Abstract
The nutritional condition of fourth instar larvae of the yellow fever mosquito, Aedes aegypti, governs female longevity and egg production, both are key determinants of pathogen transmission. As well, nutrition provisions larval growth and development and attains its greatest pace in the last larval instar in preparation for metamorphosis to an adult. These developmental processes are regulated by a complex endocrine interplay of juvenile hormone, neuropeptides, and ecdysteroids that is nutrition sensitive. We previously determined that feeding for only 24 h post-ecdysis was sufficient for fourth instar Ae. aegypti larvae to reach critical weight and accumulate sufficient nutritional stores to commit to metamorphosis. To understand the genetic basis of metamorphic commitment in Ae. aegypti, we profiled the expression of 16 genes known to be involved in the endocrine and nutritional regulation of insect metamorphosis in two ways. The first set is a developmental profile from the beginning of the fourth instar to early pupae, and the second set is for fourth instars starved or fed for up to 36 h. By comparing the two sets, we found that seven of the genes (AaegCYP302, AaegJHE43357, AaegBrCZ4, AaegCPF1-2, AaegCPR-7, AaegPpl, and AaegSlif) were expressed during metamorphic commitment in fourth instars and in fed but not starved larvae. Based on these results, the seven genes alone or in combination may serve as molecular indicators of nutritional and metamorphic status of fourth instar Ae. aegypti larvae and possibly other mosquito species in field and laboratory studies to gauge sub-lethal effects of novel and traditional cultural or chemical controls.
Keywords: larval-pupal molt, metamorphosis, development, hormone, gene expression, mRNA profiles
1. Introduction
Holometabolous insects emerge from eggs as larvae to feed, and their growth is facilitated by molts into ever-larger exoskeletons. Last instar larvae exhibit the greatest increase in food intake and growth to enable metamorphosis and to deposit nutritional stores for adult use. Two more molts are required to complete metamorphosis, first to the pupal stage where the larval tissues are remodeled into an adult form revealed in the last molt. The processes enabling insect growth and development are regulated through diverse signal pathways activated by juvenile hormone (JH), ecdysteroid hormones (ECD), and neuropeptides. From studies of lepidopteran species, e.g. tobacco hornworm Manduca sexta and silkworm Bombyx mori, it is known that larval and metamorphic molts are mediated by the brain neuropeptide, prothoracicotropic hormone (PTTH), which activates the prothoracic glands (PG) to synthesize and release ECD (Marchal et al., 2010). A rising titer of ECD in the absence of JH commits the fourth instar to the metamorphic molt into the pupal stage (Nijhout, 1994), whereas the presence of JH during this same ECD rise in earlier instars maintained their larval nature after each molt.
These hormones clearly play a major role in regulating molting and metamorphosis, but studies of M. sexta have shown that both food ingestion and accumulation of mass during two phases of the last larval instar are important cues for metamorphic readiness and likely affect JH and ECD secretion. In the first phase, food intake and subsequent growth allow larval M. sexta to reach a critical mass and commit to metamorphosis (Nijhout, 2003). Additional food intake results in continued larval growth, and this second phase has a major impact on the adult stage, especially for females, because extra provisioning results in larger adult body size that is correlated with greater reproductive success (Bronson, 1985; Iyengar and Eisner, 1999; Tu and Tatar, 2003). Studies of the fruit fly, Drosophila melanogaster, contributed to the same conclusions and further suggested that the insulin signaling pathway plays a central role in the control of nutrition-dependent growth (Mirth and Riddiford, 2007). Likewise, the nutritional condition of larval mosquitoes dictates many important adult traits such as body size, emergent nutrient reserves and egg production (Telang and Wells, 2004; Telang et al., 2006).
Similar to the model insects examined, an important physiological determinant of female mosquito size and fertility is the critical mass that larvae must attain during last instar development to commit to metamorphosis. We recently determined for the yellow fever mosquito, Aedes aegypti, that fourth instar larvae must feed for a minimum of 24 hrs to reach the critical mass (2.7–3.2 mg) needed to complete metamorphosis to an adult in standardized rearing conditions (Telang et al., 2007). Within 30 hrs post ecdysis, protein and reserves of glycogen and lipid reach their maximum values in the larvae, as does the hemolymph ECD titer.
Over the course of development in an ever-changing aquatic environment, it is likely that mosquito larvae face periods of food shortage or competition that ultimately affect adult development and provisioning for longevity and reproduction (Metcalfe and Monaghan, 2001; Telang et al., 2006; Reiskind and Lounibos, 2009). In response, mosquito larvae can either accelerate growth to emerge as smaller adults or slow their development. Larval mosquitoes, including Ae. aegypti, can withstand starvation for two weeks (Rasnitsyn and Yasyukevich, 1989; Barrera, 1996; Telang et al., 2007) while retaining their capacity to pupate when food becomes available again. We further examined this response by feeding newly molted fourth instars for different periods of time but sampling them at the same age. Using this design, we determined that a larva’s capacity to metamorphose depends on its nutritional condition, not just its chronological age or ECD titer (Telang et al., 2007). In particular, lipid reserves are a critical determinant, as shown for other insects (Wigglesworth, 1942; Gilpin and McClelland, 1979; Rion and Kawecki, 2007; Ballard et al., 2008).
To gain insights into the molecular basis of nutritional and hormonal regulation of mosquito metamorphosis, we employed this same feeding-restriction design to determine expression profiles for 16 genes possibly related to both aspects of this regulation in fourth instar Ae. aegypti. Some of the genes encode enzymes or proteins known to be directly involved in the regulation of ECD and JH action or affected by their action in model insects and in Ae. aegypti (Cho et al., 1995; Chen et al., 2004; Margam et al., 2006). A set of genes implicated in the regulation of feeding behavior and in nutrient sensing in D. melanogaster (Sarov-Blat et al., 2000; Colombani et al., 2003; Melcher et al., 2007) were also examined in this study. Our use of a feeding-restriction design allowed us to identify metamorphic marker genes that are regulated by the starved or fed status of fourth-instar mosquitoes. Products of such genes are expected to ultimately determine the success of female reproduction and longevity and pathogen transmission. Targeting the expression of these key genes by environmental, chemical, or genetic interference has great promise for mosquito population control and reducing transmission of pathogens, such as dengue virus and filarid worms, by Ae. aegypti.
2. Materials and Methods
2.1 Animals
All stages of Ae. aegypti (UGAL strain) were reared at 27°C, 16 h light: 8 h dark. Larvae were reared in 34 cm × 21 cm × 5 cm metal pans at a density of 200 in 500 ml distilled water and fed a mixture of ground rat chow/lactalbumin/brewer’s yeast (1:1:1 w/w). Under these conditions, individuals of this strain completed larval development in 6 to 7 days and were pupae on day 7 or 8 (hatch day being day 1). Pupae and remaining late fourth instars were collected on day 7. Adults were offered 10% sucrose solution on the third day post-eclosion and provided distilled water all other days. For egg production, females were fed on an anesthetized rat until engorged and allowed to lay eggs on moistened paper towel strips.
2.2 Larval staging and feeding regimens
Under our standard rearing conditions, a majority of Ae. aegypti larvae are late third instars 4 days post-hatching. In the late morning of this day, third instars with darkly tanned head capsules were picked from rearing pans and placed as single larvae in a well containing 1.6 ml distilled water and no food in a 24 well culture plate. The next morning, newly molted fourth instars were transferred and held individually in new wells containing fresh 1.6 ml distilled water. Another group of newly molted fourth instars were set up in feeding or starvation regimens. For the “starved” regimen, fourth instars received no food for 36 h. In the “intermediate” regimen, fourth instars were given food for the first 12 h and then the solution in the well with food was replaced with water alone for 24 h. In the “fed” regimen, fourth instars had access to food for 36 h. These regimens were used because previously we determined that fourth instars fed for 36 h completed metamorphosis and had significantly greater nutrient reserves and ecdysteroid titer at 36 h than those starved or given access to food for 12 h or less, which were developmentally arrested (Telang et al., 2007).
