Abstract
Presynaptic inhibition is a widespread mechanism for regulating transmitter release in the CNS. Presynaptic inhibitors act as a high-pass filter, but the functional consequence of this filtering during the synaptic processing of behaviorally relevant activity remains unknown. Here we use analytical approaches to examine the effects of presynaptic inhibition on synaptic output in response to activity patterns from CA3 pyramidal cells during the performance of a complex behavioral task. We calculate that presynaptic inhibition enhances the contrast between background activity and responses to environmental cues and that neuronal responses to location are subject to stronger contrast enhancement than neuronal responses to olfactory information. Our analysis suggests that presynaptic inhibition also enhances the importance of integrative inputs that respond to many behavioral cues during the task at the expense of specific inputs that respond to only a few of these cues.
INTRODUCTION
Presynaptic inhibition is a form of activity-dependent neuromodulation, which has a larger effect on postsynaptic potentials (PSPs) preceded by periods of inactivity (Burke and Hablitz 1994; Dunwiddie and Haas 1985; Haas et al. 1987; Mallart and Martin 1968; Manabe et al. 1993). Although much is known about the mechanisms underlying presynaptic inhibition (Blackmer et al. 2001; Brody and Yue 2000; Elmslie et al. 1990; Luscher et al. 1997; Sakaba and Neher 2003; Scanziani et al. 1992; Wu and Saggau 1995), and the effects of presynaptic inhibition during spike trains (Brenowitz et al. 1998; Kreitzer and Regehr 2000; Ohliger-Frerking et al. 2003; Tsodyks and Markram 1997; Varela et al. 1997), little is known about the effects of presynaptic inhibition on behaviorally relevant neural computations.
Because presynaptic inhibitors act as high-pass filters, one suggested role for these inhibitors is that they improve contrast during behaviorally relevant changes in afferent activity. Although this idea seems plausible in general terms, it has not been subjected to a quantitative test to determine whether the filter has the appropriate characteristics to fulfill this role during patterns of afferent activity that encode information about relevant behaviors. We have recently defined the filter generated by presynaptic inhibition during complex patterns of activity (Ohliger-Frerking et al. 2003), and the patterns of activity that encode behaviorally relevant cues during the performance of a complex cognitive task (Frerking et al. 2005). This allows us to perform, for the first time to our knowledge, a rigorous analytical test of the hypothesis that presynaptic inhibition can alter synaptic signal processing during afferent activity generated by behaviorally relevant cues.
During behavioral tasks in vivo, CA3 pyramidal cells fire in complex patterns with interspike intervals (ISIs) ranging over several orders of magnitude. We have characterized the filtering imposed by GABAB receptor (GABABR) activation with the exogenous agonist baclofen during these complex patterns at the Schaffer collateral synapse between CA3 pyramidal cells and CA1 pyramidal cells (Ohliger-Frerking et al. 2003). Briefly, this filter is well-described by a monotonic relationship between the inhibition for a given excitatory PSP (EPSP) and the ISI between the spike generating that EPSP and the one preceding it (Fig. 1A) (data from Ohliger-Frerking et al. 2003). This relationship is a sufficiently complete description of the filter to accurately predict the inhibitory effect of GABABRs on individual PSPs during novel complex spike trains (Ohliger-Frerking et al. 2003).
FIG. 1.
