Abstract
Omsk hemorrhagic fever virus (OHFV) and Russian spring-summer encephalitis virus (RSSEV) are tick-borne flaviviruses that have close homology but different pathology and disease outcomes. Previously, we reported that C57BL/6 and BALB/c mice were excellent models to study the pathology and clinical signs of human RSSEV and OHFV infection. In the study described here, we found that RSSEV infection induced robust release of proinflammatory cytokines (IL-1α, IL-1β, IL-6 and TNF-α) and chemokines (MCP-1, MIP-1β, RANTES and KC) in the brain at 9 and 11dpi, together with moderate to low Th1 and Th2 cytokines. In contrast, OHFV infection stimulated an early and prominent induction of IL-1α, TNF-α, IL-12p70, MCP-1, MIP-1α and MIP-1β in the spleen of infected mice. Collectively our data suggest that a differential host response to infection may lead to the alternate disease outcomes seen following OHFV or RSSEV infection.
Keywords: OHFV, RSSEV, TBE, Omsk hemorrhagic fever, Russian spring-summer encephalitis, pathogenesis, host response
Introduction
Omsk hemorrhagic fever virus (OHFV) and Russian spring-summer encephalitis virus (RSSEV) are tick-borne flaviviruses of the genus Flavivirus, family Flaviviridae. These viruses are NIAID Category C priority pathogens and potential biothreat agents. Although these two virus groups are antigenically closely related with ~90% homology in their amino acid sequences, they cause vastly different disease outcomes in humans. OHFV can cause haemorrhagic fever syndromes in humans, but clinical expression ranges widely between mild to moderate clinical courses (Lvov et al., 1988). RSSEV is a member of the tick-borne encephalitis (TBEV) serocomplex, and it causes potentially fatal neurological infection. It is endemic across Asia including northern, western and southwestern regions of China (Lu et al., 2008), northern Japan (Takashima et al., 1997) and Russia.
Cytokines play a crucial role in the regulation of the immune response in clearing virus and protecting the host. However, overproduction of cytokines and chemokines may play a deleterious role. The innate and adaptive immune responses, with special reference to the cytokine profile, during OHFV or RSSEV infection is largely unknown. However, it has been reported that various proinflammatory mediators such as TNF-α, IL-1α and IL-6 were elevated in human TBE patients (Atrasheuskaya et al., 2003; Kondrusik et al., 2001; Timofeev et al., 2002; Toporkova et al., 2008). The decline of these proinflammatory cytokines together with an elevation of IL-10 was also associated with recovery (Atrasheuskaya et al., 2003; Kondrusik et al., 2001). However, the role of cytokines in OHFV and RSSEV infection in humans or animal models was never indicated.
Very few studies have been conducted to determine the role of the cellular immune response during OHFV or RSSEV infection. In TBE patients, T-cell subsets were significantly elevated in CSF as compared to the peripheral blood (Gelpi et al., 2005; Tomazic and Ihan, 1997). Similarly, the number of lymphocytes in the peripheral blood did not show a significant increase as a result of OHFV or RSSEV infection of BALB/c C57BL/6 mice (Tigabu et al., 2009). Adoptive transfer of CD4+ T-cells improves the survival of SCID mice after TBEV infection (Ruzek et al., 2009). Although the mechanism behind is not yet clear, CD4+ T-cells play a protective role in TBEV infection in mice. However, mice lacking CD8+ T-cells showed extended survival and decreased mortality, indicating a major contribution of the host immune system to the development of TBE (Ruzek et al., 2009).
Understanding the role of the host immune response in disease pathogenesis is important for the development of immune protection with minimal negative effects. BALB/c and C57BL/6 mice have been used as a reliable model to study disease progress following OHFV or RSSEV/TBEV infection (Hayasaka et al., 2009; Holbrook et al., 2005; Ruzek et al., 2009; Tigabu et al., 2009). Previously, we have shown that infected BALB/c and C57BL/6 mice have clinical signs and pathology similar to those seen in humans. RSSEV-infected mice have severe encephalitis manifested by paralysis, ranging from hind limb paresis to complete flaccid paralysis. However, OHFV infected mice have viscerotropic disease with limited signs of neurological disease (Holbrook et al., 2005; Tigabu et al., 2009).
