Abstract
Although TLR are often studied on DC because of their ability to bridge innate and adaptive defenses, TLR are also expressed by epithelial cells. Because the majority of cancers are carcinomas, and thus of epithelial origin, we wanted to know whether a carcinoma and DC responded similarly to a TLR agonist. We found the mammary carcinoma 4T1 and CD11c+ DC both secreted proinflammatory chemokines in response to the TLR4 agonist lipopolysaccharide (LPS). However a clear dichotomy existed. DC, but not 4T1 secreted IL-1β, TNF-α, and upregulated CD80 and CD86 expression following LPS treatment. A potential reason for differential responsiveness was that DC expressed greater levels of TLR4, CD14, Myd88, and TRAM. Despite the low level of TLR signaling proteins, the carcinoma were able to elicit a range of responses contingent upon the source, dose, length, and frequency of TLR agonist treatment. Thus, carcinoma and DC are distinctly responsive to LPS.
Keywords: Dendritic cells, Tumor cells, Toll-like receptors, LPS
1. Introduction
TLR have a domain of leucine-rich repeats, a toll/interleukin 1 (IL-1) receptor (TIR) domain, and exist either on the cell surface or in intracellular compartments of white blood cells, fibroblasts, and epithelial cells [1, 2]. Different TLR generally recognize pathogens through pathogen-associated molecular patterns (PAMPs). For example, TLR4 binds lipopolysaccharide (LPS), a key cell wall component of gram-negative bacteria; TLR3 recognizes double-stranded RNA from viruses; and TLR5 detects bacterial flagellin [3]. These receptors may function alone such as with TLR5 for flagellin, together such as with TLR2/TLR6 for diacyl lipopeptide recognition, or with additional proteins such as with TLR4 in association with MD-2 (Ly96), and CD14 for LPS recognition [1, 3]. Downstream of these receptors signaling cascades converge, promoting expression of inflammatory cytokines and type I interferons (IFN) the purpose of which are to assist in initiating a response against a pathogen [1–3]. Indeed, TLR signaling has been implicated in host protection by initiating antibacterial and antiviral responses [4, 5]. However, some detrimental effects have also been associated with TLR signaling such as with atherosclerosis and colitis [6, 7]. As a result, there is a concerted effort to delineate the extent to which different cells respond to different TLR agonists.
Since DC are capable of bridging innate and adaptive defenses there is great interest in characterizing how TLR agonists impact the capabilities of these cells. Toward this aim investigators have explored how TLR agonists directly impact DC. For instance, Messmer et al., [8] reported that LPS was capable of inducing DC maturation resulting in MHC Class II, CD80, and CD86 expression. Shen et al., [9] explored the signaling cascade induced in DC by LPS, and reported that Myd88 dependent and independent signaling was required. Additionally, investigators have explored how TLR agonist treated DC impact subsequent adaptive immune responses. For example, Wiethe et al., [10] reported that LPS and anti-CD40 treated DC, but not TNF-α treated DC, resulted in a Th1 response that assisted in controlling a Leishmania major infection, and Sato and Iwasaki [11] reported that an effector T cell response to herpes virus required TLR on stromal cells as well as DC. Because DC are differentially responsive to multiple distinct TLR agonists, there is also a growing interest in deciphering the cooperation that exists among TLR signaling cascades [12–15]. Thus, much remains to be learned with regard to DC and TLR.
Since epithelial cells express TLR, are responsive to TLR agonists [16–18], and since most tumors are epithelial in origin [19], there is great interest in characterizing how TLR agonists impact the capabilities of these cells. For instance, TLR agonists have been reported to slow tumor growth or progression [20, 21]. Hirabayashi et al., [20] reported that a TLR3 agonist could slow the growth of several different breast cancer cell lines in vitro, and there are a number of instances in which TLR7 and TLR8 agonists were beneficial in fighting cancer in vitro and in vivo [21]. On the other hand, in some cases TLR agonists may augment tumor growth or progression. For instance, LPS has been reported to influence β1 integrin expression [22], stimulate endothelial sprouting [23], and signaling through TLR4 has been shown to increase motility of melanoma cells in vitro [24]. Such studies suggest that TLR agonists may influence angiogenesis or metastasis. Because of these findings, and because TLR agonist treated DC are also being explored to fight cancer [25], we were interested in comparing the responsiveness of DC and tumor cells to a TLR agonist.
For these reasons we began comparing responsiveness of CD11c+ bone marrow derived DC and 4T1 murine mammary carcinoma cells to LPS. We found that genes encoding multiple TLR were expressed in each type of cell, and that both types of cells secreted the proinflammatory chemokines CCL2 and CXCL1 in response to LPS. Yet, DC alone secreted IL-1β, TNF-α, and upregulated CD80 and CD86 expression following LPS treatment. Analysis of gene expression revealed that TLR4 and CD14, which are utilized for LPS recognition, and Myd88 and TRAM, which are used immediately downstream of the LPS receptors, were expressed at greater levels by DC. In an attempt to determine whether 4T1 was capable of eliciting a greater response to LPS we explored varying treatment conditions. Indeed, despite low levels of TLR signaling proteins, 4T1 was able to make a range of LPS dependent responses. For instance, treatment with LPS from E. coli serotype 0128:B12 enhanced tumor growth rate while treatment with LPS from E. coli serotype 026:B6 decreased tumor growth rate. Yet, LPS from E. coli serotype 026:B6 was capable of enhancing tumor growth if treatment continued for 72 rather than 24 hours. Collectively, these data demonstrate that responses to TLR agonists are multifactorial in nature depending upon not only the level of TLR signaling proteins, but also the source of agonist, dose, length, and frequency of treatment.
2. Materials and methods
2.1. Cells and mice
4T1 murine mammary carcinoma cells were maintained in complete RPMI (cRPMI) (RPMI 1640, Lonza, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum (Lonza), glutamine (2 mM, Lonza), penicillin (100 U/mL, Lonza), streptomycin (100 μg/mL, Lonza), nonessential amino acids (Sigma, St. Lois, MO), 2-mercaptoethanol (5 × 10−5 M, Sigma), and sodium pyruvate (1 mM, Lonza). For in vivo analysis of TLR agonist treated tumor cells 1 × 106, 1 × 105, or 5 × 104 cells were cultured with or without TLR agonists in 75 cm2 culture flasks for 24, 48, or 72 hours respectively. To determine whether frequency of treatments influenced tumor growth rate the cells were either treated one time with LPS or every 24 hours. After 24–72 hours the cells were harvested, counted, washed three times in sterile saline, and adjusted to 5 × 105cells/ml. Next, 100 μl of cells were injected into the left hind flank of mice. Tumors were measured every 2 to 3 days and the volumes calculated (L x W2/2). Mice were housed in a thoren caging system (Thoren Caging Systems Inc., Hazelton, PA). Food and water were provided ad libitum. All mice were used in accordance with an Institutional Animal Care and Use Committee approved protocol that followed the guidelines for ethical conduct in care and use of animals.
