang IK, Daniel WG, Brady TJ. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 22.Nandalur KR, Baskurt E, Hagspiel KD, Finch M, Phillips CD, Bollampally SR, Kramer CM. Carotid artery calcification on CT may independently predict stroke risk. AJR Am J Roentgenol. 2006;186:547–552. doi: 10.2214/AJR.04.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Li. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 25.Abedin M, Tintut Y, Demer LL. Vascular calcification mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 26.Wardlaw JM, Chappell FM, Best JJ, Wartolowska K, Berry E, NHS Research and Development Health Technology Assessment Carotid Stenosis Imaging Group Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet. 20066;367:1503–1512. doi: 10.1016/S0140-6736(06)68650-9. [DOI] [PubMed] [Google Scholar]
- 27.Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, Koroshetz W, Marler JR, Booss J, Zorowitz RD, Croft JB, Magnis E, Mulligan D, Jagoda A, O'Connor R, Cawley CM, Connors JJ, Rose-DeRenzy JA, Emr M, Warren M, Walker MD. Brain Attack Coalition. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]
- 28.Nonent M, Serfaty JM, Nighoghossian N, Rouhart F, Derex L, Rotaru C, Chirossel P, Guias B, Heautot JF, Gouny P, Langella B, Buthion V, Jars I, Pachai C, Veyret C, Gauvrit JY, Lamure M, Douek PC, CARMEDAS Study Group Concordance rate differences of 3 noninvasive imaging techniques to measure carotid stenosis in clinical routine practice: results of the CARMEDAS multicenter study. Stroke. 2004;35:682–686. doi: 10.1161/01.STR.0000117251.65222.DA. [DOI] [PubMed] [Google Scholar]
- 29.Bartlett ES, Symons SP, Fox AJ. Correlation of carotid stenosis diameter and cross-sectional areas with CT angiography. AJNR Am J Neuroradiol. 2006;27:638–642. [PMC free article] [PubMed] [Google Scholar]
- 30.Hong C, Becker CR, Schoepf UJ, Ohnesorge B, Bruening R, Reiser MF. Coronary artery calcium: absolute quantification in nonenhanced and contrast-enhanced multi-detector row CT studies. Radiology. 2002;223:474–480. doi: 10.1148/radiol.2232010919. [DOI] [PubMed] [Google Scholar]
- 31.Miralles M, Merino J, Busto M, Perich X, Barranco C, Vidal-Barraquer F. Quantification and Characterization of Carotid Calcium with Multi-detector CT-angiography. Eur J Vasc Endovasc Surg. 2006;32:561–567. doi: 10.1016/j.ejvs.2006.02.019. [DOI] [PubMed] [Google Scholar]