2.3 RNA purification and cDNA synthesis
To examine transcript expression over the course of fourth instar development, individual newly molted larvae were given a 2% solution of larval food, and 12, 24, 36 and 48 h later, larvae were removed and chopped into head, thorax, and abdomen parts for total RNA extraction. Pupae from the same larval cohort were similarly processed at 12 h post ecdysis (PE). The cephalothorax of each pupa was separated into distinct head and thoracic regions with extra care. To examine transcript expression in response to feeding or starvation, fourth instars were set up in three feeding regimens (see subsection 2.2), and only at 36 h later were larvae cut into body regions for total RNA extractions.
In both experiments regarding fourth instar development and feeding regimens, groups of ten larvae were removed from their wells at specific time points and rinsed in fresh water. Heads, thoraces, and abdomens of each larval group were dissected in saline solution (128 mM NaCl, 4.7 mM KCl, and 1.9 CaCl2), pooled in RNAlater (Sigma), incubated at 4°C overnight and stored at -80°C until processed. Total RNA was extracted and treated with DNAase I using the RNeasy mini kit (Qiagen), and RNA integrity was checked on a 1% agarose gel. RNA was quantified using the Nanodrop ND-1000 (Thermo Scientific), and cDNA was synthesized from total RNA (0.3 μg for each body region sample/20 μl final volume) using iScript cDNA kit (BioRad).
2.4 Reverse transcription-polymerase chain reaction (RT-PCR)
For the first phase of our investigation, reverse transcriptase – PCR was used to make two determinations for the genes of interest during the period of fourth instar to early pupal development and in response to the dietary regimens: 1) distribution of transcripts in the different body regions and 2) qualitative changes in transcript abundance. From published gene/transcript sequences or sequences submitted to GenBank, primers were designed using PrimerSelect (Lasergene, DNASTAR; Table 1 for gene/primer sequences). Specific transcript products were amplified from body region cDNA (1 μl of cDNA mixture equivalent to 15 ng total RNA) with gene-specific primers and Titanium Taq DNA polymerase (Clontech) by PCR (total volume 20 μl) under the following conditions: 94°C for 2 min; 35 or 40 cycles of 94°C for 30 sec, anneal temperature for 30 sec (see Table 1 for gene specific Tm), 72°C for 30 sec; 72°C for 7 min. The integrity of cDNA obtained from the body regions of every sample in a cohort was evaluated by PCR amplification of Ae. aegypti actin products (Ibrahim et al., 1996) as above, and if no actin products resulted, the cDNA was not used. PCR mixtures (10 μl vol) were run on a 1% (w/v) agarose/TAE (40 mM Tris/acetate, 2 mM EDTA, pH 8.0) gel, and products were visualized by ethidium bromide stain and stored as digital images (GeneSnap, Syngene or Gel Logic 200, Kodak). All PCR products of the expected size designed for candidate genes were gel purified, cloned, and sequenced to confirm their expected sequence. Results are summarized for 3 different cohorts of similarly staged and treated animals.
Table 1.
Primers used for RT-PCR analysis of transcript expression during fourth instar Aedes aegypti development and in response to feeding regimens.
| Protein/Gene | GenBank gene or EST (ref)a | Forward primer (ref)b | Reverse primer | Amplicon | Tm (°C) |
|---|---|---|---|---|---|
| Actin | U20287 (Ibrahim etal., 1996) | 5′-ACCAACTGGGATGACATGGAGAAG -3′ | 5′-GTAGACAGTTTCGTGGATACCGCA -3′ | 609 BP | 60 |
| Prothoracicotropichormone, PTTH | DV370510.1 | 5′-GTTCATCAACAATGAAGTTAGTGTTC -3′ | 5′-TTGTGTTCCGATCGCATAGC -3′ | 684 bp | 60 |
| 22-hydroxylase, CYP302a1 | AY947549 (Sieglaff et al., 2005) | 5′-TCTTACACTACAGCATTCGCC -3′(Sieglaff et al., 2005) | 5′-CTGGAAGCCCTGATCCGTCGG -3′ | 285 bp | 60 |
| Ecdysteroid receptor EcR-B, | U02021(Cho et al., 1995) | 5′-AGGCCAACGCAAACAGCATTCAG -3′ | 5′-GATCGCCGCACACCAGACACAG -3′ | 273 bp | 60 |
| Juvenile hormone acid methyltransferase JHAMT | DQ409061 | 5′-GCACGCGTTCTTGGGACAG -3′ | 5′-CCGACCGGATTCTCACAGTA -3′ | 371 bp | 50 |
| Juvenile hormone esterase, JHE | XM_001650430(Nene et al., 2007) | 5′-GGTCCAATCAGGCCTTTTTAGTG -3′ | 5′-CGGGAGCGGTTTCGGTGTC -3′ | 381 bp | 65 |
| Methoprene-tolerant protein, Met | AY955097 | 5′-GAACGGGCGACTTTGTGTATCTGA -3′ | 5′-GACGGCCTTTGAACTTGGTGCTAT -3′ | 365 bp | 58 |
| Broad complex isoform Z4, BrCZ4, | AY499540 (Chen et al., 2004) | 5′-CACCGGCACGAGAGCAATAAGACG -3′ | 5′-GGAACGCACGGAAGGAGATGGAG -3′ | 263 bp | 65 |
| Cuticle protein CPR22, | AAGE02020823.1 | 5′-GCCGTGGTCGCTGCCAATTCC -3′ | 5′-GGCACCCTCAGCACGGAAACCA -3′ | 302 bp | 65 |
| Cuticle protein CPF1–2 | AAEL000896 | 5′-CAGTGTCGTACGCCGCTCCA -3′ | 5′-TGTATCATTTGTGGTTTCCGTAGGCAGT -3′ | 595 bp | 65 |
| Cuticle protein CPR-7 | XM_001655238 | 5′-CGGAACGCTCAAGGACGGCAAA -3′ | 5′-TGCAATGGTCAACAACACTCAAAATCCATC -3′ | 217 bp | 53 |
| Hexamerin-1-γ subunit, Hex-1-γ | U86079 (Gordadze et al., 1999) | 5′-GTGCTGGGCCGAACAATACG -3′ | 5′-CGGCAATCGACATCATCATCTTC -3′ | 289 bp | 60 |
| AMP-activated protein kinase α subunit, AMPK-α | AY870330 | 5′-CGGCATTCGGTCCCAGTCTAAAC -3′ | 5′-CATCCCGCCGAACCCAATC -3′ | 286 bp | 60 |
| Glycine cleavage system, H subunit(Pumpless), Ppl, | AY431133 | 5′-TCTGTCCCGGGTTGAGTAGG -3′ | 5′-TCGACCGCGGAGTTCTTCT -3′ | 400 bp | 57 |
| Cation amino acid transporter (Slimfast), Slif | AY654299 (Attardo et al., 2006) | 5′-CAAGCTGGCCCGTGTCCTGA -3′ | 5′-AAAGCGGCAAACTCCCCTATCGTA -3′ | 255 bp | 57 |
| Takeout, To | AF458100 (Bohbot and Vogt, 2005) | 5′-GATTCACCGGCAACCCCACCAA -3′ | 5′-GGATGTCCTGCCAGTTCTCGTTCA -3′ | 330 bp | 57 |
References given with gene or EST ID indicate primers designed to published sequences. Otherwise, primers were designed to sequences submitted to GenBank.