DC were generated from male OTII transgenic mice as previously described [12]. Briefly, bone marrow from the femurs and tibias were cultured at 1 × 106 cells/ml in cRPMI with GM-CSF (20 ng/ml, Peprotech, Rocky Hill, NJ) and IL-4 (10 ng/ml, Peprotech) with one ml/well in 24-well tissue culture dishes (Costar, Fisher). The media with GM-CSF and IL-4 was replaced every 2 to 3 days. Following 7 to 8 days, CD11c+ DC were purified using CD11c microbeads and MS positive selection columns (Miltenyi Biotec, Auburn, CA). CD4+ T cells were purified from spleens of male OTII mice using CD4 microbeads and magnetic cell separation (Miltenyi Biotec). Purity of the cells, which was ≥ 95%, was verified by flow cytometry.
2.2. TLR agonists
For TLR2 and TLR4 agonists peptidoglycan (PGN) from Staphylococcus aureus (Sigma), and LPS from Escherichia coli serotype 026:B6 (Sigma), Escherichia coli serotype 0128:B12 (Sigma), Pseudomonas aeruginosa serotype 10 (Sigma), Salmonella enteritidis (Sigma), or Salmonella typhimurium (Sigma) were used. Stocks were prepared in Hank’s Balanced Salt Solution (HBSS, Lonza), aliquoted, and stored at −20°C in sterile microcentrifuge tubes. PGN was used at a final concentration of 5μg/ml, and LPS was used at a final concentration of 1, 10, or 100 ng/ml. An equal volume of HBSS was used as a negative control.
2.3. DC antigen presentation
Cytokine release and proliferation assays were used to determine whether DC were competent antigen presenting cells. For this purpose 100 μL of CD11c+ DC at 2 × 106 cells/ml were added to 96-well flat bottom plates (Falcon). The DC were pulsed with 0.001, 0.01, 0.1, or 1.0 μM ovalbumin (OVA) peptide (ISQAVHAAHAEINEAGR, Sigma), or an equivalent volume of HBSS, and incubated for 2 hours at 37°C, 5% CO2. Next, 100 μL of OVA specific CD4+ T cells from OTII mice at a concentration of 1 × 106 cells/ml were added to each well and the cells were incubated at 37°C, 5% CO2 for 48 hours. Eighteen hours before analysis the cells were pulsed with 110 μM 5-bromo-2′-deoxy-uridine (BrdU, Roche Diagnostics, Mannheim, Germany).
To assess cytokine release, supernatants were harvested after 48 hours, centrifuged at 450 x g to remove particulate materials, and stored at −20°C for ELISA. The samples were assayed for IFN-γ using a Quantikine Sandwich ELISA (R&D Systems, Minneapolis, MN).
Proliferation was assessed using a non-radioactive proliferation assay according to manufacturer’s instructions (Roche Diagnostics). Briefly, the 96-well plates containing the cells were centrifuged for 8 minutes at 450 x g, and allowed to dry for 2 hours at 60°C. The cells were fixed with 200 μL of precooled (−20°C) fixative/well and washed 3 times with 200 μL of wash medium (phosphate buffered saline (PBS) + 10% bovine serum albumin (BSA))/well. The cells were then incubated with 100 μL of nucleases/well for 30 min. at 37°C, washed 3 times with 200 μL of wash medium/well, and 100 μL of anti-BrdU-POD was added to each well. Following a 30 min. incubation at 37°C, and 3 washes with 200 μL wash medium/well, 100 μL peroxidase substrate was added to each well and absorbance was read at 405 nm (reference wavelength 490 nm) at 30 min. on a microplate reader (Phenix Research Products, Hayward, CA).
2.4. TLR reverse transcriptase-PCR analysis
TLR expression by 4T1 and CD11c+ DC was determined by RT-PCR. For analysis, mRNA was isolated (Oligotex direct mRNA micro kit, Qiagen, Valencia, CA) from 5 × 105 to 1 × 106 cells. All of the mRNA was precipitated, resuspended in 25 μl diethyl pyrocarbonate (Sigma) treated H2O and used for cDNA synthesis. Complementary DNA was made with random primers (0.5 μg, Promega, Madison, WI), dithiothreitol (2 mM, Promega), dNTP (0.2 mM each, Promega) and M-MLV reverse transcriptase (200 units, Promega) in a 1 hour reaction at 42°C. An aliquot (3 μl) of cDNA was amplified in a reaction with 1.25 mM MgCl2 (Promega), dNTP (0.2 mM each, Promega), specific primers (20 μM), and GoTaq Flexi DNA Polymerase (2.5 units, Promega). The reaction conditions consisted of 35 cycles of 94°C for 15 sec., 59°C for 30 sec., and 74°C for 45 sec., on a MJ Research thermocycler (MJ Research, Waltham, MA). The TLR specific primers, which have been previously described [12], were synthesized by Integrated DNA Technologies (Coralville, IA) and analyzed for specificity with the NCBI Blast Program. PCR products were analyzed on a 2% agarose gel using the Alpha Innotech Gel Documentation System (Alpha Innotech Corp., San Leandro, CA).
2.5. ELISA
To determine whether TLR agonist treatment induced secretion of proinflammatory mediators, chemokine and cytokine specific ELISAs were used. For this purpose, 1 ml of 4T1 or CD11c+ DC at 1 × 106 cells/ml were added to each well in 24-well tissue culture plates and treated with HBSS, LPS, or PGN as described above. After 24 hours, supernatants were harvested, centrifuged at 450 × g to remove particulate materials, and stored at −20°C. For analysis, samples were assayed for CCL2, CCL5, CXCL1, IL-1β, and TNF-α using Quantikine Sandwich ELISAs (R&D Systems).
2.6. Flow cytometry
Flow cytometry was used to evaluate whether LPS modulated costimulatory molecule expression. For this purpose, 5 × 105 4T1 or CD11c+ DC that were treated with HBSS or LPS for 24 hours at 37°C, 5% CO2 were harvested, resuspended in labeling buffer (1 × PBS, 0.5% BSA, 2 mM EDTA), and phenotyped for CD80 (FITC) and CD86 (PE) expression. The antibodies were purchased from BD Pharmingen (San Diego, CA). Prior to antibody labeling, cells were incubated with Fc block (BD Pharmingen) for 15 min. on ice, washed, centrifuged, and resuspended in 1 mL labeling buffer. The cells were then aliquoted with 1 μg of antibody or isotype control, and incubated for 30 min. on ice. Following a wash with labeling buffer, the cells were fixed with 3.7% formaldehyde (Sigma) for 10 min. on ice, washed again, and resuspended in 0.5 ml HBSS. The samples were sent for analysis to the Cell Science Core Facility at Penn State College of Medicine, Hershey Medical Center (Hershey, PA).
2.7. Gene array analysis
Gene arrays were used to examine gene expression profiles of 4T1 and DC before and after LPS exposure. For this purpose Oligo GEArray Mouse Toll-like Receptor Signaling Pathway Microarrays (SuperArray Bioscience Corp., Frederick, MD) were used. First, total RNA was isolated from 1 × 106 to 3 × 106 4T1 or CD11c+ DC that were treated with HBSS or LPS for 24 hours at 37°C, 5% CO2 using the ArrayGrade Total RNA Isolation Kit (SuperArray Bioscience Corp.). The 260/280 for all RNA samples was at least 1.80. Identical amounts of total RNA (1.6–2.5 μg) isolated from the cells were used to make complementary DNA, and subsequently complementary RNA (cRNA) was synthesized, labeled, and purified using the TrueLabeling-AMP 2.0 kit (SuperArray Bioscience Corp.) according to manufacturer’s instructions. The labeling reaction using 10 mM biotinylated-UTP (Perkin Elmer, Waltham, MA) was carried out for 4 hours. Purified biotinylated cRNA samples had a 260/280 of at least 1.80.
