Abstract
Purpose
To investigate the effect of ferritin protein overexpression on superparamagnetic iron oxide (SPIO) particle labeling of C6 rat glioma cells, and track the labeled cells in vivo using magnetic resonance imaging (MRI).
Materials and Methods
A plasmid of H-chain of murine ferritin gene was constructed and transfected into C6 cells. The parental and the transfected C6 cells labeled with SPIO were bilaterally inoculated subcutaneously into nude mice. The mice were imaged by multiple T2-weighted MR scans after C6 cell inoculation. The mice were killed 2 weeks later, and the concentration of iron in the tumor tissue was measured by inductively coupled plasma.
Results
The iron concentration in xenografts derived from SPIO-labeled C6 cells that were transfected with ferritin plasmid was significantly higher than that in xenografts from parental C6 cells that were labeled with SPIO but not transfected (p=0.034, N=5). Ferritin-transfected C6 cells showed an improved T2 contrast in vivo compared with parental cells labeled with SPIO but not transfected.
Conclusion
Coordinating ferritin with SPIO can lead to a longer MRI cellular tracking period.
Keywords: Ferritin reporter, Superparamagnetic iron oxide (SPIO) particle, Cell tracking, MRI
Introduction
With the rapidly increasing number of reports on cellular therapy [1], there is an urgent need to develop techniques that can track transplanted therapeutic cells in living subjects over time. At present, most cell therapy protocols require histological analysis to determine viable engraftment of the transplanted cells. The development of noninvasive, real-time, and sensitive technologies to monitor transplanted cells will greatly aid clinical implementation of cellular therapy. Magnetic resonance imaging (MRI), with superior three-dimensional spatial resolution and multiple contrast mechanisms, has proved to be a powerful tool in the clinic which reveals detailed anatomical and functional information. MRI is also the method of choice in many research studies on tracking cells in vivo, and these studies usually use superparamagnetic iron oxide (SPIO) particles as the MRI contrast agents [2-6]. SPIO is biodegradable and metabolized by cells. In rapidly dividing cells, intracellular iron oxide nanoparticles disappear after five to eight divisions [7]. Because the iron oxide nanoparticles are diluted with cell division, as well as metabolized, the SPIO-induced T2 contrast may be lost over time.
Ferritin is an ubiquitous and highly conserved iron-binding protein that consists of variable amounts of heavy (21 kDa H-Ferritin) and light (19 kDa, L-Ferritin) chains and plays a major role in iron homeostasis. The H-chain of ferritin has a ferroxidase activity that oxidizes Fe2+ to Fe3+, while the L-chain of ferritin is associated with iron nucleation and protein stabilization. Twenty-four ferritin subunits assemble to form the apoferritin shell, and each apoferritin molecule can sequester up to 4,500 iron atoms. Because of the crystalline nature of the ferrihydrite core, ferritin has an anomalously high superparamagnetism and a marked effect on magnetic resonance (MR) relaxation rates. More recently, ferritin has been used as an endogenous reporter for MRI [8-10]. Endogenous reporter genes generate contrast themselves by accumulating MR-detectable iron and can prevent the dilution of MRI contrast. However, there are some limitations with the use of metalloprotein-based reporter gene in MRI cell tracking, including insufficient endogenous iron concentration and intrinsically low detection sensitivity.
We postulate that the overexpression of ferritin protein in cells could sequester iron atoms degraded from SPIO, and ferritin could coordinate with SPIO to improve the duration of cells tracked by MRI. In this study, a plasmid of murine ferritin H-chain was constructed and transfected into C6 rat glioma cells. Ferritin H-chain gene-transfected C6 and parental C6 cells were each labeled with SPIO and inoculated bilaterally into nude mice. T2-weighted MRI was performed at selective time points after cell implantation, and the iron content in xenografts was measured with inductively coupled plasma (ICP) analysis.
