Abstract
Ribose 5-phosphate (R5P) is a sugar known to undergo the Maillard reaction (glycation) at a rapid rate. In a reaction with the lysines of bovine heart cytochrome c, R5P generates superoxide (O2-) that subsequently reduces ferri-cytochrome c to ferro-cytochrome c. The rate equation for the observed cytochrome c reduction is first order in respect to cytochrome c and half order in respect to R5P. The addition of amines to the cytochrome c-R5P system greatly increases the O2- generation with rates of approximately 1.0 μM min-1 being observed with millimolar levels of R5P and amine at 37 °C. Pre-incubation of R5P with the amine prior to cytochrome c addition further enhances the rate of cytochrome c reduction approximately 2-fold for every 30 minutes of incubation. While clearly accounting for a portion of the reduction of cytochrome c, O2- is not the sole reductant of the system as the use of superoxide dismutase only partially limits cytochrome c reduction, and the contribution of O2- proportionally decreases with longer amine-R5P incubation times. The remainder of the cytochrome c reduction is attributed to either the Amadori product or a cross-linked Schiff base created when a Maillard reaction-derived dicarbonyl compound(s) reacts with the amine. It is believed these compounds directly transfer electrons to ferri-cytochrome c and subsequently become stable free-radical cations. ATP, a putative regulator of cytochrome c activity, does not inhibit electron transport from O2- or the cross-linked Schiff base but does prevent R5P from reacting with surface lysines to generate superoxide. The spontaneous reaction between R5P and amines could serve as an alternative system for generating O2- in solution.
Keywords: Ribose 5-phosphate, Superoxide, Cytochrome c, Glycation, Maillard reaction
1. Introduction
Cells contain efficient systems for protecting against oxidative damage known to contribute to numerous muscular and neurodegenerative diseases and perhaps to the aging process. Synthesized directly by an electron transfer to O2, the preliminary molecule in the formation of the reactive oxygen species group is superoxide (O2-), a free radical generally regarded as a weak oxidant, but one that leads to more damaging species like H2O2 and hydroxyl radicals (•OH). High cellular activities of superoxide dismutases (SODs) reduce O2-levels by converting these radicals into H2O2, which in turn is eliminated by cellular catalases and peroxidases. Superoxide formation arises from many biological sites with mitochondrial reactions, such as from NADH dehydrogenase activity and ubisemiquinone autoxidation, being regarded as a major source that generates substantial amounts of cellular O2- levels. In vitro, O2- is typically generated for SOD investigations by a xanthine oxidase (XO) system using acetaldehyde as a substrate. These systems produce O2- at rates of several micromoles of O2-per minute per Unit of XO activity while simultaneously generating H2O2.1 Pulse radiolysis of aerated solutions is an alternative way of manufacturing O2- at the 10–200 μM level.2 Cytochrome c is commonly used to monitor the level of O2- in solution. The rate of electron transfer of from O2- to ferri-cytochrome c is sufficiently fast (k = 2.6 × 105 M-1 s-1)2 to accurately trap a high percentage of the superoxide ions prior to their dismutation to H2O2. A simple analysis at 550 nm is proportional to the amount of cytochrome c reduced by the available O2-.
Autooxidation of sugars and Amadori products, the latter derived from a Maillard reaction of sugars and amines, are also known to be sources of O2-, H2O2, and •OH.3-6 The mechanism of O2- formation is believed to be via an enediol or eneaminol radical derived from an electron transfer from an enediol or enaminol transitional form to an appropriate metal ion or other electron acceptor.4 The enediol or eneaminol radical cation subsequently reacts with O2 to generate O2-. With a dismutation rate constant of 2 × 105 M-1 s-1 at pH 7,7 the O2-spontaneously produces H2O2 at a relatively rapid rate. Using methylglyoxal in a Maillard glycation reaction, Lee et al.8 and Yim et al.6, 9, 10 also reported the direct transfer of an electron to O2 from a cross-linked Schiff base (via a dicarbonyl), ultimately forming a stable free-radical cation. Stepuro et al.11 likewise observed reduction of cytochrome c by high levels of glucose after extended times (up to 2 weeks) at 37 °C. They ascribed the reduction of cytochrome c to a combination of O2- and post-Amadori glycation products, again implicating dicarbonyls and ketoamines in the process. Reactions performed in vacuum still reduced cytochrome c showing that only a portion of the ferro-cytochrome c reduction was due to O2- and that glyoxidation reactions with O2 were not required in the production of the glycation reductant.
Ribose 5-phosphate (R5P) is a sugar that undergoes the Maillard reaction series at faster rates than most other common sugars.12, 13 The high reactivity of this molecule is thought to be due to a comparatively higher level of acyclic form (needed for the initial amine reaction) along with its ability to auto-catalyze the reaction via its intramolecular phosphate group. We have investigated R5P's potential to generate O2- in solutions containing amines and have obtained rates approaching those seen in XO systems. We also provide evidence of formation of an advanced glycation end-product that can directly pass an electron to cytochrome c by a mechanism that is not inhibited by ATP. These reactions illustrate the potential of glycation reactions to be significant contributors in the generation of reactive oxygen species in cellular systems.