In the current study, we investigated the extent to which cellular reactivity and cytokine production during experimental RSSEV and OHFV infection may contribute to the difference in pathology and disease outcome of these two viruses. We identified a robust and predominant proinflammatory response, along with a modest Th1 response during RSSEV infection in the brain. In contrast, OHFV primarily caused an early inflammatory response in the visceral organs with minimal effect on the brain.
Results
RSSEV infection induced the release of proinflammatory cytokines in the brain
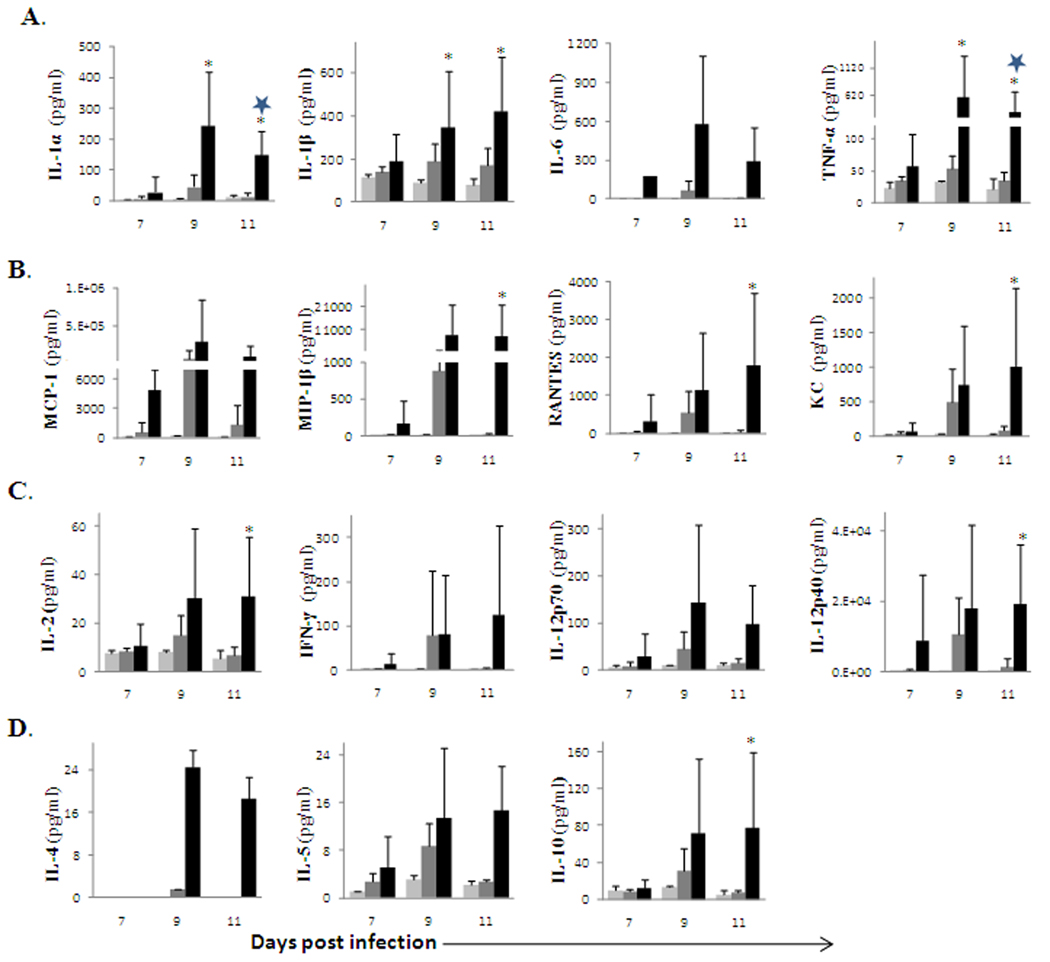
We recently showed that OHFV caused viscerotropic disease, while RSSEV caused encephalitis in the BALB/c and C57BL/6 mouse models (Holbrook et al., 2005; Tigabu et al., 2009). In the present study, we investigated the role of cytokines following RSSEV or OHFV infection by determining the profile and levels of proinflammatory cytokines using a Bioplex assay. The brain was analyzed for cytokines (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10 IL-12, IL-13, and IL-17) and chemokines (KC, G-CSF, GM-CSF, MCP-1α, MIP-1α, MIP-1β and RANTES). A general trend towards an increase in the expression of proinflammatory cytokines was observed in the brain of RSSEV infected BALB/c mice as compared to mock or OHFV infection (Fig.1). The concentrations of proinflammatory cytokines and chemokines were elevated in RSSEV infection at the later time of infection. The expression of IL-1α was enhanced 5-fold and 8-fold (p<0.05) in RSSEV infected mice on 9 and 11 days post infection (dpi), respectively, when compared with OHFV infected mice. Similarly, the level of IL-1β was elevated 2-fold at day 9 and 3-fold at 11 days post RSSEV infection, respectively, when compared to OHF infection. The increase in TNF-α was as great as 11-fold and 9-fold (p<0.05) on 9 and 11 dpi, respectively, when compared with the OHFV infection. In the case of IL-6, none of the mock and only very few OHFV-infected mice released detectable level of IL-6 in the brain. In contrast, RSSEV infected mice had very enhanced level of IL-6 starting from 7 dpi (Fig 1A). Amongst the proinflammatory molecules investigated, increases in the expression of MCP-1, MIP-1β and RANTES were the highest (Fig 1B). These chemokines elevated dramatically in RSSEV infection starting from 7 dpi, and this enhanced level was maintained and reached