References given with forward primer indicate published forward and reverse primers were used in PCR analyses. Otherwise, primers were designed to either banked or published sequences.
2.5 Quantitative real-time PCR (qPCR)
In the second phase of our investigation, qPCR was used to quantify changes in gene expression in larvae subjected to the feeding manipulations. Only genes showing the most consistent and apparent differences in product amount after RT-PCR of the samples were chosen for qPCR. The same body cDNA samples used for the RT-PCR study (1 μl of cDNA mixture equivalent to 15 ng total RNA) and gene specific primers (250 nM final concentration/10 μl reaction) were added to a master mix containing IQ™ SYBR® Green Supermix (Biorad) and pure water. Primers were designed using PrimerSelect (Lasergene, DNASTAR; See Table 2 for gene/primer sequences). A non-template control (NTC) was included, and each sample body cDNA and NTC reaction had four internal replicates to minimize pipetting differences. Reactions were conducted on a Chromo4 cycler (Biorad) using the program: 95°C for 3 mins; 45 cycles of 95°C for 20 s, anneal temperature for 20 sec (see Table 1 for gene specific Tm), 72°C for 20 s; followed by melt curve analysis. Melt curve analysis was used to confirm amplification of single products in each qPCR that contained cDNA template, and those samples that did not display a product specified by melt curve analysis were excluded from further analysis. Reaction products were collected and analyzed on a 1% (w/v) agarose/TAE (40 mM Tris/acetate, 2 mM EDTA, pH 8.0) gel to confirm product size. The threshold cycle (CT), the cycle number at which each sample yields a detectable fluorescent signal, and PCR efficiency values for all samples were obtained from the Opticon Software (Biorad). We normalized transcript abundance in each sample relative to qPCR expression of ribosomal S7. We chose the well-nourished 36 h fed, 36 h old fourth instar group as the control sample (calibrator). Transcript expression values for the food restricted larvae (0 h fed, 36 hr old and 12 h fed, 36 hr old fourth instar groups) were then reported as an increase or decrease relative to the calibrator (well-nourished 36 h fed, 36 hr old fourth instar group) for each body region using the Pfaffl (2001) method. Specifically, we used the following formula to determine the expression ratio between sample and calibrator: Expression Ratio = (Etarget) ΔCT, target(calibrator-test)/(Eref) ΔCT, ref (calibrator-test), where E is PCR efficiency.
Table 2.
Primers used for qPCR analysis of transcript expression in response to feeding regimens.
| Transcript ID | Forward primer | Reverse primer | Amplicon |
|---|---|---|---|
| Ribosomal S7 (AY380336) | 5′-TCAGTGTACAAGAAGCTGACCGGA -3′ | 5′-TTCCGCGCGCGCTCACTTATTAGATT -3′ | 118 bp |
| JHAMT(DQ409061) | 5′-TGTTGTGCGCAGGATGTCTCTA -3′ | 5′-TTCCGATGTCAAACCCACTACTTC -3′ | 120 bp |
| JHE(XM_001650430) | 5′-AAATCAATGTGCGTCCAGAAGAAC -3′ | 5′-CCCCGCCATGAATGTAAACCA -3′ | 142 bp |
| AMPK-α(AY870330) | 5′-AGCCGCTGGTAAAGATAGGTC -3′ | 5′-TGGCGGTTCAGGATTTTTAC -3′ | 127 bp |
| Pumpless(AY431133) | 5′-CCGCGGTCGAGGAAACAC -3′ | 5′-AACTTAATGGCCGTCGCTCTTGA -3′ | 152 bp |
| Takeout(AF458100) | 5′-ACCCGGTTCCACATGCACTTCTCT -3′ | 5′-GATGGCCGGCTTCAGCTCCTTCAG -3′ | 120 bp |
2.6 Data analyses
For each gene of interest, qPCR expression ratio in relation to larval feeding regimen was analyzed by ANOVA for 3 different cohorts of similarly staged and treated animals and included body region, feeding regimen, and an interaction term in all statistical models. Differences among means are reported as significant based on values of p<0.01. Comparisons between specific groups (36/36 treatment vs 0/36 + 12/36 treatments) were further analyzed using linear contrasts. All data were statistically analyzed using JMP IN (version 6.0.2, SAS Institute Inc.). Least square means (s.e.m.) were obtained from statistical models and illustrated using GraphPad Prism 4.0 (2006; GraphPad Software Inc., San Diego, CA).
3. Results and Discussion
We first used RT-PCR to assess the expression of 16 genes at different times during the fourth instar and early pupal stages of Ae. aegypti (Fig. 1, 2A, 3, 4A). The genes were chosen because their products have known or suggested roles in growth and metamorphosis of D. melanogaster, and their function and expression in other insects is referenced below. The expression pattern of each gene is summarized from a qualitative assessment of transcript abundance based on the presence and density of the PCR products obtained from three mosquito cohorts that were reared and processed identically for RNA extraction, synthesis of body region cDNA, and PCR conditions. By dividing experimental larvae into body regions, we took the first step in determining whether the gene of interest was expressed in a tissue found in all body regions, such as the fat body or epidermis, or only in a tissue found in only one body region, such as the brain in heads. The integrity of all cDNA samples used in this study was verified by the presence of an actin product, and consistently, it was amplified in all body regions of larvae or pupae processed from the same experimental cohort (Fig. 1).
Figure 1.
Expression of genes involved in ECD biosynthesis and signaling during Ae. aegypti fourth instar development and in response to different feeding regimens. Representative images of tissue specific RT-PCR products obtained for one experimental cohort are shown along with actin loading control. H, head; T, thorax; A, abdomen. See Table 1 for gene identification and primers. Only AeaCYP302a1 showed differential expression in response to fourth instar feeding.
Figure 2.
(A) Expression of genes involved in the regulation of JH levels during Ae.aegypti fourth instar development and in response to different feeding regimens. Representative images of RT-PCR products obtained for one experimental cohort are shown. H, head; T, thorax; A, abdomen. See Table 1 for gene identification and primers. See Figure 1 for image of actin loading control. Both AeaJHAMT and AeaJHE showed differential expression in response to fourth instar feeding. qPCR analysis of AeaJHAMT (B) and AeaJHE (C) transcripts in head (white bars), thorax (gray bars) and abdomen (striped bars) of fourth instar larvae starved for 36 h (0/36), fed 12 h/starved 24 h (12/36), or fed 36 h (36/36). Bars represent normalized levels of transcripts in the body regions with fourth instars fed for 36 h chosen as calibrator. Its ratio of 1.0 indicated by dashed line. AeaJHAMT transcripts were significantly greater in thoraces of starved 36 h PE larvae (p<0.0001, from a linear contrast), whereas AeaJHE43357 expression was significantly greater in the three body regions of fed instars at 36 h PE (p<0.0001, from a linear contrast).
Figure 3.
Expression of genes involved in metamorphic commitment during Ae.aegypti fourth instar development and in response to different feeding regimens. Representative images of RT-PCR products obtained for one experimental cohort are shown. H, head; T, thorax; A, abdomen. See Table 1 for gene identification and primers. See Figure 1 for image of actin loading control. AeaBrCZ4, AeaCPF1-2, and AeaCPR-7 showed differential expression in response to fourth instar feeding.