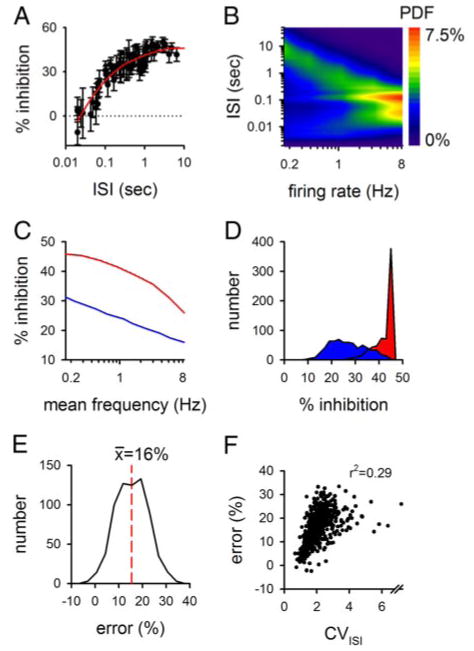
The calculated level of presynaptic inhibition is affected by temporal complexity in the afferent input train. A: filter imposed by 3 μM baclofen is shown. Data (symbols) (from Ohliger-Frerking et al. 2003) were smoothed to generate the filtering function (red line). The inhibition expected for interspike intervals (ISIs) <20 ms was set to that seen at 20 ms. At very short ISIs, presynaptic inhibition might lead to a facilitation (Brenowitz et al. 1998); however, because very short ISIs are largely independent of both the mean firing rate and cellular responses to environmental cues in these cells (Frerking et al. 2005), error during these ISIs will have little effect on our conclusions. B: model for realistic activity of CA3 pyramidal cells is shown (from Frerking et al. 2005). The relative frequency of each ISI (the probability density function or PDF) is shown in pseudocolor. C: average level of inhibition caused by 3 μM baclofen was calculated as a function of mean afferent firing rate based on the filtering characteristics in A and either constant-frequency firing (red) or the model of afferent activity in B (blue). D: average level of inhibition caused by 3 μM baclofen was calculated for 683 CA3 pyramidal cells, using either the mean firing rate (red) or the ISI distribution (blue). E: error generated in predicted inhibition by using only the mean firing rate was assessed for the cells in D by subtracting the average level of inhibition calculated using the mean firing rate from that calculated using the ISI distribution. F: error calculated in E is shown as a function of the CVISI, used here as an assay of temporal complexity. Note that the x axis in F contains a break to allow display of a single exceptional cell with a CVISI of 10.
Afferent activity patterns were generated in two ways: either using a model describing CA3 pyramidal cell firing in vivo to generate realistic trains (Fig. 1B; patterns used in C and D) (model from (Frerking et al. 2005)), or by taking patterns of activity directly from single-unit recordings of CA3 pyramidal cells in awake, behaving rats during the performance of an olfactory delayed nonmatch to sample (DNMS) task (for Figs. 2 and 3) (see Frerking et al. 2005; Wiebe and Staubli 1999 for details; single-unit recordings in Wiebe and Staubli 1999 were made in the absence of baclofen).