2. Results and discussion
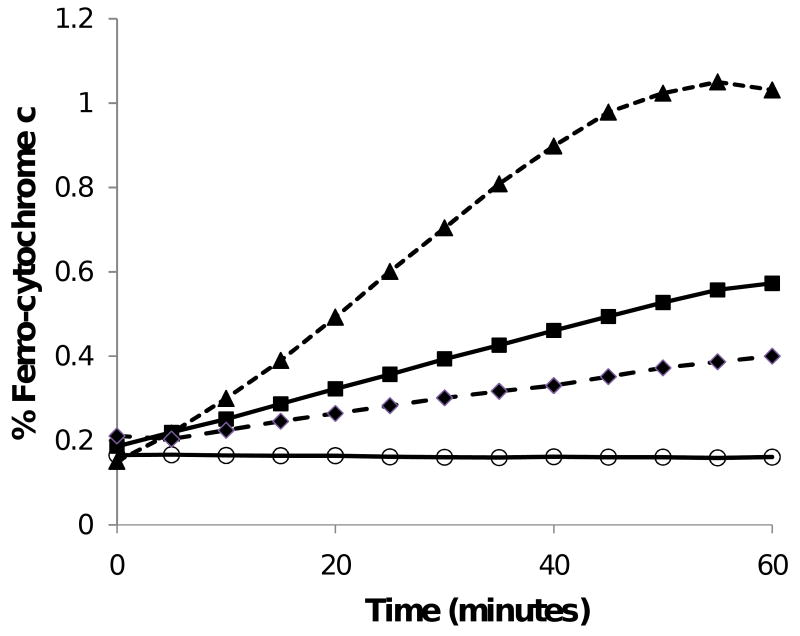
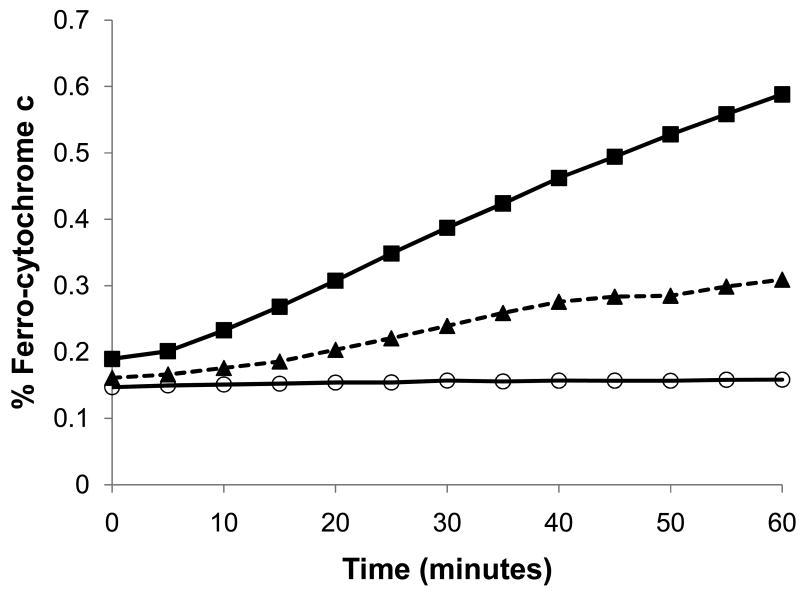
Our results (see Figure 1 and Table 1) indicate that R5P alone can reduce cytochrome c and that the addition of an amine (Lys shown) to the system greatly increases the cytochrome c reduction rate. In both cases, these results were similar to those of Stepuro et al.11 using glucose and amino acids except that observable cytochrome c reduction by the R5P reaction was immediate and required no long-term pre-incubation of sugar and amine to obtain significant reductions. The cytochrome c reduction rate observed is directly related to the concentrations of R5P and amine (in the first system the participating amines are lysines on the cytochrome c molecule itself) and on the pKa value(s) of the attacking amines. The reaction of R5P with cytochrome c (at 82 μM) at 37 °C exhibited decreasing rates with decreasing R5P concentrations, eventually failing to show full reduction of cytochrome c at R5P levels less than approximately 50 μM R5P (see Supplementary Data, Figure S1). The rate is first order with cytochrome c concentrations and half order with R5P concentrations (see Supplementary Data, Figures S2 and S3), i.e.,
Figure 1.
Reactions of R5P with bovine heart cytochrome c and with Lys at 37 °C and pH 7.5 generate products that can reduce ferri-cytochrome c. Concentrations of reactants: cytochrome c (1 mg/mL; 82 μM), R5P (10 mM), and Lys (100 mM). Cytochrome c alone (○—○); cytochrome c and R5P (■—■); cytochrome c, R5P, and SOD (76 U/mL) (◆ - - ◆); cytochrome c, R5P, and Lys (▲- - ▲).
Table 1. Rates of bovine heart cytochrome c reduction under various conditions at 37 °C, pH 7.5.
| [Lys]a (mM) |
[R5P]a (mM) |
[Cyto-chrome c]a (μM) |
Additivea (mM) |
Preincubation of Amine and R5P? | Cytochrome c Reduction Rate (μM min-1) |
O2- Generation Rateb (μM min-1) |
|---|---|---|---|---|---|---|
| 0 | 10 | 82 | None | --- | 0.42 | 0.20 |
| 100 | 10 | 82 | None | None | 1.8 | 1.10 |
| 100 | 10 | 82 | None | 0.5 h | 3.0 | 0.85 |
| 0 | 10 | 82 | Catalase (94 U/mL) | None | 0.60 | |
| 0 | 0 | 82 | 2,3-BD (10) | None | 0 | 0 |
| 100 | 0 | 82 | 2,3-BD (10) | None | 1.2 | --- |
| 100 | 0 | 82 | 2,3-BD (10) | 0.5 h | 2.4 | 0.9 |
| 100 | 0 | 82 | 2,3-BD (10) | 0.5 h | 2.4 | --- |
| ATP (10) | ||||||
| 0 | 10 | 82 | ATP (10) | None | 0.22 | --- |
| 100 | 10 | 82 | w/o ATP | 0.5 h | 2.5 | --- |
| with ATP (10) | 0.5 h | 2.5 | 2.5 | |||
| 0 | 10 | 82c | None | None | 0.52 | --- |
| 0 | 10 | 82c | ATP (10) | None | 0.24 | --- |
Concentrations of reagents listed are those when incubated with cytochrome c.
O2- generation rate was determined by the percent decrease in cytochrome c reduction rate upon addition of SOD (76 U/mL) to the system.
Yeast cytochrome c (from Sigma–Aldrich Chemical Co.)
For concentrations of R5P in the micromolar range, a rate constant of approximately 5.0 × 10-2 M-0.5 min-1 was obtained. In the presence of catalase (to reduce cytochrome c re-oxidation), a stoichiometry of two cytochrome c molecules reduced for every R5P was observed. The lack of catalase in the system at low R5P concentrations gave ratios closer to four cytochrome c molecules per R5P. We suspect the cause of the higher observed ratios was the slow re-oxidation of cytochrome c by H2O2 (formed from the dismutation of O2-), which proportionally became a larger factor at the extended times (up to 48 h) used for the lower R5P concentrations.11
The reduction of cytochrome c by a sugar is not unique to R5P; however, the sugar phosphate is faster in its reduction of cytochrome c than that observed for other sugars such as ribose, ribose plus equimolar phosphate, fructose 6-phosphate, glucose 6-phosphate, and glucose (see Table 2). Many of these solutions in long-term reactions (up to 24 h) showed biphasic reaction profiles with a fast initial reduction and then slower rates of reduction (see Supplementary Data, Figure S4). As shown by other studies, sugars like glucose, glucose 6-phosphate, and fructose 6-phosphate failed to cause full reductions at the mM levels employed.11 The R5P and ribose plus phosphate reactions did not exhibit these biphasic rate profiles. Arabinose 5-phosphate, an epimer of R5P, was nearly as effective (approximately 75%) as R5P in its ability to reduce cytochrome c while 2-deoxyribose 5-phosphate failed to cause any significant reduction of the heme protein over the first 2 h of reaction. Addition of ribose 5-phosphate isomerase, an enzyme that converts R5P to the ketose ribulose 5-phosphate (Ru5P), increased the reduction rate approximately 2-fold. These data are all consistent with the involvement of a Maillard (glycation) reaction requiring a sugar in an acyclic form and a neighboring C-2 hydroxyl group. It agrees with previous work that suggests that ribulose 5-phosphate is a more highly reactive reducing agent than R5P.14
Table 2. Rate constants for cytochrome c reduction by various sugars.