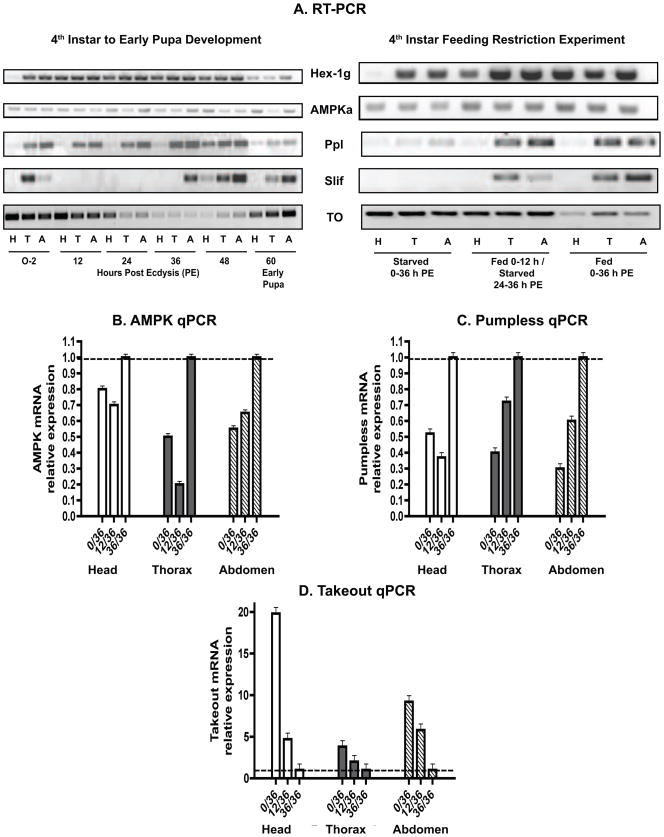
Figure 4.
(A) Expression of genes involved in nutrient sensing and feeding behavior during Ae.aegypti fourth instar development and in response to different feeding regimens. Representative images of RT-PCR products obtained for one experimental cohort are shown. H, head; T, thorax; A, abdomen. See Table 1 for gene identification and primers. See Figure 1 for image of actin loading control. AeaPpl, AeaSlif, and AeaTo showed differential expression in response to fourth instar feeding. qPCR analysis of AeaAMPK-α (B), AeaPpl (C), and AeaTo (D) transcripts in head (white bars), thorax (gray bars) and abdomen (striped bars) of fourth instar larvae starved for 36 h (0/36), fed 12 h/starved 24 h (12/36), or fed 36 h (36/36). Bars represent normalized levels of transcripts in the body regions with fourth instars fed for 36 h chosen as calibrator. Its ratio of 1.0 indicated by dashed line. Transcripts of AeaAMPK-α and AeaPpl were significantly higher in all body regions of 36 h fed larvae (p<0.0001, from a linear contrast in all tissues), whereas expression of AeaTo was significantly greater in the head (p<0.0001, from a linear contrast) and abdomen (p<0.0001, from a linear contrast) of starved 36 h fourth instars.
Next, we used the same approach to qualitatively assess which of the 16 genes is regulated directly by the ingestion of nutrients. By starving fourth instars for 36 h or feeding them for 12 h only of 36 h and processing as above for RT-PCR, we obtained an expression profile for each gene that revealed its response to feeding, as discussed below (Fig. 1, 2A, 3, 4A). This feeding regimen was chosen because we found that fourth instars fed for 36 h post ecdysis (PE) all completed metamorphosis, and ones starved for 36 h do not, nor do ones fed for 12 h PE (starved for the next 24 h) (Telang et al., 2007). Feeding fourth instars for longer than 12 h increased the percentage of larvae that pupated, thus suggesting a 12 h feeding period was a threshold for metamorphic commitment. Finally, we used qPCR to perform a quantitative and statistical assessment of transcript expression for those genes that appeared to be good candidates for metamorphic markers that may also be cued into the nutritional condition of fourth instars (Fig. 2B, 2C, 4B-D).
3.1a. Expression of genes encoding proteins involved in ecdysteroid biosynthesis and signaling
Previously, we reported that tissues in the thorax and abdomen, such as epidermis and fat body, are the source of circulating ECDs in Ae. aegypti larvae and not the PG, as in other insects (Jenkins et al., 1992; Telang et al., 2007). It is not known how ECD secretion by such tissues is regulated, but the neurohormone, PTTH, may have a role, since a homolog gene exists in mosquitoes (Riehle et al., 2002; Predel et al., 2010). For this reason, we examined AaegPTTH expression and found that its product was consistently present in the heads of fourth instar larvae during development whether starved or fed (Fig. 1). In other insects, PTTH is secreted by only a few neurosecretory cells in the brain (Marchal et al., 2010), thus the head-specific AaegPTTH expression suggests that cells in the brain also are the source in mosquito larvae.
Cytochrome P450 enzymes (CYPs) are involved in ECD biosynthesis (Huang et al., 2008), and a 22 hydroxylase, CYP302a1, is a terminal step in this pathway. In female Ae. aegypti, the gene encoding this enzyme, AaegCYP302a1, is highly expressed in ovaries, the source of ECDs in blood-fed females (Sieglaff et al., 2005). In fourth instar larvae, we found that its transcription in all body regions increased by 12 h post ecdysis (PE), which precedes the rise in ECD titer (Telang et al., 2007), and remained elevated up to 36 h PE (Fig 1), when the titer falls, suggesting the translated product is an important component of ECD biosynthesis. Expression in all body regions accords with ECD production by tissues common to all body regions, including the head, as described above.
In the feeding-restriction experiment, AaegCYP302a1 expression was almost undetectable in larvae starved for 36 h, but its expression was greatly increased in larvae fed 12 h or 36 h (Fig. 1). Production of ECD in fourth instar larvae similarly was responsive to feeding, as demonstrated in our prior study. Bodies of larvae fed 12 h or 36 h secreted three and four fold greater amounts of ECD in vitro, respectively, than the bodies of starved larvae (Telang et al., 2007). Together, these results suggest that ECD production in larvae may depend on the feeding-induced expression of at least one CYP involved in ECD biosynthesis.
The commitment to metamorphosis is activated by ECD, 20-hydroxyecdysone, through its binding to a heterodimer formed by the ecdysteroid receptor (EcR) and a related nuclear hormone receptor, ultraspiracle (USP). This complex regulates a cascade of transcription factors that affect gene expression during the larval-pupal transition in whole animals (Lan and Grier, 2004; Margam et al., 2006) and the midgut in Ae. aegypti (Nishiura et al., 2005; Wu et al., 2006; Parthasarathy and Palli, 2007). Two forms of the EcR and USP are known for Ae. aegypti. We chose AaegEcR-B as a marker for ECD signaling because its normalized expression, and that of AaegUSP-A, greatly increased only during the period 39 – 48 h PE and was high in early pupae (Margam et al., 2006).
In our study, AaegEcR-B expression was elevated in all body regions over the same period, 36 to 48 h PE (Fig. 1), which coincides with a falling ECD titer. As expected, AaegEcR-B products were present in all body regions, where it would be localized in the epidermis and other tissues remodeled during metamorphosis. Results from the feeding-restriction experiment showed that AaegEcR-B expression was similar in the bodies of starved and fed larvae at 36 h PE, but appeared to increase in larvae fed only 12 h (Fig. 1), which was surprising.
3.1b. Expression of genes encoding proteins involved in JH biosynthesis and signaling
The role of JH in mosquito larval development and metamorphosis remains undefined some 30 years after the development of a JH analog, methoprene, and its widespread use to control mosquito populations (Henrick, 2007). Presumably, JH and ECD coordinately regulate metamorphosis in mosquitoes, as in other insects (reviewed in Riddiford, 2007; Nijhout and Reed, 2008). We examined the expression of three genes presumably playing a role in JH biosynthesis, titer, and signaling in fourth instar Ae. aegypti: JH acid methyltransferase (JHAMT), a JH-specific esterase (JHE), and a putative JH receptor, respectively.