Materials and Methods
Cell Culture
C6 rat glioma cells were used in the present study. The cells were routinely maintained in F12 Kaighn’s medium (Gibco, Invitrogen, USA) supplemented with 1 mM L-glutamine, 15% donor equine serum (HyClone, Thermo Scientific, USA), 2.5% fetal bovine serum (FBS; Gibco, Invitrogen, USA), 100 units/ml of penicillin G and 100 mg/ml of streptomycin. The cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Construction and Stable Transfection of Murine FHC-pcDNA3.1 Plasmid
The total RNA was extracted from muscle tissues of mice using a Trizol reagent (Invitrogen, California, USA). cDNA was transcripted with an Oligo (dT) primer using Reverse Transcription System (Promega, Madison, USA) according to the protocol provided by the manufacturer. The primer sets for amplification of murine ferritin H-chain gene (FHC) were designed according to the mRNA sequence of GenBank (accession number NM-010239). The PCR products and the pcDNA3.1(–) vector (Invitrogen, USA) were digested with the restriction enzymes of NotI and BamHI (TaKaRa Biotechnology Co., Ltd., Dalian, China) in an H buffer at 37°C overnight. After being purified with a QIAquick PCR purification kit (QIAGEN, Germany), the H-chain fragment was subcloned into pcDNA3.1 vector using T4 ligase (TaKaRa Biotechnology Co., Ltd., Dalian, China).
One day before transfection, 105 C6 cells were plated in 500 μl of F12 Kaighn’s (Gibco, Invitrogen, USA) without antibiotics in a 24-well culture vessel, and the cells were 90% confluent at the time of transfection. The FHC-pcDNA3.1 plasmid was transfected into C6 cells using Lipofectamine2000 (Invitrogen, USA). The transfected C6 cells were passaged at 1:10 into fresh medium 24 h after transfection. Geniticin G418 (500 μg/ml, Gibco, USA) was added to C6 cells to select stably transfected cells.
Immunohistochemical Cell Staining
C6 cells transfected with FHC-pcDNA3.1 plasmid and parental C6 cells were cultured on poly-L-lysine-coated coverslips under standard condition for 48 h. The cells were fixed in 10% formalin in phosphate buffered solution (PBS, pH 6.5–7.5) for 30 min followed by a washing step with PBS. The slides were incubated with the specific anti-murine ferritin H-chain polyclonal antibody (Santa Cruz Biotechnology, USA) at a dilution of 1:100 at 4°C overnight. After washing with PBS (pH 7.4), the slides were incubated with secondary antibody (Dako, Produktionsvej, Denmark) for 30 min at room temperature. Color development was performed with 3,3′-diaminobenzidine (Dako). Nuclei were lightly counterstained with hematoxylin (Sigma-Aldrich, St. Louis. MO, USA).
Inductively Coupled Plasma Analysis
The intracellular concentrations of iron in cultured cells and xenografts were measured using an inductively coupled plasma spectrometer (TJA IRIS Advantage/1000 Radial ICAP, Thermo Scientific, USA). The cultured cells were harvested after digestion with 0.05% tripsin-EDTA (Gibco, USA) and rinsed with PBS buffer three times. The number of cells was calculated with a hemacytometer. The cells and xenografts were digested with 70% HNO3, incubated at 90°C for 1 h and diluted to 5 ml with pure water. Blank and four standard solutions of iron at 0.1, 1, 10, and 100 ppm were measured as well. The iron concentration of xenografts was normalized to the tissue weight.
In vivo Magnetic Resonance Imaging
Animal procedures were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Administrative Panel on Laboratory Animal Care of Stanford University. Plasmid of H-chain of murine ferritin-transfected C6 cells and parental C6 cells were incubated with SPIO particles. The SPIO was prepared by following a protocol that we recently published [11]. In brief, 15 nm oleate-coated iron oxide nanoparticles were ligand-exchanged with dopamine and were subsequently adsorbed with one layer of human serum albumin. Such particles are highly stable in a physiological environment and have proven efficiency in labeling various cell types [11]. C6 cells were incubated with SPIO at a concentration of 60 μg/ml overnight. For preparation of inoculation, the cells were rinsed with PBS, dissociated, centrifuged, and resuspended in PBS. An aliquot of cells was counted using a hemocytometer. 106 cells for each transplant were allotted, centrifuged, and resuspended in minimal serum-free F12 Kaighn’s medium. Female nude mice (6–10 weeks old, 28–30 g) were anesthetized with isoflurane. The ferritin-transfected C6 cells and parental C6 cells without or with SPIO labeling were inoculated bilaterally into nude mice in the hind limbs. T2-weighted imaging on a GE 7 Tesla microMRI was performed using a FSE (fast spin echo) T2-weighted sequence with the following parameters: TR=4000 ms, TE=40 ms, FOV=40 mm, Echo Train Length=8 ms, and NEX=6.0.