| Sugar | Rate Constanta (mM-0.5 min-1) |
|---|---|
| R5P | 5.0 × 10-2 |
| Ribose | 1.4 × 10-2 |
| Ribose and Pi | 1.6 × 10-2 |
| Glucose 6-Phosphate | 0.65 × 10-2 |
| Fructose 6-Phosphate | 1.6 × 10-2 |
| Glucose | 0.44 × 10-2 |
| Glucose and Pi | 0.37 × 10-2 |
| Arabinose 5-Phosphate | 3.7 × 10-2 |
| 2-Deoxyribose 5-phosphate | 0 |
| R5P with ribose 5-phosphate isomerase (12 kU/mL)b | 25.0 × 10-2 |
All rate constants calculated as first order in respect to cytochrome c concentration and half order in respect to sugar concentration.
Rate constant calculated based on an equilibrium amount of 30% Ru5P (in comparison to R5P
The addition of SOD to the systems, either with or without added amine, leads to significantly decreased cytochrome c reduction rates (see Figure 1 and Table 1; in Table 1 the reduction rates in the presence of SOD are listed in the “O2- Generation Rate” column), thus indicating that at least part of the cytochrome c reduction can be directly attributed to the O2- manufactured by the system. Butler et al.2 reported that O2- can directly reduce cytochrome c through an interaction with the heme edge of the front face. In our systems with R5P and cytochrome c, the use of increasing SOD activities was correlated with observed reduction rates but full inhibition of cytochrome c was not observed. Typically, we observed approximately 50– 60 % loss of reduction rate with the highest SOD activities employed. While it is unclear for our R5P-cytochrome c only system (i.e., without added amine) whether some O2- synthesized by the glycation reaction could rapidly be transferred to the heme face and conceivably escape SOD degradation, our systems with added amine also fail to show complete disappearance of cytochrome c reduction when relatively high SOD activities are used. We interpret this as indicating two different sources for the reduction of cytochrome c (see below), with one being that of the superoxide ion.
Our results with and without SOD indicate that the R5P-cytochrome c system (10 mM and 82 μM, respectively) at 37 °C generates O2- at rates of approximately 0.15 μM min-1, while the addition of relatively high concentrations of supplemental amines to the system can raise this rate to the 1 μM min-1 range. Addition of catalase (94 U/mL) to the system enhanced the speed of reduction by 27% (range 21–33%). This percent increase is even less (9%) when an exogenous amine (Lys) is added to the system. Since H2O2 present in solution re-oxidizes ferro-cytochrome c15 and thus underestimates the O2- rate of formation, these results suggest that only small amounts of H2O2 are generated from the spontaneous dismutation of the produced O2-. This is consistent with the comparative dismutation and O2- -mediated electron transfer rate constants2,7 which indicate that at 10 μM O2- concentrations, the rate of dismutation would be only about one-tenth that of the rate of cytochrome c reduction.
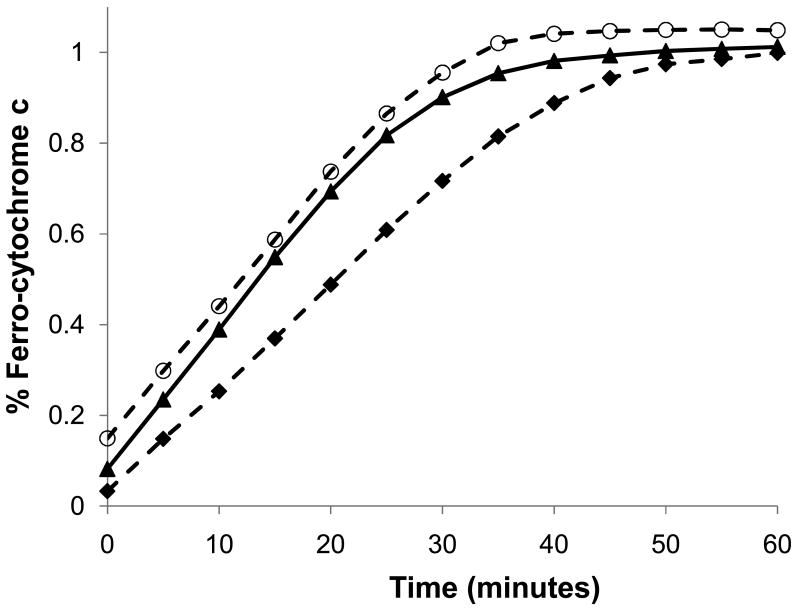
The pre-incubation of R5P with amines prior to their addition to cytochrome c yielded significantly faster reduction rates (compare Figure 2 to Figure 1). For example, a preincubation of 15 mM R5P with 150 mM Lys for one-half hour at 37 °C prior to adding the combination to cytochrome c gave an increased rate of reduction of approximately 2–3 μM min-1. These increases were directly related to length of incubation time with an approximately doubled rate for every hour of incubation at 37 °C (see Supplementary Data, Figure S5). The rates were consistent with a mechanism involving an initial first-order reaction of both R5P and Lys to generate a compound “X” that then reduced cytochrome c (see Supplementary Data, Figure S6). Consistent with the kinetics of R5P with cytochrome c, the electron transfer of X to cytochrome c was half-order, i.e.,
Figure 2.
Pre-incubation of R5P with an amine at 37 °C and pH 7.5 increases the rate of reduction of ferri-cytochrome c. Concentrations of reactants: R5P (10 mM); Lys (100 mM); cytochrome c (1.0 mg/mL; 82 μM); SOD (76 U/mL). Reactions: Lys and R5P pre-incubated for 30 min prior to addition to cytochrome c (▲– ▲); Lys and R5P pre-incubated for 30 min prior to addition to cytochrome c and SOD (◆ - - ◆); Lys and R5P pre-incubated for 30 min prior to addition to cytochrome c and catalase (○ - - ○).