Hybridizations were performed according to manufacturer’s instructions (SuperArray Bioscience Corp.) on 4 arrays at a time. Briefly, following a 2 hour pre-hybridization, 2 μg of biotinylated cRNA isolated from 4T1 +/− LPS or DC +/− LPS was added to 0.75 mL hybridization solution, and added to the arrays which were left to hybridize overnight at 60°C with 5 rpm continuous rotation in a hybridization oven (VWR, Bristol, CT). Following 15 min. washes with wash solution I (0.1 M SSC, 0.05 M SDS) and then wash solution II (0.005 M SSC, 0.025 M SDS), the arrays were cooled to room temperature, developed with streptavidin alkaline phosphatase, and chemiluminescence was captured using a CCD camera (Alpha Innotech). AlphaEase FluroChem 8800 software (Alpha Innotech) was used to record the developing arrays and for densitometric analysis. All data were normalized to the housekeeping gene GAPDH.
2.8. Quantitative reverse transcriptase PCR
Expression of select genes were analyzed by QRT-PCR. First, total RNA was isolated from 1 × 106 to 3 × 106 4T1 or CD11c+ DC using the ArrayGrade Total RNA Isolation Kit (SuperArray Bioscience Corp.). The 260/280 for all RNA samples was at least 1.80. Messenger RNA was enriched from total RNA using a mRNA isolation kit (Invitrogen, Carlsbad, CA). All of the mRNA was precipitated, resuspended in 25 μl DEPC treated H2O, and used for cDNA synthesis. Complementary DNA was generated as described above in a 1 hour reaction at 42°C. An aliquot (1 μl) of cDNA was amplified in a reaction with 1 x iQ SYBR Green Supermix (Bio-Rad Laboratories, Richmond, CA), and 200 nM gene specific primers. Each reaction was run in duplicate or triplicate. The reaction conditions consisted of 40 cycles of a two-step PCR reaction with 94°C for 10 sec., and 68°C for 30 sec., on an iQ5 Real Time PCR Detection System (Bio-RAD). Gene specific primers used included gapdh left 5′-ctt ccg tgt tcc tac ccc caa tgt-3′, gapdh right 5′-gcc tgc ttc acc acc ttc ttg atg t-3′, TLR4 left 5′-agt gcc ccg ctt tca cct ctg-3′, TLR4 right 5′-caa taa cct tcc ggc tct tgt gga-3′, TRAM left 5′-tca ggc cgg gga tcg ttt tc-3′, TRAM right 5′-cga ccc att gac cgc atc gt-3′, MyD88 left 5′-cct gac ccc act cgc agt ttg t-3′, MyD88 right 5′-tgc gcg act tca gct cct tca-3′, TIRAP left 5′-ttg cac cat acc cct gct gtc c-3′, TIRAP right 5′-aaa agc ctc cgt cct tgc ctc t-3′, CD14 left 5′-tgt cgt ggg caa caa ggg atg-3′, CD14 right 5′-aag gtg gag agg gca ggg aag a-3′. The primers were synthesized by Integrated DNA Technologies (Coralville, IA) and analyzed for specificity with the NCBI Blast Program. Standard curves were used to examine efficiency and reproducibility of each reaction, and melt curves were used to validate amplification of single products. The housekeeping gene GAPDH was used to establish relative expression (ΔΔCT).
2.9. Western blot
To analyze expression of TLR signaling proteins 5 × 106 tumor cells and CD11c+ DC were washed 3 times with 10 mL ice-cold PBS, and transferred to microcentrifuge tubes. The cells were resuspended in 150 μL of buffer A (10 mM Hepes (Sigma), 10 mM KCl (Sigma), 0.1 mM EDTA (Sigma) and 0.1 mM EGTA (Sigma)) supplemented with the protease inhibitors aprotinin, leupeptin, chymostatin, and pefabloc (Roche Molecular Biochemicals, Indianapolis, IN) and placed on ice. Following a 15 min. incubation, 10 μL of 10% Nonidet P-40 (Sigma) was added. The samples were vortexed for 10 sec., and centrifuged at 15,000 x g at 4°C for 1 minute. NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA) and dithiothreitol (1x, New England Biolabs, Ipswich, MA) were added and the samples were stored at −20°C. SDS PAGE gels (12.5%, Invitrogen) were loaded with 15 μL of proteins, electrophoresed, and transferred to Invitrolon polyvinylidene difluoride (PVDF) membranes (Invitrogen). The membranes were blocked at 4°C in PBS with 5% powdered milk and 0.05% Tween 20 (Sigma) overnight. Primary antibodies (10 μg) specific for actin, TLR4, Myd88, TRAM, CD14, or Tirap (Santa Cruz Biotechnology, Santa Cruz, CA) were added, and the blots were incubated at room temperature for 3 hours. After washing 2 times with blocking buffer, a horse-radish-peroxidase-conjugated secondary antibody (Santa Cruz) was added and the blots were incubated for 1 hour at room temperature. Following 4 washes, proteins were visualized by enhanced chemiluminesence on an Alpha Innotech Gel Documentation System.
3. Results
3.1. 4T1 and CD11c+ DC express genes encoding TLR2 and TLR4
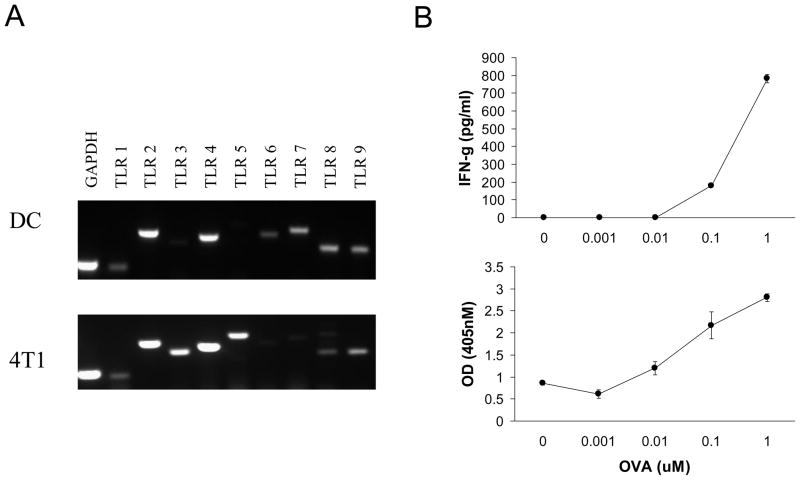
Prior to comparing the extent to which 4T1 tumor cells and bone marrow derived CD11c+ DC differed in responsiveness to TLR agonists we needed to identify TLR expressed by both cell types. For this purpose we screened TLR1 to TLR9 expression by RT-PCR. Analysis of 4T1 tumor cells revealed that TLR2, 3, 4, 5, and 9 were consistently expressed with TLR2 and TLR4 expressed at the greatest levels (Fig. 1A). To validate function prior to analyzing TLR gene expression by DC, we characterized MHC expression, and ability to present antigen. The DC expressed high levels of MHC Class I, MHC Class II (data not shown), and were capable of eliciting antigen specific T cell proliferation and cytokine secretion (Fig. 1B). For these assays CD4+ T cells were purified from spleens of OTII mice, and showed dose dependent IFN-γ secretion and proliferation when cocultured with OVA peptide pulsed DC (Fig. 1B) indicating that the DC were competent antigen presenting cells. With regard to TLR expression, these DC showed consistent expression of TLR2, 4, 7, 8, and 9 with TLR2 and TLR4 expressed at the greatest levels (Fig. 1A). Collectively, these data showed that 4T1 and CD11c+ DC expressed multiple genes encoding TLR, and that TLR2 and TLR4 were expressed by both types of cells.