Histological Examination
Prussian blue iron stain was used to assay the iron accumulation in C6 cells and xenografts. The preparation of cells for Prussian blue stain was the same as that used for the immunohistochemical staining of cells. Tumor slices (4 μm) were fixed in 10% formalin in phosphate buffered solution (PBS, pH 7.4) overnight and embedded in paraffin. Hematoxylin and eosin staining was carried out according to the standard protocol. For Prussian blue staining, the 4 μm tissue sections were deparaffinized and rehydrated using a gradient of ethanol concentrations. The sections were placed in staining solution (2% potassium ferrocyanide and 2% HCl) for 30 min, washed twice in PBS, counterstained with neutral red, and mounted on slides with neutral gum.
Statistical Analysis
Results were presented as mean ± standard deviation. The signal intensity values for T2 obtained from MR scanning and the concentrations of iron in cells and xenografts were analyzed using t-tests (SPSS10.5, Chicago, IL). A p value<0.05 was considered statistically significant.
Results
C6 Cells Overexpressing Ferritin Protein Accumulated More Iron than Parental C6 Cells In Vitro
A plasmid of H-chain of murine ferritin (FHC-pcDNA3.1) was constructed and stably transfected into C6 rat glioma cells. The overexpressed ferritin was detected in the cytoplasm of transfected C6 cells as brown particles by using immunohistochemical staining with a specific polyclonal anti-H-chain of murine ferritin antibody (Fig. 1). There was a minimal increase in iron concentration in C6 cells overexpressing ferritin protein, compared to parental C6 cells, when the cells were cultured in F12 Kaighn’s medium with 15% donor equine serum and 2.5% FBS as the only Fe sources (n=3, p=0.684; Fig. 2a). However, a significant increase in iron concentration was detected in ferritin-transfected C6 cells compared to parental C6 cells when cells were cultured with Fe supplement (ferric ammonium citrate, FAC) at a concentration of 0.2 mM (n=3, p=0.01; Fig. 2a). When incubated with FAC at an even higher concentrations (0.25, 0.5, 1.0, and 1.5 mM), transfected C6 cells accumulated substantially more iron than parental C6 cells (n=3, p=0.004, 0.021, 0.06, and 0.012, respectively; Fig. 2b). Both the transfected C6 cells and the parental C6 cells labeled with SPIO were passaged six times successively, and the intracellular concentrations of iron were measured by ICP. There was no significant difference between C6 cells and transfected C6 cells labeled with SPIO at first passage (n=3, p=0.383). However, C6 cells overexpressing ferritin accumulated significantly more iron than C6 control cells from second passage on (n=3, p=0.03, 0.02, 0.004, 0.002, and 0.002 for passages 2, 3, 4, 5, and 6, respectively; Fig. 2c).
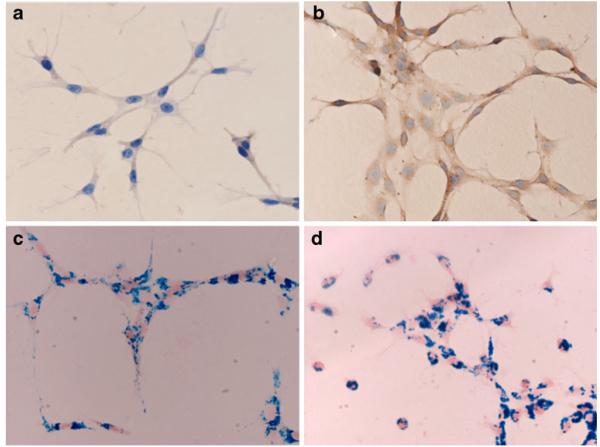
Fig. 1.