The kinetics suggest a two-step transfer of electrons from X to ferri-cytochrome c with an initial slow step followed by a rapid second transfer. The identity of X derived from R5P has not been conclusively defined in our system, but a possible candidate is the cross-linked Schiff base characterized by Yim et al.10 This mechanism supports a stoichiometry of at least two electrons being provided by the R5P-amine compound. The addition of SOD partially slowed the reduction rate; however, the percent decrease (approximately 15–30%) in the pre-incubated amine/R5P systems was less than for those systems where the amine and R5P were initiated directly in the presence of cytochrome c. This suggests that O2- is a major contributor to cytochrome c reduction initially, but that a second reductant gains relative importance with elapsed time.
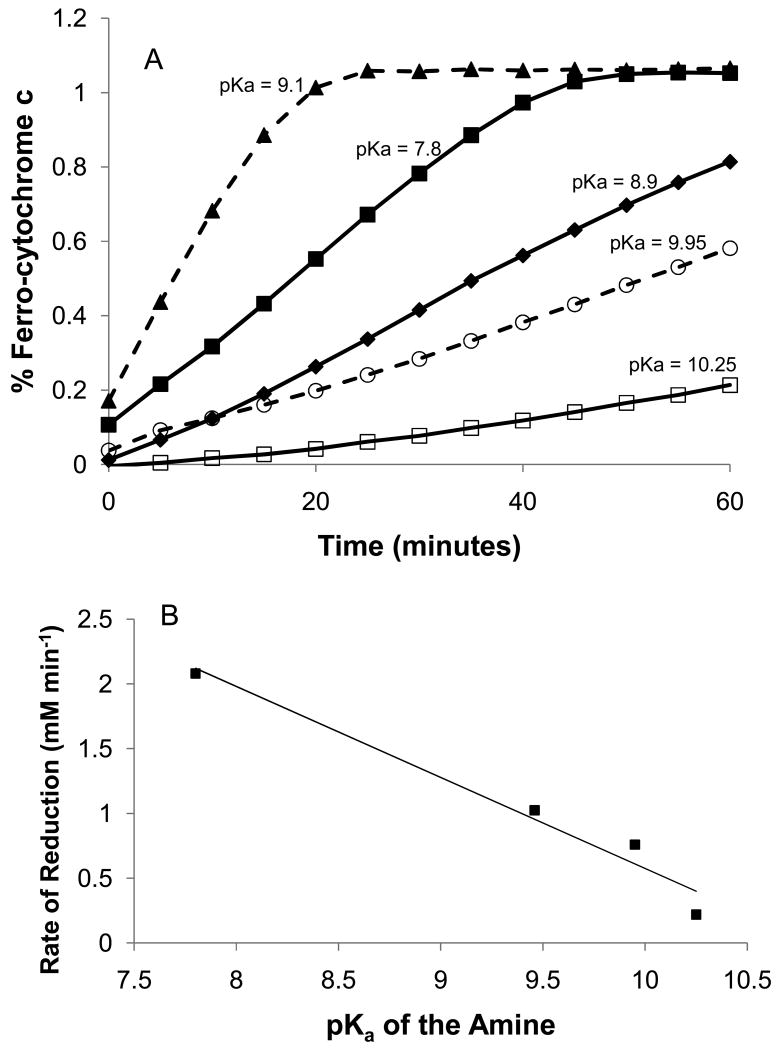
As expected, an increase in the rate of cytochrome c reduction correlated with increasing pH. The rate of reduction (without added amine) is approximately 1.4-fold greater at pH 9.4 as compared to pH 7.3. The initial reaction between the sugar and the amine to form the imine (on the way to the Amadori product, see Scheme 1) is well known to require the attacking amine to be in the unprotonated form—a state more prevalent at higher pH. That superoxide electron transfer to cytochrome c slows with increasing pH2 may partially explain why we do not observe rates directly relating to the concentration of the unprotonated amine form in solution. In further support, the cytochrome reduction rate shows a good correlation with the pKa of the attacking amine when a series of monoamines are compared (see Figure 3). As shown by Stepuro et al.11 for a series of amino acids, however, other factors must be involved that change the glycation rate and affect the subsequent cytochrome c reduction potential of these compounds. It appears that some type of synergism occurs, for example, for molecules like Lys that contain more than a single amine. With an α-amine of intermediate pKa (9.1) and an ε-amine which is highly unreactive (pKa = 10.25; similar to NAcLys), Lys would be expected to show moderate reactivity and not the highest reaction rate of the group. Both this general pKa trend for the reduction of cytochrome c by monoamines/R5P systems and the deviation from this trend by polyamines were also observed for the rate of UV–Vis absorbance increase.12,13 The cytochrome c-R5P only reaction exhibits comparatively high reactivity when considering the micromolar concentrations of ε-amine Lys groups normally thought of as unreactive (see reaction rate of N-acetyllysine, Figure 3) in these reactions. (N-acetylarginine was equally unreactive.) One or more of the protein's R-groups on the protein surface are reacting at rates similarly to free Lys of one-hundred fold higher concentration. This group(s) is not the N-terminus, which is generally a highly reactive amine in glycation reactions, as it is protected by N-acetylation in bovine heart cytochrome c.
Figure 3.
Rate of reduction of bovine heart cytochrome c is generally correlated to the pKa of the amine in reaction with R5P. Lysine, however, shows an enhanced ability to generate products with R5P that reduce cytochrome c. A) Reduction of cytochrome c with amines of different pKa values. All reactions involved a 30-min pre-incubation of amine with R5P at 37 °C and pH 7.5 prior to addition to cytochrome c. Final reaction concentrations were: Amine (100 mM); R5P (10 mM); cytochrome c (1.0 mg/mL; 82 μM). Amines depicted on the graph were Lys (▲- - ▲); Gly-Gly (■ – ■); Arg (◆ – ◆); β-Ala (○ - - ○), and N-acetylLys (□ – □). The value for the lowest pKa contained by the molecule is listed on the figure. B) Correlation of the rate of reduction (without Lys) with the pKa of the attacking amine.