Fig. 1.
4T1 and CD11c+ DC express TLR2 and TLR4. A. RT-PCR was used to assess TLR1 to TLR9 expression by 4T1 and CD11c+ DC. GAPDH was used as a positive control. B. Functional activity of CD11c+ DC. OVA specific CD4+ T cells were cultured with OVA peptide pulsed DC to evaluate the antigen presenting capability of the DC. T cell cytokine secretion and proliferation were assayed at 48 hours. The data show the average and standard deviation of triplicate wells from a representative of each experiment. All data are representative of three separate experiments.
3.2. 4T1 and CD11c+ DC respond to TLR2 and TLR4 agonists
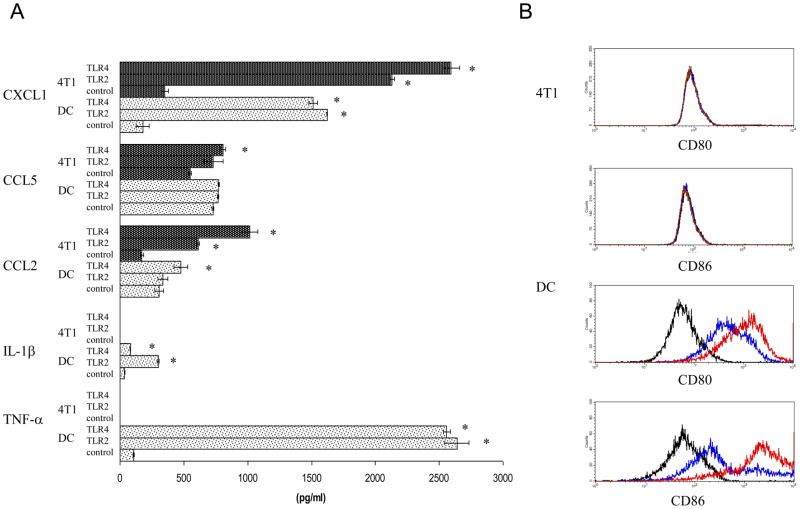
Next, we evaluated the response of 4T1 and CD11c+ DC to TLR2 and TLR4 agonists using PGN and LPS respectively. For this purpose 4T1 and DC were treated with PGN, LPS, or HBSS as a control for 24 hours, supernatants were harvested and assessed for inflammatory chemokine and cytokine levels by ELISA (Fig. 2A). Both types of cells secreted CXCL1 and CCL2 in response to PGN and LPS treatment, with CXCL1 secretion induced to the greatest extent. Baseline CXCL1 secretion by 4T1 was 352 pg/ml, and secretion increased to 2,132 pg/ml following PGN treatment, and 2,593 pg/ml following LPS treatment (Fig. 2A). Baseline CXCL1 secretion by DC was 179 pg/ml, and secretion increased to 1,622 pg/ml following PGN treatment, and 1,511 pg/ml following LPS treatment (Fig. 2A). With regard to CCL5 secretion, 4T1 exhibited a modest response (less than two fold increase) to PGN and LPS treatment, whereas DC did not increase CCL5 secretion in response to either agonist (Fig. 2A). With regard to inflammatory cytokines, DC secreted IL-1β and TNF-α following TLR agonist treatment, with TNF-α secretion induced to the greatest extent. Baseline TNF-α secretion by DC was 108 pg/ml, and secretion increased to 2,638 pg/ml following PGN treatment, and 2,558 pg/ml following LPS treatment (Fig. 2A). 4T1 secreted no detectable IL-1β or TNF-α in response to the TLR agonists at any of the doses examined (PGN 0.5 to 50 μg/ml, LPS 1 to 100 ng/ml), or at any of the time points examined (24, 48, 96 hours, data not shown).
Fig. 2.
4T1 and CD11c+ DC are distinctly responsive to LPS. A. 4T1 and DC were treated with five μg/ml PGN (TLR2), ten ng/ml LPS (TLR4), or HBSS (control) for 24 hours. Supernatants were harvested and assayed for the indicated chemokines and cytokines by ELISA. The data are the average and standard error of three separate experiments. Where indicated (*) p< 0.05 using Student’s t-test relative to control. B. 4T1 and DC were treated with ten ng/ml LPS (red), or HBSS (blue) for 24 hours and then labeled for CD80 and CD86 expression. The isotype control is shown in black. The histograms are representative of three separate experiments.
Since TLR agonists are known to induce DC maturation which concomitantly results in increased costimulatory molecule expression, we next asked whether CD80 and CD86 expression were modulated by TLR agonist treatment. For this purpose we looked at CD80 and CD86 expression on 4T1 and DC before and after treatment with LPS. 4T1 showed no detectable costimulatory molecule expression before or after LPS treatment, whereas DC upregulated both CD80 and CD86 expression following LPS treatment (Fig. 2B). Since 4T1 made a greater response to LPS than PGN, at least with respect to chemokine secretion, we did not assess whether PGN treatment upregulated expression of the costimulatory molecules. Thus, in response to TLR2 and TLR4 agonists, 4T1 secreted the chemokines CXCL1, CCL2 and CCL5, and DC secreted CXCL1, CCL2, IL-1β, TNF-α, and upregulated CD80 and CD86 expression. Collectively, these data revealed that although both 4T1 tumor cells and bone marrow derived CD11c+ DC were capable of responding to TLR agonists, they did so in distinctive manners.
3.3. Gene array analysis confirms 4T1 and CD11c+ DC are distinctly responsive to LPS
To further elucidate the differential responsiveness of tumor cells and DC to LPS we treated each cell type with the TLR agonist and used gene arrays to assess expression of genes important for TLR responses. We discuss only those genes that exhibited a greater than two-fold change in expression.
Overall, LPS modulated gene expression to a greater extent in DC than 4T1 (Tables 1 and 2). The DC response included an increase in expression of CD80 and CD86 (Table 1) correlating with the changes in protein expression shown by flow cytometry (Fig. 2B). With regard to inflammatory mediators, CXCL10 (IP-10) exhibited the greatest induction of expression (21.8 fold induction), followed by IL-12β (p40 subunit, 14.0 fold induction), then Clecsf9 (11.5 fold induction), IL-6 (11.0 fold induction), and IL-1β (7.1 fold induction) (Table 1). Several genes that encode signaling proteins were also modulated by LPS including IRF3, IRF7, and PRKR with a 6.5, 24.9, and 4.8 fold induction of gene expression respectively (Table 1). TLR3 was the only gene to show a reduction (3.4 fold) in expression by DC following LPS treatment (Table 1).
Table 1.