Immunohistochemical stain and Prussian blue iron stain of C6 cells. Immunohistochemical stain was conducted in ferritin-transfected and parental C6 cells with a specific polyclonal antibody against murine H-chain of ferritin, showing a weak positive stain (brown particles) in cytoplasm of parental C6 cells (a) and a strong positive stain in ferritin-transfected C6 cells (b). There is no obvious difference in cellular SPIO labeling between parental C6 cells (c) and ferritin-transfected C6 cells (d), based on Prussian blue iron stain.
Fig. 2.
Intracellular iron concentration in C6 cells and xenografts measured by ICP. a Ferritin-transfected C6 cells accumulated significantly more iron than the parental C6 cells, after being incubated with Fe supplement (n=3, p=0.015). b After incubation with Fe supplement at different concentrations (A 0.25 mM, B 0.5 mM, C 1.0 mM, and D 1.5 mM), transfected C6 cells accumulated substantially more iron than the parental C6 cells. c The remaining iron concentration in the transfected and parental C6 cells labeled with SPIO after various passages (P1 to P6). d No significant difference in iron concentration was detected between xenografts derived from transfected C6 and parental C6 cells (n=4, p=0.129), while a significant difference was observed in xenografts labeled with SPIO (n=5, p=0.034). Data are presented as mean ± SD.
C6 Cells Overexpressing Ferritin Protein Accumulated More Iron than Control C6 Cells Labeled with SPIO In Vivo
The ferritin-transfected C6 cells and parental C6 cells without or with SPIO label were inoculated bilaterally into nude mice in the hind limbs. To evaluate the effect of overexpressed ferritin protein on the accumulation of endogenous iron and the iron degradation from SPIO, xenografts derived from implanted C6 cells were analyzed by ICP at day 13 after tumor inoculation. The iron concentration in tumor tissues derived from ferritin-transfected C6 cells without SPIO labeling showed a minimal increase compared to that in tumor tissues from parental C6 cells without SPIO labeling (Fig. 2d, n=4, p=0.129), while the iron concentration in xenografts derived from C6 cells with both ferritin transfection and SPIO labeling was significantly higher than that in tissues derived from parental C6 cells labeled with SPIO (Fig. 2d, n=5, p=0.034). The xenografts were also subjected to histological examination and Prussian blue staining (Fig. 3). Prussian blue stain for iron with neutral red counterstain showed increased iron accumulation in glioma cells derived from C6 cells transfected by ferritin H-chain plasmid and labeled with SPIO, compared with glioma cells from parental cells labeled with SPIO.
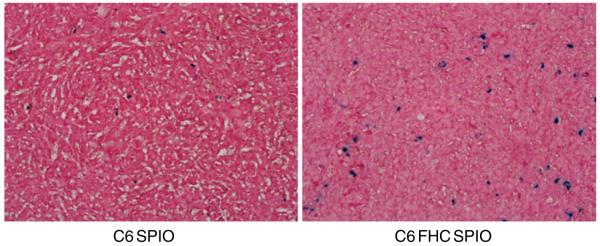
Fig. 3.
Prussian blue iron stain of tumor tissue slices showed that ferritin proteins transfected C6 cells labeled with SPIO accumulated more iron, compared with parental C6 cells labeled with SPIO (magnification: ×400).
MRI Detection of C6 Cells Overexpressing Ferritin Protein
Ferritin C6 cells and parental C6 cells without or with SPIO labeled were inoculated bilaterally into nude mice in the hind limbs. T2-weighted imaging on a GE 7 Tesla microMRI was performed using an FSE sequence 2, 6, and 13 days after inoculation. The signal intensity of T2-weighted imaging was measured and analyzed using a two-tailed paired t test. Data were represented as mean ± standard deviation. C6 cells overexpressing ferritin protein without SPIO label showed minimal contrast compared with parental C6 cells at different time points (Fig. 4a; n=4, p=0.379, 0.105 and 0.056 on days 2, 6, and 13, respectively). On the other hand, ferritin overexpressing C6 cells labeled with SPIO significantly reduced signal intensity in T2-weighted images for MRI (Fig. 4b; n=5, p=0.012, 0.003 and 0.021 on days 2, 6, and 13, respectively).
Fig. 4.