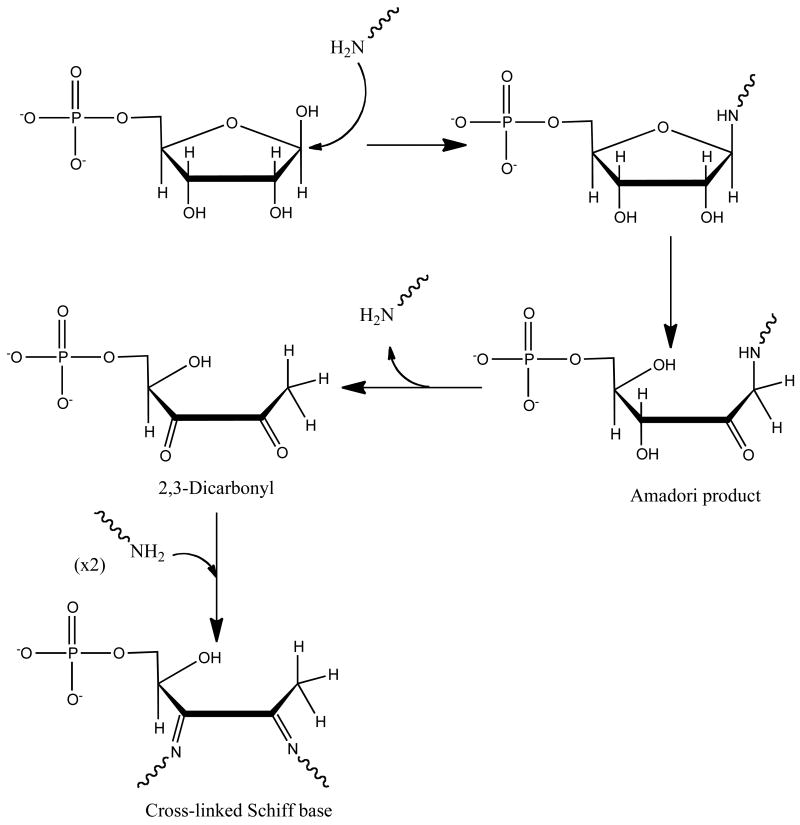
The initial time period for cytochrome c reduction in the presence of R5P, with or without amine, reveals a lag period that we associate to a period of slow O2- formation. This initial lag period is missing in the system where the amine is preincubated with R5P prior to its addition to cytochrome c (see Figure 1 and 3). This suggests an initial slow formation of an intermediate prior to a faster O2- formation step. Two candidates for intermediates that may serve as the electron donor in the O2- formation step are the Amadori product and a dicarbonyl rearrangement compound that readily forms in Maillard reactions (see Scheme 1). LC–MS analysis of a reaction of amine with R5P reveals the eventual production of both compounds, although no evidence of the dicarbonyl product was observed in the time frame used for cytochrome c reduction (<2 h) (see Supplementary Data, Figure S7). (We acknowledge, however, that the μM levels required to promote cytochrome c reduction could have escaped LC–MS detection.) Our first detection of measurable amounts of dicarbonyl compound(s) was at approximately 24–48 h. Conversely, a product consistent with the Amadori product (or initial Schiff base) does not increase in amount over the first one-half hour, but then significantly increases over the subsequent time frame, doubling in concentration from one hour to two hours of incubation at 37 °C. This is consistent with our observations of a lag-time prior to O2- generation. Stepuro et al.11 assessed the potential of early glucose-derived glycation products (like the Amadori product) to act as reductants of heme proteins and concluded that these compounds contribute little to the protein reduction. We therefore propose that a portion of R5P's fast action in this process is partially based on R5P's unique ability to generate O2- from early glycation products.
An interesting observation was made during our investigations of pre-incubating amines with R5P. Based on the rate constant of reaction between superoxide and cytochrome c, we initially anticipated we would see a burst of cytochrome c reduction for the pre-incubated system due to the superoxide concentration developing over the one-hour period. While an increased rate of cytochrome c reduction was seen upon the addition of the solution to cytochrome c, no rapid (first 30 s) burst of reduction was evident (see Figure 2). This indicates that O2- levels in the incubation mixture were not accumulating. Modeling O2- breakdown using the dismutation rate of 2.0 × 105 M-1 s-1 with our observed rate of proposed superoxide synthesis based on glycation reveals that O2- dismutation is comparatively fast, and a steady-state O2- level of less than 1 μM would be reached. Thus in our incubation system, the burst of O2- would be undetectable. We thereupon expected a buildup of H2O2 in the order of approximately 50–70 μM over the course of the one-hour incubation period; this would subsequently be expected to reduce the observed cytochrome c reduction once the incubation mixture was mixed with the cytochrome c.11 This was clearly not the case. When catalase was added to the amine-R5P incubation system to eliminate H2O2 just prior to its addition to cytochrome c, we observed only a modest change (9%) in the cytochrome c reduction rate. A chemiluminescence assay using horseradish peroxidase measured H2O2 levels after an hour of Lys incubation with R5P to be approximately 70 μM, a value consistent with the amount of superoxide we believed to be generated during this timeframe. Thus, it appears that while we observe a buildup of H2O2, it does not greatly affect our cytochrome c reduction rates. Hence the rate of H2O2-derived re-oxidation of cytochrome c must be relatively slow compared to the rates of the reduction. This is in contrast to the results of Wang et al.15 who claim that cytochrome c can act as a catalyst to remove H2O2 from solution. Kim et al.16 showed that a 2-hour incubation of cytochrome c at 37 °C with H2O2 levels as low as 100 μM can result in the formation of cross-linked dimers, trimers, etc. We allowed a Lys-R5P system to incubate for times up to 24 h and exposed this mixture to cytochrome c for 2 hours. We did not observe any significant oligomer formation for shorter Lys-R5P incubations, but we clearly saw trimer formation (band at 38 kDa) for those reactions incubated for 24 h. This suggests buildups of H2O2 of approximately millimolar levels by these amine-R5P systems.
To further evaluate the oxidative potential of H2O2 on reacted cytochrome c, we reduced the protein with either R5P or ascorbate, removed the excess R5P or ascorbate by filtration and washing, and added H2O2 (150 μM) to the ferro-cytochrome c. The hydrogen peroxide immediately re-oxidized the ascorbate-treated cytochrome c in a rapid manner while approximately 50% of R5P-treated cytochrome c was resistant to H2O2 re-oxidation. (The protein could be efficiently re-oxidized by ferricyanide addition.) We conclude, therefore, that H2O2 generated in our 1–2 hour systems has only modest effects on the reoxidation of cytochrome c that had been treated with R5P or R5P-derived products.
Yim et al.9 noted that a cross-linked Schiff base could directly pass electrons to cytochrome c without the need of superoxide. Our experiments using SOD conclusively show that at the initiation of the reaction, the reducing agent is at least partially due to O2-. As the rate of O2- electron transfer to ferricytochrome c is approximately 10-fold faster than the rate of O2- dismutation, the overall efficiency of cytochrome c reduction by the manufactured superoxide is high. We propose the electron during this O2- -generating reaction comes from the Amadori product formed initially in the reaction. Given an adequate amine-R5P preincubation, the rapid reduction of cytochrome c is likely due to the formation of a cross-linked Schiff base that can directly interact with cytochrome c without O2- involvement. Hence, the addition of SOD to the system has less effect on the rate of cytochrome c reduction.