Genes modulated in CD11c+ DC by LPS.
| Gene Name | DC control | DC + LPS | Expression ratiob |
|---|---|---|---|
| CD80 | 0.017 +/− 0.01a | 0.056 +/− 0.02 | 3.4 |
| CD86 | 0.119 +/− 0.07 | 0.377 +/− 0.11 | 3.2 |
| Clecsf9 | 0.011 +/− 0.00 | 0.128 +/− 0.04 | 11.5 |
| CXCL10 | 0.007 +/− 0.00 | 0.156 +/− 0.16 | 21.8 |
| IFN-β | 0.007 +/− 0.00 | 0.020 +/− 0.01 | 2.8 |
| IL-12α (p35) | 0.026 +/− 0.02 | 0.058 +/− 0.02 | 2.2 |
| IL-12β (p40) | 0.059 +/− 0.04 | 0.824 +/− 0.04 | 14.0 |
| IL-1α | 0.005 +/− 0.00 | 0.024 +/− 0.01 | 4.6 |
| IL-1β | 0.124 +/− 0.08 | 0.875 +/− 0.08 | 7.1 |
| IL-6 | 0.020 +/− 0.00 | 0.224 +/− 0.03 | 11.0 |
| IRF3 | 0.006 +/− 0.00 | 0.036 +/− 0.03 | 6.5 |
| IRF7 | 0.039 +/− 0.02 | 0.981 +/− 0.06 | 24.9 |
| Ly96 | 0.073 +/− 0.04 | 0.148 +/− 0.05 | 2.0 |
| IκB-ε | 0.031 +/− 0.01 | 0.067 +/− 0.03 | 2.2 |
| PRKR | 0.012 +/− 0.00 | 0.056 +/− 0.02 | 4.8 |
| PTGES | 0.005 +/− 0.00 | 0.015 +/− 0.00 | 2.9 |
| COX2 | 0.005 +/− 0.00 | 0.059 +/− 0.02 | 11.5 |
| RIPK2 | 0.014 +/− 0.01 | 0.033 +/− 0.01 | 2.4 |
| TLR3 | 0.019 +/− 0.03 | 0.006 +/− 0.00 | − 3.4 |
Average and standard error of the integrated density value (IDV) from three separate experiments.
Ratio of the IDV from DC treated with LPS/control.
Table 2.
Genes modulated in 4T1 tumor cells by LPS.
| Gene Name | 4T1 control | 4T1 + LPS | Expression ratiob |
|---|---|---|---|
| CCL2 | 0.036 +/− 0.01a | 0.117 +/− 0.03 | 3.3 |
| G-CSF | 0.087 +/− 0.02 | 0.301 +/− 0.06 | 3.5 |
| Btk | 0.009 +/− 0.01 | 0.004 +/− 0.00 | − 2.1 |
| IFN-α | 0.009 +/− 0.01 | 0.004 +/− 0.00 | − 2.5 |
| Ly64 | 0.008 +/− 0.01 | 0.004 +/− 0.00 | − 2.2 |
| Mapk11 | 0.011 +/− 0.01 | 0.004 +/− 0.00 | − 2.7 |
| TLR6 | 0.008 +/− 0.00 | 0.004 +/− 0.00 | − 2.0 |
Average and standard error of the IDV from three separate experiments.
Ratio of the IDV from 4T1 treated with LPS/control.
4T1 showed more modest changes in gene expression following LPS treatment. Only CCL2 and G-CSF increased in expression (both 3 fold induction), whereas five genes showed reduced expressing following LPS treatment (Table 2). The reductions in gene expression were also modest with Btk, IFN-α, Ly64, Mapk11, and TLR6 all exhibiting two-fold reductions (Table 2).
Next, we compared expression of genes downstream of Myd88 and TRIF. Both IL-1β and IL-12β, gene products downstream of Myd88, were expressed at greater levels by DC following LPS treatment; expression increased 7.1 and 14.0 fold respectively (Table 1). Both IFN-β and CXCL10, gene products downstream of TRIF, were expressed at greater levels by DC following LPS treatment; expression increased 2.8 and 21.8 fold respectively (Table 1). In contrast, these genes were not upregulated in 4T1 following LPS treatment. Thus, in response to LPS treatment, of the 113 genes assessed with the arrays two genes showed elevated expression and five genes showed reduced expression by 4T1, whereas eighteen genes showed elevated expression and one gene showed reduced expression by DC. Moreover, none of the genes were modulated in the same way by the tumor cells and DC. Collectively, these data confirmed that tumor cells and DC respond in distinct manners to LPS.
3.4. Comparison of TLR signaling potential between 4T1 and CD11c+ DC
To determine whether TLR signaling cascades differed between the tumor cells and DC we compared expression of genes involved in TLR signaling. Genes encoding surface receptors and co-receptors that were analyzed with the arrays, and known to be involved in LPS signaling, included TLR4 and CD14. Based upon array analysis TLR4 showed 3.5 x greater expression by DC; the integrated density values (IDV) for TLR4 were 0.049 +/− 0.01 for DC, and 0.014 +/− 0.00 for 4T1 (Table 3). CD14 showed 11.4 x greater expression by DC, the IDV for CD14 were 0.182 +/− 0.09 for DC, and 0.016 +/− 0.00 for 4T1 (Table 3).
Table 3.
Relative expression of genes involved in the TLR signaling cascade.
| Gene Name | DC | 4T1 | Expression ratiob |
|---|---|---|---|
| TLR4 | 0.049 +/− 0.01a | 0.014 +/− 0.00 | 3.5 |
| CD14 | 0.182 +/− 0.09 | 0.016 +/− 0.00 | 11.4 |
| TIRAP | 0.018 +/− 0.00 | 0.093 +/− 0.02 | 0.2 |
| Myd88 | 0.123 +/− 0.01 | 0.078 +/− 0.01 | 1.6 |
| IRAK4 | 0.013 +/− 0.00 | 0.013 +/− 0.00 | 1.0 |
| TRAF6 | 0.016 +/− 0.00 | 0.008 +/− 0.00 | 2.0 |
| TAKI | 0.003 +/− 0.00 | 0.007 +/− 0.00 | 0.4 |
| NF-kBp50 | 0.191 +/− 0.02 | 0.109 +/− 0.01 | 1.8 |
| NF-kBp65 | 0.049 +/− 0.01 | 0.030 +/− 0.00 | 1.6 |
| MEKK1 | 0.114 +/− 0.02 | 0.047 +/− 0.01 | 2.4 |
| MKK3 | 0.197 +/− 0.02 | 0.618 +/− 0.07 | 0.3 |
| MAPK11 | 0.138 +/− 0.02 | 0.142 +/− 0.02 | 0.9 |
| TRAM | 0.017 +/− 0.00 | 0.005 +/− 0.00 | 3.4 |
| TRIF | 0.004 +/− 0.00 | 0.006 +/− 0.00 | 0.7 |
| TBK1 | 0.045 +/− 0.00 | 0.051 +/− 0.01 | 0.9 |
| IRF3 | 0.006 +/− 0.00 | 0.010 +/− 0.00 | 0.6 |
Average and standard error of the integrated density value (IDV) from three separate experiments.
Ratio of the IDV from DC/4T1.