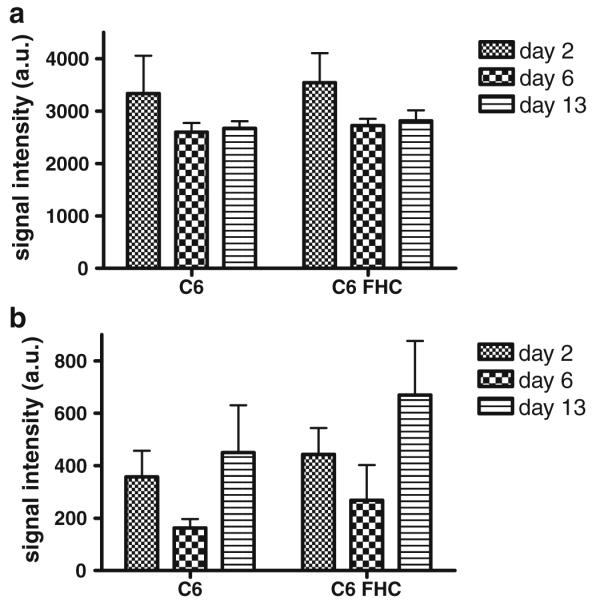
In vivo MRI. a C6 cells overexpressing ferritin protein showed minimal signal intensity reduction compared with parental C6 cells at different time points (n=4; p=0.379, 0.105 and 0.056 on days 2, 6, and 13, respectively). b Overexpression of ferritin protein in C6 cells labeled with SPIO significantly reduced signal intensity in T2-weighted images for MRI (p=0.012, 0.003 and 0.021 on days 2, 6, and 13, respectively). Data are presented as mean ± SD.
Discussion
As a widely available noninvasive imaging modality with high resolution, exquisite tissue contrast, and superb anatomical detail, magnetic resonance imaging has found extensive applications in stem cell imaging in both pre-clinical and clinical settings [2, 3, 6, 12-16]. MRI of SPIO nanoparticles, which can be internalized by cells to generate strong MRI contrast in T2- and T2*-weighted images, has become a valuable tool for studying the in vivo fate of transplanted stem cells and precursor cells. Cellular uptake of SPIO may be induced by fluid phase endocytosis or macropinocytosis. Internalized iron oxide particles remain transiently in the endosome/lysosome compartment. The effects of SPIO labeling on cellular expression of transferrin receptor and ferritin have been investigated in different types of cells [17]. Ferritin gene transcripts and protein were significantly increased in HeLa cells labeled with SPIO, compared with unlabeled cells. Ferritin expression from SPIO-labeled mesenchymal stem cells (MSCs) grown to confluence demonstrated an insignificant increase in ferritin mRNA expression, compared with that in corresponding unlabeled cells. However, the ferritin protein levels were significantly increased at each time point compared with unlabeled MSC whole cell lysate. Some of the iron oxide-loaded endosomes fused with lysomes and, at a pH of 4.5 maintained with appropriate buffers, resulted in the dissolution of the iron oxide nanoparticles, which suggests that the iron from the nanoparticles can be released into the intracellular compartment and participate in cellular iron metabolism [18]. The increased expression of ferritin in SPIO-labeled cells is a response to the intracellular iron increase that is due to the iron degraded from SPIO in cytoplasm. Although magnetic cell labeling is robust and allows the detection of a few cells, once the cells divide, there is an exponential loss of the iron oxide.
Several candidate MRI reporter genes, including one that encodes the iron storage protein ferritin, have been reported [8-10]. Although a primary application for MR reporter genes is in monitoring gene therapy [8-10], an alternative application is tracking stem or progenitor cells after transplantation. The results shown here suggest that endogenous expression of ferritin reporter gene can prevent the dilution of MRI signal. However, the use of ferritin MRI reporter gene to track cells is limited by several factors. First, the cellular contrast of MRI produced by endogenous iron is weak and the sensitivity of detection is low. Second, sufficient endogenous iron needs to be present, with different tissues having different iron concentrations. Third, the T2 signal attenuation induced by ferritin increases linearly with field strength, making it a more efficient T2 contrast agent at higher fields. The sensitivity of detection is lower at clinical field strengths (1.5 and 3 T).