To evaluate the contribution of the reduction of cytochrome c by a cross-linked Schiff base and to assess the possibility that the source of the O2- was a reaction between an amine and a dicarbonyl, we performed similar reactions replacing R5P with 2,3-butanedione (2,3-BD). These studies are similar in nature to those performed by Lee et al.8 using methylglyoxal and an amine (Ala or albumin). In a cytochrome c-2,3-BD only reaction, no reduction of cytochrome c was observed over the 1–2 h of reaction (Table 1). We interpret this as indicating that while the 2,3-BD was reacting with the protein's Lys residues, the dicarbonyl reaction failed to generate O2- in a manner similar to R5P. Since it would be held in place at a location away from the heme edge, any formation of a cross-linked Schiff base would be unable to migrate to the heme edge to allow electron transport. The addition of free Lys to the 2,3-BD-cytochrome c reaction system, however, caused observable cytochrome c reduction (1.2 μM min-1, see Table 1). Pre-incubation (30 min) of the Lys and 2,3-BD increased the reduction rate by 2-fold to 2.4 uM min-1. The addition of SOD to these systems slowed the reduction rate (approximately 40%) showing that some superoxide was being generated. The results of the 2,3-BD reduction reaction with amine are compatible with the reaction scheme of Yim et al.6 for reduction of cytochrome c via a cross-linked Schiff base and for concurrent formation of superoxide. It is noteworthy that these researchers6, 8 always used a second amine (either alanine or BSA) in the reaction and did not report on the direct reduction of cytochrome c by their dicarbonyl (methylglyoxal). Pande and Myer,17 however, noted long-term reduction of cytochrome c by relatively high levels of 2,3-BD when borate was included in the solution. They ascribed the reduction to a modification of Arg38 but do not speculate as to the mechanism of the electron transfer to the heme group.
Several interesting questions arose from the 2,3-BD results. The fact that the R5P reaction caused cytochrome c reduction while no reduction was observed for the comparable 2,3-BD-cytochrome c only system suggests that R5P (and other sugars) are capable of generating O2- in a fashion differently than via the dicarbonyl mechanism of Yim et al.10 Furthermore, since the addition of SOD did not fully quench the observed reduction in the R5P-cytochrome c only system, we speculate that a portion of the O2- is produced near the heme edge site and thus escapes SOD degradation. Alternatively, it is possible that a R5P-cytochrome c adduct (perhaps formed on Lys72, Lys87, or Lys88) proximal to the heme edge could swing into position and directly transfer an electron. Finally, it is interesting that the rate of cytochrome c reduction by the Lys (100 mM)/2,3-BD (10 mM) system is approximately equal to that of the Lys (100 mM)/R5P (10 mM) system despite significantly less dicarbonyl being present in the latter system. Obviously, some feature of the Lys/R5P Maillard reaction above that of the dicarbonyl significantly improves its action to transfer electrons to cytochrome c. We speculate that the negative charge that is contained by the phosphate group of R5P aids in its attraction to the cationic heme edge site on cytochrome c.
Cytochrome c has an ATP binding site at the upper left edge of the front face.18 This site is known to strongly bind polyphosphates and nucleotide triphosphates and has weaker binding to monophosphates and Pi. The ATP binding site shares several critical lysines (Lys87, Lys88, and Lys72) at its boundary with the site surrounding the heme edge—the site thought to be the docking location for cytochrome c's interaction with ubiquinol cytochrome c reductase and cytochrome c oxidase. A ring of positively charged residues at this heme docking site is thought to guide the protein to the negatively charged surfaces of its mitochondrial electron chain partners, bringing heme edges close enough for electron transfer.19 ATP association with the protein has been speculated to alter the positions of the boundary lysines, removing the attractive surface needed for mitochondrial partner binding. The cationic site surrounding the heme edge is also thought to attract other smaller negative reductants such as O2- and other anions. Of importance here, Butler et al.2 reported that modification of Lys72, Lys87, and Lys88 significantly slowed the kinetics of the electron transfer between O2- and ferri-cytochrome c.
The rate of reduction of cytochrome c by R5P is decreased in the presence of ATP (Figure 4 and Table 1; also see Supplementary Data, Figure S8). At ATP concentrations equimolar to R5P concentration, the rate of cytochrome c reduction decreases approximately 30–50%. This rate change is ATP concentration-dependent (a 1:10 ATP:R5P concentration ratio slows reduction by only approximately 10%, see Supplementary Data, Figure S8); however, the rate does not decrease to zero even at the highest ATP concentrations employed. (A maximum of approximately 60% decrease in rate has been observed.) GTP affects the R5P-promoted reduction rate similarly to ATP, but AMP and Pi show relatively weaker effects of one-third and one-sixth, respectively, as compared to ATP (see Supplementary Data, Figure S8). When ATP is added along with SOD to the cytochrome c-R5P only reaction, full inhibition of the reduction is observed (Figure 4 and Table 1). The effect of ATP on the reduction of cytochrome c by R5P may be explained by a slower reaction of R5P with highly reactive lysines that associate with the ATP and/or by a weaker interaction between the reductant (O2- or cross-linked Schiff base) with the heme edge surface that is now reduced in positive charge due to Lys87, Lys88, and Lys72 being complexed with the ATP. To test this, we examined the effect of ATP on mixtures that contained an added amine. When Lys was added to the cytochrome c-R5P system, some ATP inhibition was observed, but its effect did not supplement that shown when SOD was added. In the system pre-incubated with amine, ATP showed no effect on the reduction rate, with or without SOD addition (Table 1). Similarly, 2,3-BD and Lys reaction systems were not affected by ATP in their ability to generate reductants. We interpret this as indicating that ATP does not affect O2- or the cross-linked Schiff bases from reducing cytochrome c, but it does affect the glycation events that lead to O2- generation in the cytochrome c-R5P only reaction. (This also contributes a small portion of the reduction observed in the amine-containing system that has not been pre-incubated.) Thus, the initial O2- - generating reaction of R5P onto the surface of ATP most likely occurs at Lys72, Lys87, or Lys88. A site around Lys 87, Lys88, and Lys91 has been well defined as a phosphate-binding site of mammalian cytochrome c20 and one that would attract the R5P molecule. This site has been implicated as the location for cytochrome c reduction by a combination of ferrous ion and phosphate.20 The reducing/oxidizing hexacyanide reagents are also thought to work at this site.21 It is interesting to speculate that a similar electron transfer could occur between R5P and ferri-cytochrome c if it bound to this site, and that this could account for the cytochrome c reduction not affected by adding SOD to the system.
Figure 4.