Within the Myd88 dependent pathway TIRAP, Myd88, IRAK4, TRAF6, and TAK1 were analyzed. Although each gene was detected from both cell types, two of the genes (TIRAP and TAK1) were expressed at greater levels by the tumor cells, two (Myd88 and TRAF6) were expressed at greater levels by DC, and one (IRAK4) was expressed at similar levels by both (Table 3). Downstream of TAK1 are the MAPK and NFκB pathways. Since the arrays included many different NFκB and MAPK family members we focused on members of these pathways that were expressed at the greatest levels by both cell types. For NFκB the genes encoding p50 and p65 subunits were expressed at the greatest levels by both 4T1 and DC, and DC showed slightly higher levels of expression of these genes (Table 3). For the MAPK members we compared a MKKK (MEKK1), a MKK (MKK3), and a MAPK (MAPK11). Although MAPK11 was expressed at similar levels between the cell types, MEKK1 showed greater expression by the DC, and MKK3 was expressed at greater levels by the tumor cells (Table 3).
Within the Myd88 independent (TRIF dependent) pathway TRAM, TRIF, TBK1, and IRF3 were analyzed. Although each gene was detected from both cell types, three of the genes (TRIF, TBK1, and IRF3) were expressed at greater levels by the tumor cells, and one (TRAM) was expressed at greater levels by DC (Table 3). Based upon array analysis TRAM showed 3.4 x greater expression by DC, the IDV for TRAM was 0.017 +/− 0.00 for DC, and 0.005 +/− 0.00 for 4T1 (Table 3). Collectively, these data revealed that TLR4, CD14, Myd88, and TRAM, genes encoding proteins important at the beginning of the TLR signaling pathway, were expressed at greater levels by DC.
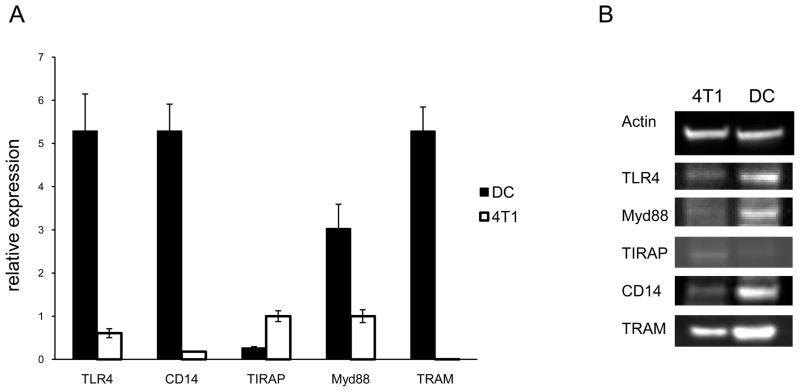
3.5. Comparison of TLR signaling potential using QRT-PCR and western blot
In order to validate some of the differences that were evident with the arrays we used QRT-PCR and western blot. Results which were in agreement with the array data included greater expression of TLR4, CD14, Myd88, and TRAM by the DC, and greater expression of TIRAP by 4T1 (Fig. 3A). Western blot analysis revealed that the differences observed at the RNA level were also evident at the protein level (Fig. 3B). Collectively, these data showed that expression of genes and proteins important for LPS recognition and response differed between the DC and tumor cells, with many of the genes and proteins expressed at greater levels by the DC.
Fig. 3.
Comparison of TLR signaling potential using QRT-PCR and western blot. A. RNA was extracted from an equal number of 4T1 and DC for QRT-PCR. Relative expression was set to GAPDH. B. Proteins were isolated from an equal number of 4T1 and DC for western blot analysis. All data are representative of three separate experiments.
3.6. Tumor cells are capable of eliciting varying responses to LPS
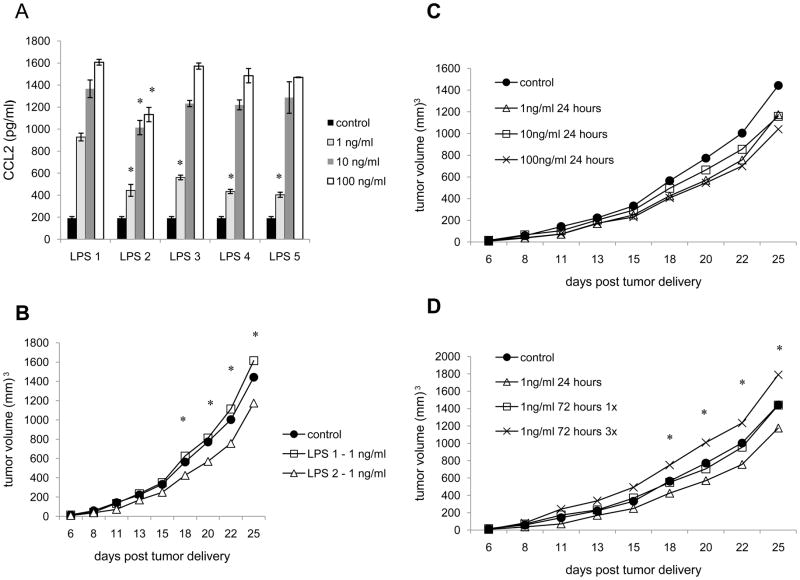
Because it is well established that DC respond to TLR agonists, and because of the poor responsiveness (Table 2), and the low level of TLR signaling proteins expressed by 4T1 relative to DC (fig. 3), we felt compelled to further explore the extent to which TLR agonist could influence the tumor cells. Thus, to explore whether the tumor cells were capable of making a greater response to LPS we used different sources of LPS, and varied the doses, lengths, and frequencies of treatment. To begin with we treated 4T1 with LPS from E. coli serotype 0128:B12, E. coli serotype 026:B6, Pseudomonas auruginosa, Salmonella enteritidis, and Salmonella typhimurium (Fig. 4A). Surprisingly, the greatest differences were found between the tumors treated with LPS from two different serotypes of E. coli. While treatment with 1, 10, and 100 ng/ml of LPS from E. coli serotype 0128:B12 induced secretion of 928, 1,366, and 1,607 pg/ml of CCL2 respectively, treatment with LPS from E. coli serotype 026:B6 induced secretion of 444, 1,014, and 1,132 pg/ml of CCL2 respectively (Fig. 4A). Moreover, LPS from different gram (−) bacteria exerted differential effects on tumor growth rates. We compared the growth rates of tumors following treatment with 1, 10, and 100 ng/ml of the 5 different types of LPS (Fig. 4B and data not shown). Again the greatest differences were evident upon treatment with LPS from the 2 different serotypes of E. coli. Whereas treatment with 1ng/ml of LPS from E. coli serotype 0128:B12 caused a modest increase in tumor growth rate relative to untreated 4T1, treatment with 1 ng/ml of LPS from E. coli serotype 026:B6 resulted in a modest, but significant, decrease in tumor growth relative to untreated 4T1 (Fig. 4B). After 25 days of tumor growth control 4T1 tumors averaged 1,442 mm3, tumors treated with LPS from E. coli serotype 0128:B12 averaged 1,614 mm3, and tumors treated with LPS from E. coli serotype 026:B6 averaged 1,175 mm3. The growth rates of tumors treated with the different serotypes of E. coli were significantly different at days 18 to 25. These data indicate that LPS from different gram (−) bacteria, as well as different serotypes of the same species, can induce different levels of chemokine secretion and differentially influence tumor growth rate.
Fig. 4.