Despite the strong appeal of a molecular MRI reporter system and SPIO for stem cell research, no attempt has been reported of combining ferritin MRI reporter with SPIO in tracking stem cells. We speculate that overexpressed ferritin protein by transfection in treated cells could sequester cytoplasmic iron degraded from SPIO, as an exogenous iron source, in addition to endogenous iron from the labile iron pool. This could enhance cellular contrast and result in a shortening of T2 and T2* of the cells in the tissue and produce a persistent decrease in signal intensity in areas containing SPIO-labeled cells.
In the present study, a plasmid of H-chain of murine ferritin was transfected into C6 cells and then labeled with SPIO. The cooperative effect between ferritin protein and SPIO was studied in vitro and in vivo. There was no obvious difference in cellular SPIO labeling between C6 rat glioma cells transfected with H-chain of ferritin and control C6 cells (Fig. 1c and d). The concentration of iron in transfected C6 cells showed a minimal increase compared to parental C6 cells when incubated with a routine medium (p=0.684; Fig. 2a), while the concentration of iron was significantly increased in transfected C6 with Fe supplement (p=0.01; Fig. 2a). The results indicated that overexpressed ferritin protein worked, and the H-chain of murine ferritin could sequester more iron independently. We then tested the relationship between accumulation of iron associated with overexpressed ferritin protein and concentration of Fe supplement (0.2, 0.25, 0.5, 1.0, 1.5 mM). Our data showed that the intracellular iron concentration is not linearly associated with iron supplement concentration. In this study, 0.25 mM Fe supplement was the appropriate concentration for ferritin protein to sequester more iron. The intracellular concentration of iron in transfected and parental C6 cells showed no difference at first passage 2 days after SPIO labeling (n=3, p=0.383). This can be interpreted as meaning that only a small portion of SPIO degraded 2 days after labeling. The concentration of intracellular iron sharply decreased after second passage at day 4, and C6 cells overexpressing ferritin accumulated more iron than parental C6 cells from the second to sixth passages (Fig. 2c). Our data indicated that the intracellular iron concentration in cells labeled with SPIO decreased rapidly during the first several passages. The concentration of iron in xenografts from transfected C6 cells was minimally increased compared to that from parental C6 cells without SPIO labeling (n=4, p=0.129). Although overexpressed ferritin in implanted C6 cells has the potential to sequester relatively large amount of iron, the concentration of endogenous iron is limited, and there is not enough iron entering ferritin protein under normal physiological condition. The concentration of iron in transfected C6 cells labeled with SPIO was significantly higher than in the control C6 cells labeled with SPIO (n=5, p=0.034). This is consistent with the results from in vivo MRI of inoculated mice. C6 cells overexpressing ferritin protein showed minimally enhanced visualization, compared with parental C6 cells 13 days after implantation (Fig. 3a, b; n=4, p=0.056). Ferritin-transfected C6 cells labeled with SPIO significantly reduced signal intensity in T2-weighted images for MRI at the same time point (Fig. 3c, d; n=5, p=0.021). Although we cannot deduce from this study the detailed mechanisms of interaction between overexpressed ferritin and iron degraded from SPIO, it is possible that more iron clustering in the transgenic cells leads to the effect on relaxivity.
It is worth noting that mammalian ferritins are composed of light and heavy polypeptide subunits. The H polypeptide has a potent ferroxidase activity and L polypeptide plays a role in iron nucleation and protein stability [19]. In addition, expression of mutant light chain induced iron overload in transgenic mice has been recently reported [19]. In this study, however, we transfected only H-chain of murine ferritin into C6 cells. Our future work will include testing the effects of co-transfection of H and L-chain of ferritin, or the effects of L-chain transfection alone, on iron nucleation and MRI tracking. Furthermore, the current study tracked only rapidly dividing C6 cells. A topic of our future research will also be whether an increase of ferritin levels in slowly dividing or nondividing stem cells would also help retain iron to facilitate T2-weighted MR.
Conclusion
Our data indicate that expression of ferritin can sequester iron degraded from labeled SPIO in cells. The cooperative effect leads to the retention of more iron in inoculated cells and to reduced signal intensity in T2-weighted images for MRI. This novel in vivo MR cell tracking method may have potential benefits for research in cellular and gene therapy.