The corpora allata (CA) are the source of JH III in mosquito adults (Li et al., 2003) and presumably larvae, although the only report is for the CA of Toxorhynchites brevipalpus larvae in a study that focused on the ring gland of D. melanogaster (Richard et al., 1989). JHAMT metabolizes JH acid at the final step of JH biosynthesis (Shinoda and Itoyama, 2003), and its expression is localized in the CA of female Ae. aegpyti (Mayoral et al., 2009). This study also reported that its transcript levels were high in early fourth instar and absent in pupae. The same trend was noted in our study (Fig. 2A). In early fourth instars, expression was predominantly in heads and less so in thoraces, which was surprising, because the CA are located dorsally at the beginning of the thorax. This may be a result of the CA being drawn into the head/neck region as it was stretched to sever the head for RNA extraction.
In the feeding-restriction experiment, AaegJHAMT expression was induced in larvae starved 36 h post ecdysis (PE) but absent in those fed for the same time, with an intermediate expression in larvae of the same age but fed only for the first 12 h PE (Fig. 2A). As confirmed by qPCR analysis, AaegJHAMT transcripts were significantly greater in starved 36 h PE larvae (p<0.0001, from a linear contrast), and clearly localized in the thoraces (Fig. 2B), indicating JH biosynthesis occurs in starved larvae, as reported for the CA from starved M. sexta larvae (Lee and Horodyski, 2002). Feeding for longer than 12 h appears to block expression of this enzyme, suggesting JH biosynthesis likely declines prior to metamorphic commitment in fourth instar larvae.
In mosquitoes as in other insects, JHE metabolizes circulating JH at critical periods during development (Lassiter et al., 1995), and of the three JHEs characterized for mosquitoes, only two are expressed in larvae (EAT43357 and EAT43353; Bai et al., 2007). Their study and ours showed that AaegJHE43357 expression occurred only 36 – 48 h PE in all body regions of fourth instars (Fig. 2A), which is consistent with its reported expression in midgut and fat body. This expression pattern appears to be the same as that of AaegEcR-B, which suggests JH clearance is a necessary prerequisite for ECD signaling to set the commitment to metamorphosis, as demonstrated for lepidopteran larvae (Cymborowski et al., 1982; Browder et al., 2001). In the feeding-restriction study, AaegJHE43357 expression was significantly greater in fed instars at 36 h PE (Fig. 2C, p<0.0001, from a linear contrast in all tissues), than in those fed 12 h PE and undetectable in starved larvae. Feeding clearly has a positive effect on AaegJHE43357 expression, thus suggesting that this enzyme plays a role in JH clearance as JH biosynthesis ceases.
A key component of the JH signaling pathway is Met protein (methoprene tolerant gene in Drosophila), an insect homolog of the aryl hydrocarbon receptor (Willis, 2007). Its expression in the beetle, Tribolium castaneum, is required for the anti-metamorphic effect of JH, suggesting it may be the long sought JH receptor (Konopova and Jindra, 2007). That study identified a Met homolog in Ae. aegypti, which we found to be present in all body regions of fourth instars during development, but its expression was not affected by feeding (Fig. 2A). Similarly, TcMet transcripts were present throughout T. castaneum development (Konopova and Jindra, 2007; Parthasarathy et al., 2008). In D. melanogaster, Met products were present throughout all stages but showed tissue specificity (Pursley et al., 2000).
3.2 Expression of genes encoding proteins involved in metamorphosis commitment
Few genes are known to be specifically expressed in the last instar during the commitment to metamorphosis. One such gene family is the Broad complex (BrC), which encodes isoforms of a transcription factor, and studies of D. melanogaster and M. sexta showed that BrC expression serves as a molecular marker for pupal commitment (Riddiford et al., 2003). In Ae. aegypti, expression of four BrC isoforms (Z1–4) was first characterized in females, and the isoforms have different modulatory effects on ECD induction of egg maturation (Chen et al., 2004; Zhu et al., 2007). We examined BrCZ4 because its expression was the highest in Ae. aegypti females and fourth instars (Chen et al., 2004; Wu et al., 2006). AaegBrCZ4 products were clearly evident at 24 h and 36 h post ecdysis (PE) in all body regions of the fourth instar (Fig. 3), likely reflecting its expression in fat body, epidermis, and midgut, as previously reported (Wu et al., 2006; Parthasarathy and Palli, 2007). The timing of its expression is coincident with the ECD peak and AaegJHE43357 expression, but before and after this period, expression was essentially undetectable (Fig. 3). In the feeding-restriction experiment, AaegBrCZ4 expression was almost undetectable in larvae starved for 36 h, but its expression greatly increased in larvae fed 12 h or 36 h (Fig. 3), thus indicating it is a nutrition-dependent marker for metamorphic commitment.
Cuticle proteins (CP) form the exoskeleton and other tissue-specific internal matrices throughout insect development, and expression of some CP genes is stage specific and responsive to ECD signaling. A large set of CP genes and their expression patterns are now determined for the mosquito, Anopheles gambiae (Togawa et al., 2008; Cornman and Willis, 2009). Three Ae. aegypti CP genes were chosen for this study based on the temporal specific expression of their homologs in fourth instar An. gambiae larvae: AgamCPR22, early marker; AgamCPF1-2, late marker; and AgamCPR-7, early marker (Togawa et al., 2008). Expression of each AaegCP gene generally matched that of the An. gambiae homolog during fourth instar development, and products for each gene were observed in all body regions (Fig. 3). By 36 h PE, expression of the early marker, AaegCPR22, was greatly reduced, and the late marker, AaegCPF1-2, was elevated. The other early marker, AaegCPR-7, shifted from body-wide expression to exclusive expression in the abdomen late in the instar and in early pupae, thus offering an abdomen specific marker for metamorphosis commitment. Although the rise or fall in expression differed for the three AaegCP genes, it coincided with the ECD titer peak between 24 and 36 h PE, thus suggesting a response to ECD signaling. As well, these changes were responsive to feeding (Fig 3). In particular, expression of the late marker, AaegCPF1-2, was clearly greater in larvae fed for 36 h than in ones fed for 12 h or starved for 36 h. The absence of products in 12 h fed larvae suggests expression of this gene may be more responsive to feeding or nutrient intake than to ECD signaling, because the ECD titer in these larvae is significantly greater than that in starved ones (Telang et al., 2007).
3.3 Expression of genes encoding proteins involved in nutrient sensing and feeding behavior
Sufficient nutritional stores must be acquired in the fourth instar to initiate the first metamorphic molt in Ae. aegypti (Telang et al., 2007), so we examined two genes that likely reflect nutritional condition. The first one encodes a hexamerin, and isoforms of this protein are secreted by the fat body into the hemolymph of larvae and later taken up by the fat body for storage to be used during metamorphosis (Telfer and Kunkel, 1991; Burmester and Scheller, 1999). Accumulation of hexamerins varies in response to larval nutritional condition in the non-blood feeding autogenous mosquito Ochlerotatus atropalpus (Telang and Wells, 2004), and two hexamerins have been identified in Ae. aegypti larvae (Gordadze et al., 1999; Zakharkin et al., 2001). We examined expression of AaegHex-1γ, which encodes a subunit of the Hex-1 protein (Gordadze et al., 1999). During fourth instar development, AaegHex-1γ products were present in all body regions, but its expression was reduced in early pupae (Fig. 4A). This pattern of AaegHex-1γ expression is similar to that previously reported for Ae. aegypti (Gordadze et al., 1999) and Oc. atropalpus (Zakharkin et al., 2001). The feeding-restriction experiment did not show a notable effect of starvation or feeding on AaegHex-1γexpression, which was surprising (Fig. 4A).