FIG. 2.
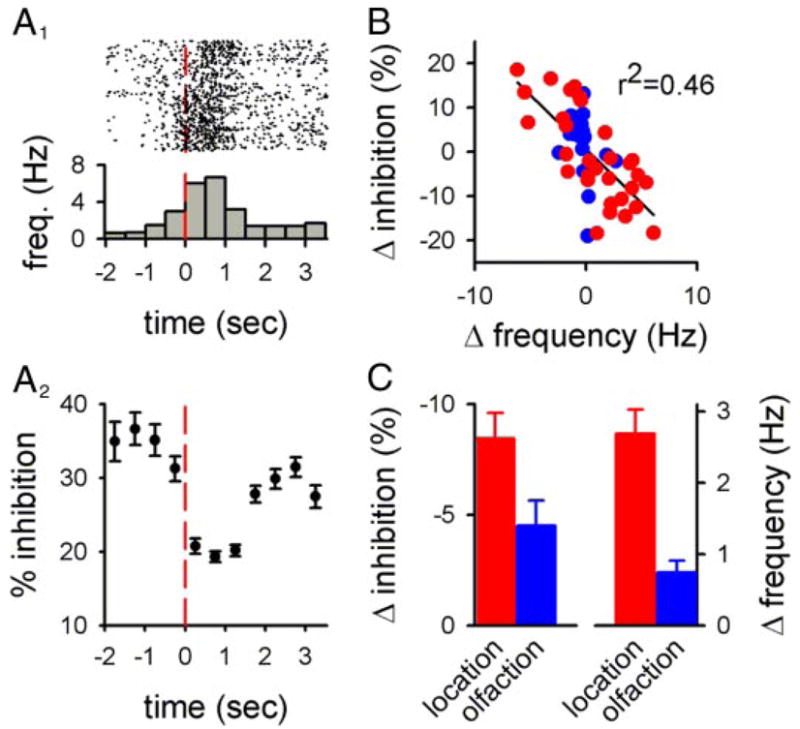
Presynaptic inhibition is expected to enhance contrast between behaviorally relevant afferent activity and background firing. A: inhibition induced by 3 μM baclofen was calculated as a function of time during the response of a cell to leaving the sample arm (at time = 0) during the delayed nonmatch to sample (DNMS) task. Raster plots for 100 trials are shown (A1, top) as well as the average response to the cue (A1, bottom) and the presynaptic inhibition that was calculated using the ISIs for the spikes in each time bin and the filtering function in Fig. 1A (A2). The contrast between background firing and the neural response in this experiment was calculated as the difference in the appropriate parameter between the 1st 2 time bins and the 2 time bins at the peak of the neural response. B: similar analysis to A was repeated in 53 cells for afferent responses to different cues that were associated with the location of the rat in the maze (red symbols) or with olfactory cues during the task (blue symbols). C: olfactory cues were subject to less inhibition-mediated contrast enhancement than were location-based cues. Both the change in frequency and the change in inhibition were expressed on an absolute scale in C to include responses that were a decrease in frequency.
FIG. 3.
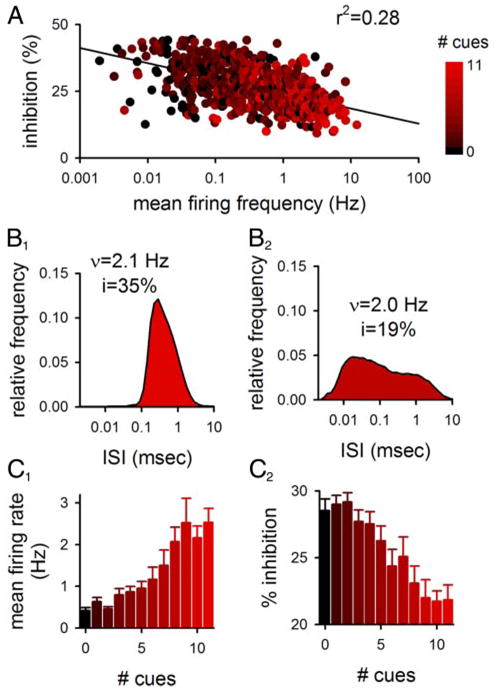
Integrative cells that respond to several cues are subject to less presynaptic inhibition than specific cells that respond to only a few cues. A: for 683 single-unit recordings, the ISI distribution was used to calculate the expected overall level of presynaptic inhibition shown as a function of each cell’s mean firing rate. Color denotes the number of cues to which the cell responded. B: ISI distributions of 2 cells are shown with similar mean firing rates but different overall levels of calculated presynaptic inhibition. Colors correspond to the scale in A. C: data in A were regrouped according to cue number to show that cells that responded to multiple cues had a higher firing rate (C1) and were calculated to be less subject to presynaptic inhibition (C2) than cells that responded to few or no cues (n = 24–129 for different numbers of cues).