Acknowledgments
The authors would like to thank Dr. Laura Jean Pisani at the Stanford School of Medicine for MRI technical support, and Dr. Hui Mao at the Emory University School of Medicine for helpful discussion of experimental details. This work was supported, in part, by the National Basic Research Priorities Program 973 Project (CB705707) from the Ministry of Science and Technology of China, China Nanjing Medical Science and Technology Research Project (No. 06Z37), the National Natural Science Foundation of China (30970813, 30930028), and the Intramural Research Program of the NIH, including the National Institute of Biomedical Imaging and Bioengineering. Dr. Jiandong Wang acknowledges financial support from Siemens Ltd. China Medical Solution for study at Stanford.
References
- 1.Menon LG, Kelly K, Yang HW, et al. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27:2320–2330. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 2.Farrell E, Wielopolski P, Pavljasevic P, et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem Biophys Res Commun. 2008;369:1076–1081. doi: 10.1016/j.bbrc.2008.02.159. [DOI] [PubMed] [Google Scholar]
- 3.Kraitchman DL, Gilson WD, Lorenz CH. Stem cell therapy: MRI guidance and monitoring. J Magn Reson Imaging. 2008;27:299–310. doi: 10.1002/jmri.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kustermann E, Himmelreich U, Kandal K, et al. Efficient stem cell labeling for MRI studies. Contrast Media Mol Imaging. 2008;3:27–37. doi: 10.1002/cmmi.229. [DOI] [PubMed] [Google Scholar]
- 5.Neri M, Maderna C, Cavazzin C, et al. Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells. 2008;26:505–516. doi: 10.1634/stemcells.2007-0251. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Hu J, Zhou L, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 7.Arbab AS, Bashaw LA, Miller BR, et al. Characterization of biophysical and metabolic properties of cells labeled with super-paramagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229:838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 8.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7:109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 10.Cohen B, Ziv K, Plaks V, et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007;13:498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Wang J, Niu G, et al. Human serum albumin coated iron oxide nanoparticles for efficient cell labeling. Chem Commun. 2010;46:433–435. doi: 10.1039/b917195a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M, Gurney PT, Chung J, et al. Manganese-guided cellular MRI of human embryonic stem cell and human bone marrow stromal cell viability. Magn Reson Med. 2009;62:1047–1054. doi: 10.1002/mrm.22071. [DOI] [PubMed] [Google Scholar]
- 13.Modo M, Beech JS, Meade TJ, Williams SC, Price J. A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. Neuroimage. 2009;47(Suppl 2):T133–T142. doi: 10.1016/j.neuroimage.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Cheng EC, Long RC, Jr, et al. Noninvasive Monitoring of Embryonic Stem Cells in vivo with MRI Transgene Reporter. Tissue Eng Part C Methods. 2009;15:739–747. doi: 10.1089/ten.tec.2008.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapon C, Jackson JS, Aboagye EO, et al. An in vivo multimodal imaging study using MRI and PET of stem cell transplantation after myocardial infarction in rats. Mol Imaging Biol. 2009;11:31–38. doi: 10.1007/s11307-008-0174-z. [DOI] [PubMed] [Google Scholar]
- 16.Brekke C, Williams SC, Price J, Thorsen F, Modo M. Cellular multiparametric MRI of neural stem cell therapy in a rat glioma model. Neuroimage. 2007;37:769–782. doi: 10.1016/j.neuroimage.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Pawelczyk E, Arbab AS, Pandit S, Hu E, Frank JA. Expression of transferrin receptor and ferritin following ferumoxides-protamine sulfate labeling of cells: implications for cellular magnetic resonance imaging. NMR Biomed. 2006;19:581–592. doi: 10.1002/nbm.1038. [DOI] [PubMed] [Google Scholar]
- 18.Arbab AS, Wilson LB, Ashari P, et al. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: implications for cellular magnetic resonance imaging. NMR Biomed. 2005;18:383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- 19.Vidal R, Miravalle L, Gao X, et al. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci. 2008;28:60–67. doi: 10.1523/JNEUROSCI.3962-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]