The second gene of interest encodes the catalytic subunit of AMP-activated protein kinase (AMPK), AaegAMPK-α, which together with the regulatory β and α subunits forms a functional AMPK complex. Multiple genes encode each subunit in mammals, but only single gene homologues have been identified in D. melanogaster (Pan and Hardie, 2002) and in databases for Ae. aegypti. AMPK is considered to be a master regulator that switches off ATP-consuming anabolic pathways, such as protein, fatty acid and glycogen synthesis and activates ATP-producing catabolic processes such as fatty acid oxidation and glycolysis (Hardie, 2007). Therefore, activation of AMPK expression would promote utilization of nutrient reserves to provide ATP, whereas its suppression would promote accumulation of reserves.
During fourth instar development, AaegAMPK-α products were present in all body regions (Fig. 4A), thus reflecting expression in tissues common to all body regions, such as the fat body. The presence of AaegAMPK-α transcripts throughout fourth instar development and early pupal stage (Fig. 4A) matched that of SNF4Aγ protein, the Drosophila orthologue of the mammalian AMPK γ subunit. SNF4Aγ protein was localized in larval fat body and highly expressed in later larval and pupal stages (Lippai et al., 2008). The feeding-restriction experiment did not show a notable effect of starvation or feeding on AaegAMPK-α expression, as determined by RT-PCR (Fig. 4A). By qPCR analysis, transcripts of AaegAMPK-α were significantly higher in all body regions of 36 h fed larvae (p<0.0001, from a linear contrast in all tissues) than in starved or 12 h fed larvae (Fig 4B). After 36 h of feeding, fourth instars had attained critical weight, accumulated sufficient metabolic reserves (protein, glycogen, and lipid), and committed to metamorphosis (Telang et al., 2007), including tissue remodeling (Wu et al., 2006). Thus, elevated AaegAMPK-α expression may indicate suppression of anabolic pathways or involvement in remodeling, as suggested for Drosophila SNF4Aγ (Lippai et al., 2008).
Three genes, pumpless (Ppl), slimfast (Slif), and takeout (To), implicated in the regulation of feeding behavior and nutrient sensing in D. melanogaster were also examined in this study. Ppl-deficient larvae do not feed or grow yet they do not show normal physiological starvation responses such as upregulation of genes involved in gluconeogenesis or lipolysis (Melcher et al., 2007). Ppl is expressed specifically in larval fat body and encodes a protein with high homology to protein H subunit of the glycine cleavage system involved in glycine catabolism in vertebrates (Zinke et al., 1999; Melcher et al., 2007). Feeding wild-type larvae diets high in glycine and other amino acids also caused cessation of feeding and growth (Melcher et al., 2007). Thus ppl is thought to be an amino acid-dependent signal from the fat body of D. melanogaster that acts on the brain to suppress food intake.
We observed AaegPpl products specifically in the thorax and abdomen throughout the fourth instar (Fig. 4A). In the feeding-restriction experiment, AaegPpl showed little to no expression in starved larvae and was evident in fed 12 h and 36 h larvae (Fig. 4A). In the qPCR analysis, AaegPpl expression was evident in heads, and it was significantly reduced in all body regions of starved and fed 12 h, 36 h old fourth instars in comparison to its upregulation in fed 36 h larvae (Fig. 4C; p<0.0001, from a linear contrast in all tissues). Similarly, ppl expression was reduced in the fat body of starved D. melanogaster larvae (Zinke et al., 1999). Although starvation suppressed Ppl expression in both dipterans, its putative role in the suppression of food intake is not supported by its expression in early fourth instar Ae. aegypti, which are actively feeding.
Slimfast encodes a cation amino acid transporter, and down regulation of its expression in the fat body of D. melanogaster resulted in larval growth defects that mimicked effects induced by amino acid starvation or inactivation of Target of Rapamycin (TOR) signaling (Colombani et al., 2003). Its homolog in Ae. aegypti was shown to be highly expressed in newly eclosed females and required for amino acid stimulation of vitellogenin expression in isolated fat body (Attardo et al., 2006).
We observed AaegSlif products predominantly in the thorax and abdomen only in newly eclosed and fed late fourth instars (metamorphosis-committed, 36 – 48 h post ecdysis) and newly eclosed (early) pupae (Fig. 4A). This tissue or body region specific expression was similar to that observed in the AaegPpl profile. AaegSlif was not expressed in larvae starved for 36 h (Fig. 4A). This pattern is similar to its expression profile in female Ae. aegypti (Attardo et al., 2006) in that its expression was highest in newly eclosed females, low in older non-blood fed ones, and did not increase even in blood fed females until 48 h post ingestion, when blood digestion is complete. In all instances, low or no AaegSlif expression was evident when extracellular amino acid levels presumably were low. Together these expression patterns suggest that the AaegSlif transporter may act as a sensor of intracellular amino acid levels in fat body for TOR signaling and regulation of development and reproduction in insects. Other amino acid transporters, such as AaegiCAT2 (Attardo et al., 2006), may serve as sensors of extracellular levels and further inform TOR signaling (Hundal and Taylor, 2009).
Takeout is thought to be a member of a novel gene family found only in insects (So et al., 2000), but the function of its protein is not known. Expression of To in D. melanogaster is linked to feeding regulation, dependent on circadian control, and induced by starvation (Sarov-Blat et al., 2000). During fourth instar development, AaegTo products were evident in all body regions of newly molted and 12 h larvae but reduced in 24 to 48 h old larvae, and appeared again in early pupae (Fig. 4A). In the feeding-restriction experiment, AaegTo expression was much higher in starved and 12 h fed larvae than in 36 h fed larvae (Fig. 4A). This pattern was notably different than that obtained for AaegPpl and AaegSlif. AaegTo expression early in the fourth instar and in the early pupae may reflect an unsatiated state, which is shared with the starved and underfed larvae. Our qPCR assays revealed that the expression of AaegTo was significantly upregulated in the head (p<0.0001, from a linear contrast) and abdomen (p<0.0001, from a linear contrast) of starved 36 h fourth instars than in 12 h or 36 h fed larvae (Fig. 4D). Differences were less apparent in thoraces (p=0.06, from a linear contrast).
High levels of To expression in the gut, crop, and heads of D. melanogaster, specifically in the antennae and fat body above the brain, suggest it has an important role in chemosensory and nutritional signaling in response to food (Sarov-Blat et al., 2000; Bohbot and Vogt, 2005; Meunier et al., 2007). Presence of AaegTo transcripts in both the head and abdomen of mosquito larvae suggests it may have a similar role, and its expression in the CA of female Ae. aegypti (Noriega et al., 2006) points to a role in coordinating feeding and JH biosynthesis.
4. Concluding remarks
The purpose of this study was to identify marker genes that show increased expression in fed and metamorphosis-committed Ae. aegypti fourth instar larvae. We chose 16 genes known to be involved in or affected by the endocrine and nutritional regulation of metamorphosis in D. melanogaster and other insects. By comparing the expression of each gene through the fourth instar and early pupal stages to that of fourth instars starved or fed for 36 h, we identified seven genes that were expressed during the time of metamorphic commitment and responsive to feeding: AaegCYP302, AaegJHE43357, AaegBrCZ4, AaegCPF1-2, AaegCPR-7, AaegPpl, and AaegSlif. These genes alone or in combination may serve as molecular indicators of nutritional and metamorphic status of fourth instar Ae. aegypti larvae and possibly other mosquito species in field and laboratory studies to gauge sub-lethal effects of novel and traditional cultural or chemical controls.