Responsiveness of tumor cells to LPS is dependent upon the source of agonist, dose, length, and frequency of treatment. A. 4T1 were treated with LPS from (1) E. coli serotype 0128:B12, (2) E. coli serotype 026:B6, (3) Salmonella enteritidis, (4) Salmonella typhimurium, or (5) Pseudomonas aeruginosa and supernatants were harvested 24 hours later for chemokine analysis. B. 4T1 were treated with LPS from (1) E. coli serotype 0128:B12 or (2) E. coli serotype 026:B6 for 24 hours prior to injection into Balb/c mice. C and D. 4T1 were treated with LPS (2) from E. coli serotype 026:B6 prior injection into Balb/c mice. All data represent the average of three separate experiments. For in the vivo growth experiments 5 mice/group were used for each experiment. Where indicated (*) p< 0.05 using Student’s t-test.
Next, we evaluated LPS from E. coli serotype 026:B6 further. While treatment with 1, 10, and 100 ng/ml of LPS resulted in a decreased growth rate relative to untreated 4T1 (Fig. 4C), differences among 4T1 treated with different doses of LPS were not significantly different. Yet varying length and frequency of treatment resulted in significant differences (Fig. 4D). While treatment with 1 ng/ml for 24 hours resulted in a modest, but significant decrease in tumor growth relative to untreated 4T1, the effect was lost if treatment continued for 72 hours (Fig. 4D). After 25 days of tumor growth the tumors that were treated with 1ng/ml of LPS for 24 hours averaged 1,175 mm3, whereas the tumors that were treated for 72 hours averaged 1,439 mm3, the same size as untreated 4T1. Moreover, if treatment occurred every 24 hours for 72 hours, a modest, but significant increase in tumor growth occurred (Fig. 4D). After 25 days of growth tumors that were treated with 1ng/ml of LPS every 24 hours for 72 hours averaged 1,789 mm3. The differences between growth rates of tumors treated for 24 hours 1 time and 72 hours 3 times, and the differences between the growth rates of tumors treated for 72 hours 1 time and 72 hours 3 times were significantly different at days 18 to 25. Thus, whether a tumor shows increased or decreased growth following TLR agonist treatment depends upon not only the source of TLR agonist, but also the dose, length and frequency of treatment.
4. Discussion
While some studies have explored how different types of cells respond at the genetic level to TLR agonists [8, 26, 27], the ultimate aim of this study was to enhance the understanding of the way a tumor cell responds to a TLR agonist by comparing it to the way a professional antigen presenting cell responds to the same agonist, and by examining the response to varying treatment conditions. Although both types of cells were capable of secreting proinflammatory chemokines in response to LPS, there were stark differences in secretion of proinflammatory cytokines and expression of the costimulatory molecules CD80 and CD86. Gene expression analysis suggested that DC and 4T1 may exhibit differential responsiveness to LPS because of differences in TLR signaling capabilities; TLR4, CD14, Myd88, and TRAM were expressed at greater levels by DC than 4T1. However, beside the differential expression of these proteins, there were other differences between the DC and tumor cells revealed in this study.
Some of the genes that encode TLR signaling proteins (TAK1, MKK3, and IRF3) were expressed at greater levels by the tumor cells than by the DC. To determine whether expression of these genes increased in the DC following LPS treatment, which may facilitate LPS responsiveness, we compared expression before and after LPS treatment. Expression of TAK1, MKK3, and IRF3 increased following treatment of DC with LPS (data not shown) suggesting that the lower baseline level of expression in the DC did not impair LPS responsiveness. These data also suggest that the greater level of expression of these genes by the tumor cells did not negatively influence LPS responsiveness. On the contrary, there may be other reasons for the elevated expression of these genes by the tumor cells. For example, TGF-β is often expressed by cancer cells, and MKK3 and TAK1 are involved in the TGF-β signaling cascade [28, 29]. Also, Neil and Schiemann [30] recently reported that TAK1 was required for the ability of TGF-β to foster tumor growth. Thus, elevated MKK3 and TAK1 expression may be a consequence of TGF-β expression and autocrine signaling by the tumor cells. TIRAP and IRF3 are other examples of genes that were expressed at greater levels by the tumor cells, the results of which may be unrelated to TLR signaling. For instance, TIRAP is negatively regulated by caspase-1 which is also used for activating IL-1β and IL-18 [31]. Thus, low TIRAP expression by DC may be related to the expression of caspase-1 necessary for proinflammatory cytokine secretion by these cells. An inverse correlation between IRF3 and A20 expression has also been previously reported [32]. Thus, the greater level of IRF3 expression by 4T1 may be related to the lower level of A20 expression in these cells compared to the DC (data not shown). Accordingly, the greater levels of TIRAP, TAK1, MKK3, and IRF3 expression by 4T1 compared to DC may be unrelated to LPS responsiveness. Moreover, there have been no reports that these proteins exert a negative influence on TLR signaling.
Although the tumor cells were less responsive than DC to LPS, CXCL1 and CCL5 secretion by the tumor cells in response to LPS may be indicative of Myd88 dependent and TRIF dependent responses respectively since secretion of CXCL1 has been reported to be Myd88 dependent, and CCL5 secretion has been reported to be TRIF dependent [4, 33]. In particular, CXCL1 production by macrophages following treatment with LPS was dependent upon Myd88 [33], and CCL5 production by macrophages following exposure to Pseudomonas aeruginosa was dependent upon TRIF [4]. Another potential explanation for the differential responsiveness of these cells to LPS may be related to TGF-β expression by the tumor cells. Naiki et al., [34] reported that TGF-β can inhibit Myd88 expression. However, we have explored this possibility using siRNA targeting TGF-β and found that neither the level of Myd88 nor the tumor cell’s response to LPS were altered (data not shown).
It is also necessary to investigate a wider range of treatment conditions. For instance, while we could detect mRNA encoding IL-6 after 24 hours of treatment with LPS, we could not detect IL-6 protein secretion until 96 hours later (data not shown). Yet, Huang et al., [35] reported that tumor cells secreted IL-6 after 24 hours of LPS treatment. In that study however, the authors used a different tumor cell line (MC26) and a higher dose of LPS (1,000 ng/ml) [35]. These data are reminiscent of some of the early studies with LPS in tumor settings that showed success or failure was dependent upon the dose, route, and timing of LPS delivery [36, 37]. Thus, further evaluating a larger range of LPS doses and a time dependent analysis of gene expression would be valuable in deciphering the full extent of LPS responsiveness by the tumor cells. Such studies may also further elucidate why the tumor cells and DC respond differently to the same TLR agonist.
Another complicating factor in unraveling why DC and tumor cells are differentially responsive to LPS is the wide variety of DC and tumor cells which exist. While extending this type of investigation to other subsets of DC as well as other types of tumor cells would help determine the overall implications of our findings, there remains much to be done with the CD11c+ DC and 4T1 tumor cells used in this study. For instance, in order to validate a role for TLR4, CD14, Myd88, and TRAM in the differential responsiveness of the cells to LPS, siRNA can be used to drive down expression of these genes in DC, or the genes may be over expressed by the 4T1 tumor cells prior to monitoring responsiveness to LPS. Additionally, the differences we observed could be attributed to the different genetic backgrounds of the cells since 4T1 was derived from Balb/C3H mice, while DC were from OTII mice. Exploring whether the genetic background influences responsiveness to TLR agonists warrants investigation and may help explain results obtained using TLR agonists in different model systems. Finally, if one is to fully understand the implications of TLR agonists in a tumor setting, analysis of the agonists must extend beyond different DC subsets, different types of tumor cells, and genetic backgrounds to include characterizing stromal cell responsiveness as well. The practicality of intratumoral delivery of TLR agonists will benefit from a clearer understanding of the ways in which these agonists impact different cell types. The more we understand about how these agonists differentially impact varying cell populations the better position we may be in for making more informed decisions about transitioning TLR agonists into a clinical setting.