ATP inhibits the reduction of cytochrome c by R5P and R5P-derived products. All reactions carried out at 37 °C and pH 7.4. Concentrations: cytochrome c (1.0 mg/mL; 82 μM); R5P (10 mM); ATP (10 mM); SOD (76 U/mL). Reactions: Cytochrome c and R5P (■ – ■); cytochrome c, R5P, and ATP (▲ - - ▲); cytochrome c, R5P, ATP, and SOD (○ - - ○).
Reactions using yeast cytochrome c likewise showed heme group reduction in the presence of R5P. At comparable levels of cytochrome c and R5P, the observed reduction of yeast cytochrome c was 24% greater than that observed for bovine heart cytochrome c (Table 1). As with the bovine form, addition of ATP (10 mM) similarly inhibited the reduction rate by about 50%. As purchased, yeast cytochrome c (mostly iso-1-cytochrome c with a small (∼5%) amount of iso-2-cytochrome c) contains a trimethylated Lys-72 positioned at the heme edge shown to be important in the cross-linked Schiff base reduction interaction with cytochrome c.2 (We confirmed the presence of this form by LC–MS analysis; a molecular mass of 12,710 Da matches the value of iso-1-cytochrome c with a trimethyl modification.) Butler et al.2 showed that 4-carboxy-2,6-dinitrophenyl (CDNP) modification of Lys72 in horse heart cytochrome c greatly decreased the ability of this protein to be reduced by superoxide. CDNP modification alters the charge of the Lys and greatly changes the sterics around this position with an addition of a bulky group. That the trimethylation modification of Lys72 failed to inhibit the reduction of cytochrome c by R5P suggests that the important aspect of this moiety in Lys72's interaction with O2- is its positive charge. The role of this trimethylation modification of yeast cytochrome c has long been debated. We speculate that one important purpose of the modification is to guard this group against possible glycation events which would alter the charge. Another interesting observation of the reduction of yeast cytochrome c with R5P was a lack of the lag time associated with the non-pre-incubated bovine heart cytochrome c. The reasons and implications for this are not entirely clear.
Our results show that glycation events can contribute to the overall production of reactive oxygen species in the cell. While this investigation examined the glycation events using R5P, other highly reactive cellular sugars, such as glyceraldehyde 3-phosphate, and lower reactive sugars of relatively high cellular concentration, such as glucose 6-phosphate, could combine to generate significant amounts of O2- and H2O2 that would require processing by the various cellular SODs and catalases/peroxidases. The generation of O2- by this system can serve as an alternative to systems employing XO. R5P-derived cross-linked Schiff bases formed by Maillard reactions are potent reductants of cytochrome c. The process and the implications of these events with membrane-bound cytochrome c are currently unknown.
3. Experimental
3.1 General methods
Bovine heart cytochrome c, yeast cytochrome c, bovine erythrocyte superoxide dismutase, bovine liver catalase, ribose 5-phosphate isomerase, horseradish peroxidase, luminol [208-309-4], ATP [987-65-5], arabinose 5-phosphate [89927-09-3], and R5P [207671-46-3] were purchased from Sigma–Aldrich Chemical Co. and were used as supplied. Amines, sugars, and all other reagents were of high purity. Cytochrome c reduction was measured at 37 °C using a BioTek Synergy microplate reader and solutions (200 μL total volume) containing 0.33 mM EDTA.2, 15 Solutions of cytochrome c, R5P (or other sugar) and additives were prepared separately, and each were adjusted to pH 7.3–7.5. The concentrations of each reagent are those after combining in the warmed microplate well. Unless stated differently, the concentration of cytochrome c was 82 μM and the concentration of R5P was 10 mM. In some situations, exogenous amine and R5P were combined prior to the start of the reaction and were held at 37 °C in a heating block for given time intervals (0–24 h) to generate superoxide and reducing Maillard compound. These solutions constituted two-thirds of the volume placed in the microwell during the reduction rate analysis. In these studies, the concentrations of amine and R5P reported are those in the incubation mixture with cytochrome c.
The rate of reduction was determined by measuring the 550 nm absorbance increase as controlled by the 526 nm isobestic point absorbance. (A formula: % ferricytochrome c = (2.42 − A550/A526)/1.61 was derived based on extinction coefficients.22) The bimolecular rate constant for reduction of cytochrome c by O2- (k = 2.6 × 105 M-1 s-1 at pH 7.8)2 is sufficiently fast to make the assumption that O2- formation is equivalent to cytochrome c reduction. Comparison of this rate in our system to the rate of O2- spontaneous dismutation suggests an efficient transfer of electron from O2- to cytochrome c with little production of H2O2. (Our data using catalase confirm this.) Values given for the rate of O2- formed were computed by comparing the reaction in the absence vs. the presence of SOD. Reactions with amines were typically carried out in mM levels of both sugar and amine. Pre-incubations of amine and R5P were performed at 37 °C; concentrations listed were those of solutions after mixing with cytochrome c. Where indicated, 2,3-BD was used as a dicarbonyl compound in place of exogenous amine.
Where indicated, SOD and was added at the start of the R5P/cytochrome c reaction to rapidly degrade any O2- generated by the system. The change in the cytochrome c reduction rate with the added SOD (i.e., versus that without SOD) was attributed to that reduction of cytochrome c caused directly by O2-. This rate is referred to in the text and in Table 1 as the “O2- generation rate.” Catalase was similarly added where indicated at the start of the cytochrome c reduction reaction.
The reduction reactions of cytochrome c with R5P without an additional amine will be referred to as “cytochrome c-R5P only” reactions. Those with an added amine will termed “with amine” represent those reactions where the amine, R5P, and cytochrome c are added together simultaneously. Those labeled “pre-incubated with amine” indicate reactions where the amine and R5P are incubated at 37 °C for a period of time prior to addition to cytochrome c.
3.2 Assessment of H2O2 generated by the system
H2O2 was determined by a chemiluminescent method (without enhancer) of Diaz et al.23 Cytochrome c (82 μM) or exogenous amine (100 mM) was incubated with R5P (10 mM) at 37 °C for given lengths of time prior to addition to the luminol-containing reagent and subsequent luminescence analysis by the microplate reader. H2O2 concentrations of the mixtures were determined by comparison to the luminescence generated by prepared H2O2 standards.