To relate presynaptic inhibition to relevant behavioral cues, the filter imposed by presynaptic inhibition was applied to each spike during the activity patterns during ≤500 repeated trials of the task; the number of spikes varied as a function of each cell’s firing rate but was between 100 and 100,000. The level of presynaptic inhibition during a time bin was determined by using the filter to calculate the level of inhibition for each spike during the time bin and then averaging those levels of inhibition together. For calculations of contrast between different time periods (e.g., before and during a response to a behavioral cue), average levels of inhibition and mean firing rates were calculated for each time period, and the difference between these parameters for each period was determined. Time bins used for these calculations ranged in size from 100 ms to 2 s, depending on the duration of the neural response to the behavioral cue under study, and the number of spikes that were included in each bin. In this regard, the width of the time bins were chosen so that ≥10 spikes from the total number of behavioral trials were included in each bin, and were chosen as regions in which the mean frequency of firing was stable (see example in Fig. 2A).
To assess whether the effects of presynaptic inhibition depend on the temporal structure of the input train, we first calculated the average level of presynaptic inhibition during modeled patterns of realistic activity. For these activity patterns, the mean level of inhibition was calculated to be roughly linear as a function of firing frequencies and was less than the maximal level of inhibition imposed by the filter at long ISIs (Fig. 1C, blue line). This contrasts with the nonlinear dependence of inhibition on mean frequency for constant-frequency input patterns (Fig. 1C, red line).
To test this idea further with spike trains taken directly from single-unit recording, we compared the mean level of presynaptic inhibition imposed by the filter for different CA3 pyramidal cells by calculating based on the mean frequency of firing (Fig. 1D; red curve) or based on the ISI distribution of the cell (Fig. 1D; blue curve). Using only the mean frequency, the inhibition is calculated to be stronger and less variable than the inhibition calculated by accounting for temporal complexity of afferent activity. The calculated error caused by omitting temporal complexity from consideration was an overestimation of the degree of inhibition for >97% of single-unit recordings examined (Fig. 1E). The predicted error caused by failing to account for temporal complexity increased with the coefficient of variation of the ISI distribution for each cell (CVISI), which was used here as a quantitative assay for temporal complexity (r2 = 0.30; Fig. 1F). These results indicate that the temporal structure of afferent activity during complex spike trains can affect presynaptic inhibition in ways that cannot be predicted using constant frequency trains with the same mean firing rate even if the filtering function relating presynaptic inhibition to activity is identical for constant frequency trains and for complex spike trains.
We next examined the effects of presynaptic inhibition on synaptic output during activity patterns that encode relevant behavioral information. Activity patterns used in this analysis were firing patterns that changed as a function of specific behavioral cues during the DNMS task taken directly from single-unit recordings. As an example, we analyzed the effects of presynaptic inhibition on activity seen in a CA3 pyramidal cell as the rat left the sample arm of the Y-maze (Fig. 2A: red line at time = 0 indicates when the rat left the sample arm). In this cell, the behavioral event was associated with a large, transient increase in firing (Fig. 2A1). The level of presynaptic inhibition imposed by the filter in Fig. 1A was calculated for each spike during each trial and binned as a function of time. The level of inhibition at the peak of the response was significantly less than the level of inhibition prior to the response (Fig. 2A2; P < 0.00001). This difference in the calculated inhibition represents a predicted enhancement of the contrast between the response to the event and the background activity preceding it.
We repeated this analysis for 53 pyramidal cells that responded during different cues during the task that were related either to location (when the rat passed specified positions in the maze) or to olfaction (when the rat was exposed to an odor or when the odor was removed). The difference in calculated inhibition between the peak of the response and background activity was inversely related to the difference in firing frequency between the peak of the response and background activity (Fig. 2B; P < 10−7), indicating that the strength of the contrast enhancement caused by presynaptic inhibition is expected to depend on the strength of the neural response to the cue.