Acknowledgments
This project was supported by award number R15CA137858 from the National Cancer Institute (to R.A.K). The Department of Biology and the Excel Scholars Program at Lafayette College also supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 2.Alegre ML, Leemans J, Le Moine A, Florquin S, De Wilde V, Chong A, Goldman M. The multiple facets of toll-like receptors in transplantation biology. Transplantation. 2008;86:1–9. doi: 10.1097/TP.0b013e31817c11e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 4.Airhart CL, Rohde HN, Hovde CJ, Bohach GA, Deobald CF, Lee SS, Minnich SA. Lipid A mimetics are potent adjuvants for an intranasal pneumonic plague vaccine. Vaccine. 2008;26:5554–5561. doi: 10.1016/j.vaccine.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Trippler M, Pei R, Lu M, Broering R, Gerken G, Schlaak JF. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol. 2009;51:1037–1045. doi: 10.1016/j.jhep.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M. Toll-like recetor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009;120:2462–2469. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Lee B, Lee HS, Bae EA, Lee H, Ahn YT, Lim KS, Huh CS, Kim DH. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-kappaB activation in experimental colitis. Int J Colorectal Dis. 2009;24:231–237. doi: 10.1007/s00384-008-0618-6. [DOI] [PubMed] [Google Scholar]
- 8.Messmer D, Messmer B, Chiorazzi N. The global transcriptional maturation program and stimuli-specific gene expression profiles of human myeloid dendritic cells. Int Immunol. 2003;15:491–503. doi: 10.1093/intimm/dxg052. [DOI] [PubMed] [Google Scholar]
- 9.Shen H, Tesar BM, Walker WE, Goldstein DR. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol. 2008;181:1849–1858. doi: 10.4049/jimmunol.181.3.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiethe C, Debus A, Mohrs M, Steinkasserer A, Lutz M, Gessner A. Dendritic cell differentiation state and their interaction with NKT cells determine Th1/Th2 differentiation in the murine model of Leishmania major infection. J Immunol. 2008;180:4371–4381. doi: 10.4049/jimmunol.180.7.4371. [DOI] [PubMed] [Google Scholar]
- 11.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden JM, LaCasse CJ, Simova DV, Murphy TR, Kurt RA. Differential mediator production by dendritic cells upon toll-like receptor stimulation does not impact T cell cytokine expression. Immunol Lett. 2008;118:30–35. doi: 10.1016/j.imlet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 14.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. Human langerhans cells express a specific TLR profile and differentially respond to viruses and gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 15.Trichieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 16.Derbigny WA, Hong SC, Kerr MS, Temkit M, Johnson RM. Chlamydia muridarum infection elicits a beta interferon response in murine oviduct epithelial cells dependent on interferon regulatory factor 3 and TRIF. Infect Immun. 2007;75:1280–1290. doi: 10.1128/IAI.01525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 18.Erridge C, Spickett CM, Webb DJ. Non-enterobacterial endotoxins stimulate human coronary artery but not venous endothelial cell activation via Toll-like receptor 2. Cardiovasc Res. 2007;73:181–189. doi: 10.1016/j.cardiores.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Stewart BW, Kleihues P, editors. World Cancer Report. Lyon France: IARC; 2003. [Google Scholar]
- 20.Hirabayashi K, Yano J, Inoue T, Yamaguchi T, Tanigawara K, Smyth GE, Ishiyama K, Ohgi T, Kimura K, Irimura T. Inhibition of cancer cell growth by polyinosinic-polycytidylic acid/cationic liposome complex: a new biological activity. Cancer Res. 1999;59:4325–4333. [PubMed] [Google Scholar]
- 21.Smits ELJM, Ponsaerts P, Berneman ZN, Van Tendeloo VFI. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. The Oncologist. 2008;13:859–875. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- 22.Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expression. J Surg Res. 2001;97:14–19. doi: 10.1006/jsre.2001.6090. [DOI] [PubMed] [Google Scholar]
- 23.Pollet I, Opina CJ, Zimmerman C, Leong KG, Wong F, Karsan A. Bacterial lipopolysaccharide directly induces angiogenesis through TRAF6-mediated activation of NF-κB and c-Jun N-terminal kinase. Blood. 2003;102:1740–1742. doi: 10.1182/blood-2003-01-0288. [DOI] [PubMed] [Google Scholar]
- 24.Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, Weih F, Sleeman J, Anderegg U, Simon J. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signaling via TLR4. Exp Dermatol. 2008;17:100–107. doi: 10.1111/j.1600-0625.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 25.Conroy H, Marchall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumors. Oncogene. 2008;27:168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Shi YH, Le GW, Ma XY. Distinct immune response induced by peptidoglycan derived from lactobacillus sp. World J Gastroenterol. 2005;11:6330–6337. doi: 10.3748/wjg.v11.i40.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz F, Mages J, Heit A, Lang R, Wagner H. Transcriptional activation induced in macrophages by Toll-like receptor (TLR) ligands: from expression profiling to a model of TLR signaling. Eur J Immunol. 2004;34:2863–2873. doi: 10.1002/eji.200425228. [DOI] [PubMed] [Google Scholar]
- 28.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Met Rev. 2001;20:133–143. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 29.Kim SI, Kwak JH, Zachariah M, He Y, Wang L, Choi ME. TGF-β-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-β1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am J Physiol Renal Physiol. 2007;292:1471–1478. doi: 10.1152/ajprenal.00485.2006. [DOI] [PubMed] [Google Scholar]
- 30.Neil JR, Schiemann WP. Altered TAB1:Ikb kinase interaction promotes transforming growth factor β-mediated nuclear factor-κB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miggin SM, Palsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S, Rothwell N, Golenbock D, Fitzgerald KA, O’Neil LA. NF-kB activation by the Toll-IL-1 receptor domain protein MyD88 adaptor-like is regulated by caspase-1. Proc Natl Acad Sci. 2007;104:3372–3377. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitoh T, Yamamoto M, Miyagishi M, Taira K, Nakanishi M, Fujita T, Akira S, Yamamoto N, Yamaoka S. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol. 2005;174:1507–1512. doi: 10.4049/jimmunol.174.3.1507. [DOI] [PubMed] [Google Scholar]
- 33.De Felippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 34.Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-β differentially inhibits MyD88-dependent, but not TRAM- and TRIF- dependent, lipopolysaccharide-induced TLR4 signaling. J Biol Chem. 2005;280:5491–5495. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- 35.Huang B, Zhao J, Li H, He K, Chen Y, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitates evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 36.Andervont HB. The reaction of mice and various mouse tumours to the injection of bacterial products. Am J Cancer. 1936;27:77–83. [Google Scholar]
- 37.Parr I, Wheeler E, Alexander P. Similarities of the anti-tumour actions of endotoxin, lipid A and double-stranded RNA. Br J Cancer. 1973;27:370–389. doi: 10.1038/bjc.1973.45. [DOI] [PMC free article] [PubMed] [Google Scholar]