The nutritional content and density of mosquito larvae in temporary aquatic habitats are two major variables that directly affect the body size, teneral reserves, egg production, and longevity of females (Juliano and Stoffregan, 1994; Barrera, 1996; Juliano, 1998; Daugherty et al., 2000; Braks et al., 2004; Alto et al., 2005; Alto et al., 2008a; Murrell and Juliano, 2008; Reiskind and Lounibos, 2009). Recent studies have indicated that stressors, such as larval crowding, result in small-sized females, presumably with reduced energy reserves, that may be more competent vectors for arboviruses (Alto et al., 2005; Alto et al., 2008a; Alto et al., 2008b). These variables influence the time a mosquito larva can feed during each instar and thus the acquisition of nutrient reserves needed for growth and molting. This is especially critical in the fourth instar, when sufficient reserves must be acquired to sustain metamorphosis to the adult stage and provision female survival until a sugar or blood meal can be taken (Telang et al., 2007).
Our findings have also contributed to our ongoing goal of constructing a conceptual model for the nutritional and hormonal regulation of mosquito metamorphosis. How information about nutrient reserves is conveyed from the fat body to the brain, which then secretes neuropeptides to regulate JH biosynthesis and ECD production, is still unresolved for insect metamorphosis in general. Studies of D. melanogaster provide important evidence that the insulin signaling pathway, activated by insulin-like peptides, and the TOR pathway are important regulators of nutritional allocation, growth, and development (Mirth and Riddiford, 2007; Zhang et al., 2009). Insulin signaling to some extent appears to regulate JH and ECD secretion and action (Colombani et al., 2005; Flatt et al., 2005; Wu and Brown, 2006). Future studies based on our short-term assay for the effects of feeding or starvation on mosquito metamorphosis may provide insight into which nutrients are key stimulators of the endocrine and gene expression cascade required for metamorphosis.
Research Highlights.
Expression profiles for 16 genes in mosquito larvae reveal metamorphic markers.
Feeding induces AaegCYP302a1and AaegJHE43357expression and alters endocrine regulation.
Feeding induces expression of AaegBrCZ4, a marker of metamorphic commitment.
Feeding induced expression of AaegPpl and AaegSlif suggests a role for nutrient sensing.
Acknowledgments
The authors would like to thank Judy Willis and Toru Togawa for assistance regarding cuticle protein gene sequences and Danny Fendley for technical assistance. This collaborative work was supported by funds from the Arts & Science Dean’s Office to A.T. and NIH grant AI33108 to M.R.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2008a;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. American Journal of Tropical Medicine and Hygiene. 2008b;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- Attardo G, Hansen IA, Shiao SH, Raikhel AS. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. Journal of Experimental Biology. 2006;209:3071–3078. doi: 10.1242/jeb.02349. [DOI] [PubMed] [Google Scholar]
- Bai H, Ramaseshadri P, Palli SR. Identification and characterization of juvenile hormone esterase gene from the yellow fever mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 2007;37:829–837. doi: 10.1016/j.ibmb.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO, Melvin RG, Simpson SJ. Starvation resistance is positively correlated with body lipid proportion in five wild caught Drosophila simulans populations. Journal of Insect Physiology. 2008;54:1371–1376. doi: 10.1016/j.jinsphys.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:117–127. [Google Scholar]
- Bohbot J, Vogt RG. Antennal expressed genes of the yellow fever mosquito (Aedes aegypti L.); characterization of odorant-binding protein 10 and takeout. Insect Biochemistry and Molecular Biology. 2005;35:961–979. doi: 10.1016/j.ibmb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lounibos LP, Lourenco-De-Oliveira R, Juliano SA. Interspecific competition Bbetween two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Annals of the Entomological Society of America. 2004;97:130–139. [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biology of Reproduction. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Browder MH, D’Amico LJ, Nijhout HF. The role of low levels of juvenile hormone esterase in the metamorphosis of Manduca sexta. Journal of Insect Science. 2001 insectscience.org/1.11. [PMC free article] [PubMed] [Google Scholar]
- Burmester T, Scheller K. Ligands and Receptors: Common Theme in Insect Storage Protein Transport. Naturwissenschaften. 1999;86:468–474. doi: 10.1007/s001140050656. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu J, Sun G, Raikhel AS. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti. Journal of Molecular Endocrinology. 2004;33:743–761. doi: 10.1677/jme.1.01531. [DOI] [PubMed] [Google Scholar]
- Cho WL, Kapitskaya MZ, Raikhel AS. Mosquito ecdysteroid receptor: analysis of the cDNA and expression during vitellogenesis. Insect Biochemistry and Molecular Biology. 1995;25:19–27. doi: 10.1016/0965-1748(94)00045-j. [DOI] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic Actions of Ecdysone and Insulins Determine Final Size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Cornman RS, Willis JH. Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Molecular Biology. 2009;18:607–622. doi: 10.1111/j.1365-2583.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cymborowski B, Bogus M, Beckage NE, Williams CM, Riddiford LM. Juvenile hormone titers and metabolism during starvation-induced supernumerary larval moulting of the tobacco hornworm, Manduca sexta (L.) Journal of Insect Physiology. 1982;28:129–135. [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate Carcasses as a Resource for Competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Gilpin ME, McClelland GAH. Systems analysis of the yellow fever mosquito Aedes aegypti. Fortschritte Zoology. 1979;25:355–388. [PubMed] [Google Scholar]
- Gordadze AV, Korochkina SE, Zakharkin SO, Benes H. Molecular cloning and expression of two hexamerin cDNAs from the mosquito, Aedes aegypti. Insect Molecular Biology. 1999;8:55–66. doi: 10.1046/j.1365-2583.1999.810055.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Reviews: Molecular Cell Biology. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Henrick CA. Methoprene. Journal of the American Mosquito Control Association. 2007;23:225–239. doi: 10.2987/8756-971X(2007)23[225:M]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. Journal of Genetics and Genomics. 2008;35:1–10. doi: 10.1016/S1673-8527(08)60001-6. [DOI] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. American Journal of Physiology: Endocrinology and Metabolism. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MS, Eisinger SW, Scott AL. Muscle actin gene from Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1996;33:955–962. doi: 10.1093/jmedent/33.6.955. [DOI] [PubMed] [Google Scholar]
- Iyengar VK, Eisner T. Heritability of body mass, a sexually selected trait, in an arctiid moth (Utetheisa ornatrix) Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9169–9171. doi: 10.1073/pnas.96.16.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SP, Brown MR, Lea AO. Inactive prothoracic glands in larvae and pupae of Aedes aegypti: ecdysteroid release by tissues in the thorax and abdomen. Insect Biochemistry and Molecular Biology. 1992;22:553–559. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Stoffregan TL. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oecologia. 1994;97:369–376. doi: 10.1007/BF00317327. [DOI] [PubMed] [Google Scholar]
- Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proceedings of the National Academy of Sciences, USA. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, Grier CA. Critical period for pupal commitment in the yellow fever mosquito, Aedes aegypti. Journal of Insect Physiology. 2004;50:667–676. doi: 10.1016/j.jinsphys.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Lassiter MT, Apperson CS, Roe RM. Juvenile hormone metabolism during the fourth stadium and pupal stage of the Southern House mosquito, Culex quinquefasciatus Say. Journal of Insect Physiology. 1995;41:869–876. [Google Scholar]