We next separated this data according to the behavioral cue to which the cell responded to examine whether the contrast enhancement induced by presynaptic inhibition would be different for different cues. The calculated contrast enhancement induced by presynaptic inhibition was stronger for responses to location (Fig. 2C, red; n = 31) than for responses to olfaction (Fig. 2C, blue; n = 22; P < 0.02). This could be explained by the relationship between contrast enhancement and response magnitude shown in Fig. 2B as the responses to location were, on average, larger in magnitude than the responses to olfaction (Fig. 2C, P < 0.01). These results indicate that the contrast enhancement generated by presynaptic inhibition will more effectively enhance positional signals than olfactory signals, at least during the DNMS task.
We next tested the hypothesis that presynaptic inhibition would enhance contrast between active and inactive cells. We calculated the overall level of presynaptic inhibition for different CA3 pyramidal cells based on the ISI distributions of single-unit recordings. The mean firing rate was correlated with the calculated level of presynaptic inhibition expected for individual afferent fibers (Fig. 3A; n = 683; P < 10−10). However, cells with similar mean firing rates could have different ISI distributions and therefore different levels of inhibition (Fig. 3B).
The observation that cells that fire at a higher overall rate are subject to less presynaptic inhibition may have functional significance if the overall firing rate of the cell is correlated with the cell’s function. The overall firing rate of a cell was correlated with the number of behavioral cues to which that cell responds (Fig. 3C1), so that integrative cells that responded to several cues had a higher firing rate than specific cells that responded to few cues (r2 = 0.19; P < 0.001). This correlation could have a trivial explanation if responses were only due to increased firing; however, the correlation persisted even when the firing frequency was examined as a function of the number of cues that decreased the firing rate (r2 = 0.11; P < 0.001). When cells were separated according to the number of cues to which they responded, the calculated level of presynaptic inhibition decreased as cells went from specific to integrative (Fig. 3, A and C2; P < 0.001), indicating that presynaptic inhibition will favor the transmission of integrative signals.
In this study, we have used analytical approaches to calculate the functional effects of presynaptic inhibitors on behaviorally relevant signals. It has been suggested that presynaptic inhibition might enhance the contrast between afferent responses to behavioral cues and background firing, but the feasibility of this suggestion has not been quantitatively evaluated. Our analysis indicates that this role is not only plausible but expected based on the filter imposed by presynaptic inhibition and the patterns of activity that encode behavioral cues. Notably, presynaptic inhibition is expected to enhance contrast during the DMNS task for afferent responses to location more than it will for afferent responses to odor. Thus these results suggest that activity-dependent synaptic mechanisms can distinguish between different behavioral cues.
Surprisingly, our analysis also predicts that presynaptic inhibition will more strongly inhibit signals coming from specific cells than it will signals coming from integrative cells. This suggests that presynaptic inhibition favors transmission of integrative signals from CA3 to CA1 and raises the possibility that presynaptic inhibition may be important for filtering signals that are distributed across different afferents as well as signals that are intrinsic to single afferents.
We caution that our analysis infers properties of signal processing in vivo based on synaptic mechanisms that have been characterized ex vivo in brain slices. Brain slices have been used widely to characterize synaptic mechanisms with a level of resolution that is difficult if not impossible to achieve in vivo, but it remains possible both for this study in particular and for ex vivo work in general that systematic differences between in vivo and ex vivo conditions may compromise the conclusions obtained using ex vivo systems. We therefore emphasize that our conclusions are predictions that must ultimately be tested experimentally.
The contrast enhancement predicted here is likely to be functionally important at other synapses as well. Of particular relevance, corticothalamic synapses express synaptic dynamics similar to those described in hippocampus (Descheenes and Hu 1990; McCormick and von Krosigk 1992), and similar neuromodulator-dependent high-pass filtering has also been documented using both ex vivo and in vivo recordings (Castro-Alamancos and Calcagnotto 2001). Further consideration of the functional role of presynaptic inhibition in that system will require analysis of the spike trains observed at corticothalamic projections during relevant behavioral tasks as has been done here. We also note that contrast enhancement need not be the only functional consequence of presynaptic inhibition; in neocortex, presynaptic inhibition curtails synaptic depression (Abbott and Regehr 2004), which has been implicated in temporal coding (Tsodyks and Markram 1997), sensory adaptation (Chance et al. 1998; Chung et al. 2002), and short-term modifications in response tuning (Felsen et al. 2002). It seems reasonable to speculate that presynaptic inhibition could suppress these phenomena at synapses that express this depression, and we note with interest that both synaptic depression and sensory adaptation are altered by different behavioral states (Castro-Alamancos 2004).