Furthermore, the production of H2O2 by an amine-R5P system was assessed by the ability of μM levels of H2O2 to cross-link cytochrome c.16 Reactions of Lys (100 mM) and R5P (10 mM) were allowed to incubate at 37 °C for periods up to 24 h to generate H2O2 (derived from the dismutation of O2-). This solution was added to cytochrome c, and after a 30-min period, SDS-PAGE was performed on the samples. In comparison, cytochrome c solutions directly treated with H2O2 (various concentrations in the range of 1 μM–1 mM) for 30 min were also analyzed by SDS–PAGE. SDS–PAGE analysis was performed using the PhastGel system by Amersham. Proteins were detected using Coomassie stain, and protein masses were determined using a set of four protein standards (bovine serum albumin, ovalbumin, carbonic anhydrase, and lysozyme).
3.3 LC–MS characterization
LC–MS characterizations were performed to assess for the development of Amadori products and dicarbonyl compounds in the amine-R5P reaction and to evaluate the methylation form of yeast cytochrome c. An Agilent Series 1100 LC/MSD Trap XCT Plus LC–MS with electrospray injection was used for these characterizations. Samples (5–25 μL) were injected into a C8 Zorbax reversed phase column (4.6 × 150 mm) typically with a 50:50 H2O –2-PrOH (plus 0.1% HCO2H) solvent. (A 50:50 H2O –CH3CN solvent was used for the yeast cytochrome c study.) Electrospray injection conditions employed a drying temperature of 365 °C, a nebulizer pressure of 50 psi, and a dry gas flow of 9 L/min. Typically, a MS experiment was performed in positive-ion mode over a scan range of m/z = 50–2200 and a target mass of 300 daltons. Photodiode array and MS detection (positive-ion mode) was used to monitor eluant flow. To assess the relative concentrations the Amadori product and dicarbonyl compounds in the Lys-R5P reactions, extracted ion chromatograms (EICs) were generated using a ±0.5 Da window and combining the m/z [M + H+], [M + Na+], and [M + 2 Na+ (-H+)] masses. For the Lys-R5P reaction, EIC were generated for 359 Da, 381 Da, 403 Da for the Amadori product and 213 Da, 235 Da, and 257 Da for the free R5P dicarbonyl compound. (This advanced glycation product is a dehydrated rearrangement product of the Amadori product.) Integration of these signals gave relative peak areas that were associated with the concentration of the compound. (No standards were commercially available for quantification purposes.) During these reactions changes in theconcentrations of the reagents were assessed in a similar manner by EIC of their respective masses (Lys: 146 Da; R5P: 230 Da). The identification of the oxidative product of R5P, either the α-dicarbonyl (not dehydrated, mass = 228 Da) or 5-phosphoribonic acid (mass = 246 Da) was also attempted by extracting the appropriate signals for these compounds.
In the assessment of the methylation state of yeast cytochrome c, a target mass of m/z 1200 was employed. Signals eluting with a 410 nm absorbance were chosen for analysis and the mass of the protein was determined using the charge-state ruler function of the software.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Petroleum Research Fund (PRF #44216-B4), administered by the American Chemical Society and by the Vermont Genetics Network (VGN), a five-year IDeA Networks of Biocmedical Research Excellence (INBRE) project sponsored by the National Institutes of Health. The LC–MS instrument used in this study was funded by a grant from the Major Research Instruments program at NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Das AB, Nagy P, Abbott HF, Winterbourn CC, Kettle A. J Free Radic Biol Med. 2010;48:1540–1547. doi: 10.1016/j.freeradbiomed.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Koppenol WH, Margoliash E. J Biol Chem. 1982;257:10747–10750. [PubMed] [Google Scholar]
- 3.Jiang ZY, Woollard AC, Wolff SP. FEBS Lett. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JV, Dean RT, Wolff SP. Biochem J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt JV, Wolff SP. Free Radical Res Commun. 1991;12-13(Pt 1):115–123. doi: 10.3109/10715769109145775. [DOI] [PubMed] [Google Scholar]
- 6.Yim HS, Kang SO, Hah YC, Chock PB, Yim MB. J Biol Chem. 1995;270:28228–28233. doi: 10.1074/jbc.270.47.28228. [DOI] [PubMed] [Google Scholar]
- 7.Tajima G, Shikama K. J Biol Chem. 1987;262:12603–12606. [PubMed] [Google Scholar]
- 8.Lee C, Yim MB, Chock PB, Yim HS, Kang SO. J Biol Chem. 1998;273:25272–25278. doi: 10.1074/jbc.273.39.25272. [DOI] [PubMed] [Google Scholar]
- 9.Yim MB, Kang SO, Chock PB. Ann N Y Acad Sci. 2000;899:168–181. doi: 10.1111/j.1749-6632.2000.tb06185.x. [DOI] [PubMed] [Google Scholar]
- 10.Yim MB, Yim HS, Lee C, Kang SO, Chock PB. Ann N Y Acad Sci. 2001;928:48–53. [PubMed] [Google Scholar]
- 11.Stepuro II, Chaikovskaya NA, Vodoevich VP, Vinogradov VV. Biochemistry (Moscow) 1997;62:967–972. [PubMed] [Google Scholar]
- 12.Sandwick R, Johanson M, Breuer E. Ann N Y Acad Sci. 2005;1043:85–96. doi: 10.1196/annals.1333.011. [DOI] [PubMed] [Google Scholar]
- 13.Munanairi A, O'Banion SK, Gamble R, Breuer E, Harris AW, Sandwick RK. Carbohydr Res. 2007;342:2575–2592. doi: 10.1016/j.carres.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margoliash E, Novogrodsky A. Biochim Biophys Acta. 1959;35:130–140. doi: 10.1016/0006-3002(59)90342-7. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZB, Li M, Zhao Y, Xu JX. Protein Pept Lett. 2003;10:247–253. doi: 10.2174/0929866033479013. [DOI] [PubMed] [Google Scholar]
- 16.Kim NH, Jeong MS, Choi SY, Kang JH. Mol Cells. 2006;22:220–227. [PubMed] [Google Scholar]
- 17.Pande J, Myer JP. J Biol Chem. 1980;255:11094–11097. [PubMed] [Google Scholar]
- 18.McIntosh DB, Parrish JC, Wallace CJ. J Biol Chem. 1996;271:18379–18386. doi: 10.1074/jbc.271.31.18379. [DOI] [PubMed] [Google Scholar]
- 19.Rieder R, Bosshard HR. J Biol Chem. 1978;253:6045–6053. [PubMed] [Google Scholar]
- 20.Taborsky G, McCollum K. J Biol Chem. 1979;254:7069–7075. [PubMed] [Google Scholar]
- 21.Ilan Y, Shafferman A, Stein G. J Biol Chem. 1976;251:4336–4345. [PubMed] [Google Scholar]
- 22.Margoliash E, Frohwirt N. Biochem J. 1959;71:570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díaz AN, Sanchez FG, García JAG. Anal Chim Acta. 1996;327:161–165. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.