- Lee KY, Horodyski FM. Restriction of nutrient intake results in the increase of a specific Manduca sexta allatotropin (Manse-AT) mRNA in the larval nerve cord. Peptides. 2002;23:653–661. doi: 10.1016/s0196-9781(01)00659-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochemistry and Molecular Biology. 2003;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Lippai M, Csikos G, Maroy P, Lukacsovich T, Juhasz G, Sass M. SNF4Aγ, the Drosophila AMPK γ subunit is required for regulation of developmental and stress-induced autophagy. Autophagy. 2008;4:476–486. doi: 10.4161/auto.5719. [DOI] [PubMed] [Google Scholar]
- Marchal E, Vandersmissen HP, Badisco L, Van de Velde S, Verlinden H, Iga M, Van Wielendaele P, Huybrechts R, Simonet G, Smagghe G, Broeck JV. Control of ecdysteroidogenesis in prothoracic glands of insects: A review. Peptides. 2010;31:506–519. doi: 10.1016/j.peptides.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Margam VM, Gelman DB, Palli SR. Ecdysteroid titers and developmental expression of ecdysteroid-regulated genes during metamorphosis of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae) Journal of Insect Physiology. 2006;52:1–11. doi: 10.1016/j.jinsphys.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mayoral JG, Nouzova M, Yoshiyama M, Shinoda T, Hernandez-Martinez S, Dolghih E, Turjanski AG, Roitberg AE, Priestap H, Perez M, Mackenzie L, Li Y, Noriega FG. Molecular and functional characterization of a juvenile hormone acid methyltransferase expressed in the corpora allata of mosquitoes. Insect Biochemistry and Molecular Biology. 2009;39:31–37. doi: 10.1016/j.ibmb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. Journal of Endocrinology. 2007;192:467–472. doi: 10.1677/JOE-06-0066. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends in Ecology & Evolution. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Meunier N, Belgacem YH, Martin JR. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. Journal of Experimental Biology. 2007;210:1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu Z, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF. Insect Hormones. Princeton University Press; Princeton: 1994. [Google Scholar]
- Nijhout HF. The control of body size in insects. Developmental Biology. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Reed MC. A mathematical model for the regulation of juvenile hormone titers. Journal of Insect Physiology. 2008;54:255–264. doi: 10.1016/j.jinsphys.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Nishiura JT, Ray K, Murray J. Expression of nuclear receptor-transcription factor genes during Aedes aegypti midgut metamorphosis and the effect of methoprene on expression. Insect Biochemistry and Molecular Biology. 2005;35:561–573. doi: 10.1016/j.ibmb.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R. Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochemistry and Molecular Biology. 2006;36:366–374. doi: 10.1016/j.ibmb.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochemical Journal. 2002;367:179–186. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy R, Palli SR. Stage- and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti. Journal of Insect Physiology. 2007;53:216–229. doi: 10.1016/j.jinsphys.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Tan A, Palli SR. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval–pupal metamorphosis. Mechanisms of Development. 2008;125:601–616. doi: 10.1016/j.mod.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predel R, Neupert S, Garczynski SF, Crim JW, Brown MR, Russell WK, Kahnt J, Russell DH, Nachman RJ. Neuropeptidomics of the mosquito Aedes aegypti. Journal of Proteome Research. 2010;9:2006–2015. doi: 10.1021/pr901187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursley S, Ashok M, Wilson TG. Intracellular localization and tissue specificity of the Methoprene-tolerant (Met) gene product in Drosophila melanogaster. Insect Biochemistry and Molecular Biology. 2000;30:839–845. doi: 10.1016/s0965-1748(00)00056-4. [DOI] [PubMed] [Google Scholar]
- Rasnitsyn SP, Yasyukevich VV. On the ability of mosquito larvae (Diptera, Culicidae) to endure starvation. Entomological Review. 1989;68:143–151. [Google Scholar]
- Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Medical and Veterinary Entomology. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DS, Applebaum SW, Sliter TJ, Baker FC, Schooley DA, Reuter CC, Henrich VC, Gilbert LI. Juvenile hormone bisepoxide biosynthesis in vitro by the ring gland of Drosophila melanogaster: A putative juvenile hormone in the higher Diptera. Proceedings of the National Academy of Sciences, USA. 1989;86:1421–1425. doi: 10.1073/pnas.86.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM. Juvenile hormone action: A 2007 perspective. Journal of Insect Physiology. 2007;54:895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Hiruma K, Zhou X, Nelson CA. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochemistry and Molecular Biology. 2003;33:1327–1338. doi: 10.1016/j.ibmb.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298:172–175. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- Rion S, Kawecki TJ. Evolutionary biology of starvation resistance: what we have learned from Drosophila. Journal of Evolutionary Biology. 2007;20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 2000;101:647–656. doi: 10.1016/s0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Shinoda T, Itoyama K. Juvenile Hormone Acid Methyltransferase: A Key Regulatory Enzyme for Insect Metamorphosis. Proceedings of the National Academy of Sciences, USA. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieglaff DH, Duncan KA, Brown MR. Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 2005;35:471–490. doi: 10.1016/j.ibmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- So WV, Sarov-Blat L, Kotarski CK, McDonald MJ, Allada R, Rosbash M. takeout, a novel Drosophila gene under circadian clock transcriptional regulation. Molecular and Cellular Biology. 2000;20:6935–6944. doi: 10.1128/mcb.20.18.6935-6944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang A, Frame L, Brown MR. Larval feeding duration affects ecdysteroid levels and nutritional reserves regulating pupal commitment in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae) The Journal of Experimental Biology. 2007;210:854–864. doi: 10.1242/jeb.02715. [DOI] [PubMed] [Google Scholar]
- Telang A, Li Y, Noriega FG, Brown MR. Effects of larval nutrition on the endocrinology of mosquito egg development. The Journal of Experimental Biology. 2006;209:645–655. doi: 10.1242/jeb.02026. [DOI] [PubMed] [Google Scholar]
- Telang A, Wells MA. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. Journal of Insect Physiology. 2004;50:677–685. doi: 10.1016/j.jinsphys.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Telfer WH, Kunkel JG. The function and evolution of insect storage hexamers. Annual Review of Entomology. 1991;36:205–228. doi: 10.1146/annurev.en.36.010191.001225. [DOI] [PubMed] [Google Scholar]
- Togawa T, Dunn WA, Emmons AC, Nagao J, Willis JH. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochemistry and Molecular Biology. 2008;38:508–519. doi: 10.1016/j.ibmb.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. The storage of protein, fat, glycogen and uric acid in the fat body and other tissues of mosquito larvae. The Journal of Experimental Biology. 1942;19:56–77. [Google Scholar]
- Willis JH. Metamorphosis starts with Met. Proceedings of the National Academy of Sciences, USA. 2007;104:10297–10298. doi: 10.1073/pnas.0704255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annual Review of Entomology. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mechanisms of Development. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zakharkin SO, Headley VV, Kumar NK, Buck NA, Wheeler DE, Benes H. Female-specific expression of a hexamerin gene in larvae of an autogenous mosquito. European Journal of Biochemistry. 2001;268:5713–5722. doi: 10.1046/j.0014-2956.2001.02514.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Li CR, Momen B, Kohanski RA, Pick L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proceedings of the National Academy of Sciences, USA. 2009;106:19617–19622. doi: 10.1073/pnas.0905083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen L, Raikhel AS. Distinct roles of Broad isoforms in regulation of the 20- hydroxyecdysone effector gene, Vitellogenin, in the mosquito Aedes aegypti. Molecular and Cellular Endocrinology. 2007;267:97–105. doi: 10.1016/j.mce.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke I, Kirchner C, Chao LC, Tetzlaff MT, Pankratz MJ. Suppression of food intake and growth by amino acids in Drosophila: the role of pumpless, a fat body expressed gene with homology to vertebrate glycine cleavage system. Development. 1999;126:5275–5284. doi: 10.1242/dev.126.23.5275. [DOI] [PubMed] [Google Scholar]