Most forms of presynaptic inhibition, including GABABR activation, share a common mechanism of inhibition of voltage-gated calcium channels by the G protein βγ subunit (reviewed in Wu and Saggau 1997). Different inhibitors also have similar effects during spike trains (Kreitzer and Regehr 2000; Varela et al. 1997). We therefore think it likely that the conclusions of this study will be applicable to several presynaptic inhibitors. We also note plasticity in the efficacy of presynaptic inhibition (Bradaia et al. 2005) could have the functional effect of up- or down-regulating contrast enhancement.
Although we have referred to the GABABR-mediated inhibition as a presynaptic inhibition, GABABRs are also located postsynaptically where they hyperpolarize the cell by activating G-protein-coupled inwardly rectifying potassium (GIRK) channels (Luscher et al. 1997; Newberry and Nicoll 1984). A significant contribution of postsynaptic GABABR to the effects described here is unlikely for two reasons. First, tonic postsynaptic GABABR activation by baclofen leads to tonic hyperpolarization (Newberry and Nicoll 1984); this might modestly reduce the overall magnitude of the inhibition due to an increased driving force for EPSPs, but this effect would be activity-independent and therefore would not contribute to the shape of the filtering function. Second, neither the inhibitory effect of baclofen nor the activity dependence of that inhibition is altered in GIRK knockout mice, even though the postsynaptic hyperpolarization by baclofen is abolished (Luscher et al. 1997). Thus the activity-dependent effects that are the core of the present study are likely to be mainly if not entirely due to presynaptic effects.
The present results examine the effects caused by the continuous presence of a presynaptic inhibitor; this framework is most consistent with inhibitors that act diffusely and vary slowly in concentration. Of course, this framework would be most appropriate for exogenous agonists, applied either medicinally (e.g., baclofen) or as drugs of abuse (e.g., g-hydroxybutyrate). However, we note that while endogenous presynaptic inhibitors are released in an activity-dependent manner, many act over long distances, are subject to slow clearance, and change levels so slowly that they are, in practice, tonically active (e.g., adenosine) (reviewed in Dunwiddie and Masino 2001). These endogenous inhibitors would fit into this framework as well. For GABABRs in particular, the characteristics of endogenous activation in vivo remain unresolved; however, ambient GABA levels in CA1 in vivo are in the low micromolar range (Lerma et al. 1986), which could lead to tonic GABABR-mediated inhibition, based on the affinity of the receptor (Yoon and Rothman 1991). Moreover, activation of these receptors is not tied to short-term changes in local activity in brain slices (Ohliger-Frerking et al. 2003; Scanziani 2000), and the receptors under consideration here are located on excitatory terminals, which are not directly contacted by GABAergic release sites. Thus consistent with the framework applied here, endogenous activation of presynaptic GABABRs at Schaffer collateral synapses is unlikely to depend strongly on local short-term changes in activity.
In conclusion, our analysis suggests that presynaptic inhibition enhances contrast between background activity and behaviorally relevant responses and does so differentially for different behavioral cues. Presynaptic inhibition is also expected to enhance contrast between cells that are specific or integrative in their responses to different cues during a task. These results highlight the importance of accounting for activity-dependent effects of synaptic mechanisms during natural behaviors, and they predict functional effects of GABABR-mediated inhibition at the Schaffer-collateral synapse during behaviorally relevant activity. As these synapses are only one of several sites at which GABABRs are found in the hippocampus, further characterization of the functional effects of GABABRs acting at other sites will be of interest as will consideration of how these different effects of GABABRs combine to produce coordinated network effects on the hippocampal circuit.
Acknowledgments
We thank G. Hjelmstad, D. Rossi, and R. Wilson for comments on an earlier version of the manuscript and R. Taylor and R. Wilson for helpful discussions.
GRANTS
M. Frerking is supported by National Institute of Neurological Disorders and Stroke Grant R01NS-045101.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Bradaia A, Berton F, Ferrari S, Luscher C. beta-Arrestin2, interacting with phosphodiesterase 4, regulates synaptic release probability and presynaptic inhibition by opioids. Proc Natl Acad Sci USA. 2005;102:3034–3039. doi: 10.1073/pnas.0406632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABA(B) receptors. Neuron. 1998;20:135–141. doi: 10.1016/s0896-6273(00)80441-9. [DOI] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Relief of G-protein inhibition of calcium channels and short-term synaptic facilitation in cultured hippocampal neurons. J Neurosci. 2000;20:889–898. doi: 10.1523/JNEUROSCI.20-03-00889.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Hablitz JJ. Presynaptic depression of synaptic transmission mediated by activation of metabotropic glutamate receptors in rat neocortex. J Neurosci. 1994;14:5120–5130. doi: 10.1523/JNEUROSCI.14-08-05120.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron. 2004;41:455–464. doi: 10.1016/s0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. High-pass filtering of corticothalamic activity by neuromodulators released in the thalamus during arousal: in vitro and in vivo. J Neurophysiol. 2001;85:1489–1497. doi: 10.1152/jn.2001.85.4.1489. [DOI] [PubMed] [Google Scholar]
- Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci. 1998;18:4785–4799. doi: 10.1523/JNEUROSCI.18-12-04785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Descheenes M, Hu B. Electrophysiology and pharmacology of the corticothalamic input to lateral thalamic nuclei: an intracellular study in the cat. Eur J Neurosci. 1990;2:140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Elmslie KS, Zhou W, Jones SW. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Felsen G, Shen YS, Yao H, Spor G, Li C, Dan Y. Dynamic modification of cortical orientation tuning mediated by recurrent connections. Neuron. 2002;36:945–954. doi: 10.1016/s0896-6273(02)01011-5. [DOI] [PubMed] [Google Scholar]
- Frerking M, Schulte J, Wiebe SP, Staubli U. Spike timing in CA3 pyramidal cells during behavior: implications for synaptic transmission. J Neurophysiol. 2005;94:1528–1540. doi: 10.1152/jn.00108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Hermann A, Greene RW, Chan-Palay V. Action and location of neuropeptide tyrosine (Y) on hippocampal neurons of the rat in slice preparations. J Comp Neurol. 1987;257:208–215. doi: 10.1002/cne.902570207. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Modulation of transmission during trains at a cerebellar synapse. J Neurosci. 2000;20:1348–1357. doi: 10.1523/JNEUROSCI.20-04-01348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mallart A, Martin AR. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968;196:593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. Nature. 1984;308:450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Ohliger-Frerking P, Wiebe SP, Staubli U, Frerking M. GABA(B) receptor-mediated presynaptic inhibition has history-dependent effects on synaptic transmission during physiologically relevant spike trains. J Neurosci. 2003;23:4809–4814. doi: 10.1523/JNEUROSCI.23-12-04809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SP, Staubli UV. Dynamic filtering of recognition memory codes in the hippocampus. J Neurosci. 1999;19:10562–10574. doi: 10.1523/JNEUROSCI.19-23-10562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J Physiol. 1995;485:649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Yoon KW, Rothman SM. The modulation of rat hippocampal synaptic conductances by baclofen and gamma-aminobutyric acid. J Physiol. 1991;442:377–390. doi: 10.1113/jphysiol.1991.sp018798. [DOI] [PMC free article] [PubMed] [Google Scholar]