a peak at 11 dpi. Unlike the OHFV infected mice, the enhancement of these chemokines in the brains of RSSEV infected mice was statistically significant (p<0.05) when compared with the mock controls. Contrary to the elevated proinflammatory cytokines, RSSEV infection induced a very small amount of the Th2 and regulatory cytokines IL-4, IL-5 and IL-10 (Fig 1D). Taken together, there was an overall induction of proinflammatory cytokines and chemokines in the brain following RSSEV infection, but a limited response in OHFV infected animals.
FIG. 1.
Kinetics of inflammatory mediators and Th1/Th2 cytokines in the brain of BALB/c mice following ( ) mock, (
) mock, ( ) OHFV or (
) OHFV or ( ) RSSEV infection. Protein levels of (A) proinflammatory cytokines, (B) Chemokines, (C) Th1 cytokines and (D) Th2 cytokines were measured in the brain at the indicated time points following foot pad injection of 1000pfu OHFV or RSSEV. Data are shown as mean ± SD for 3–5 mice at each time point. *p<0.05 for the comparison between RSSEV and mock,
) RSSEV infection. Protein levels of (A) proinflammatory cytokines, (B) Chemokines, (C) Th1 cytokines and (D) Th2 cytokines were measured in the brain at the indicated time points following foot pad injection of 1000pfu OHFV or RSSEV. Data are shown as mean ± SD for 3–5 mice at each time point. *p<0.05 for the comparison between RSSEV and mock,  p<0.05 for comparison between RSSEV and OHFV.
p<0.05 for comparison between RSSEV and OHFV.
The levels of Th1 cytokines were increased moderately in the BALB/c mouse as a result of RSSEV infection (Fig 1 C). IL-2 increased to 4 and 6-fold (p<0.05) by day 9 and 11, respectively when compared to mock. IL-12p40 showed an increase of 270- and 463-fold (p<0.05) on 9 and 11 dpi, respectively. In addition, IL-12p70 increased 14-fold at day 9, but it decreased over time and was not statistically significant when compared to that of OHFV or mock infected mice. IFN-γ was also elevated at day 9 and 11; however, it was not significantly different from OHFV infected mice.
In C57BL/6 mice, similar trends of cytokine and chemokine release were observed (data not shown) where RSSEV infected mice predominantly released proinflammatory cytokines, which were higher compared to OHFV infection. However, the magnitude of elevation of IL-6, KC, IFN-γ and IL-4 in RSSEV infected C57BL/6 mice were smaller as compared to RSSEV infected BALB/c mice.
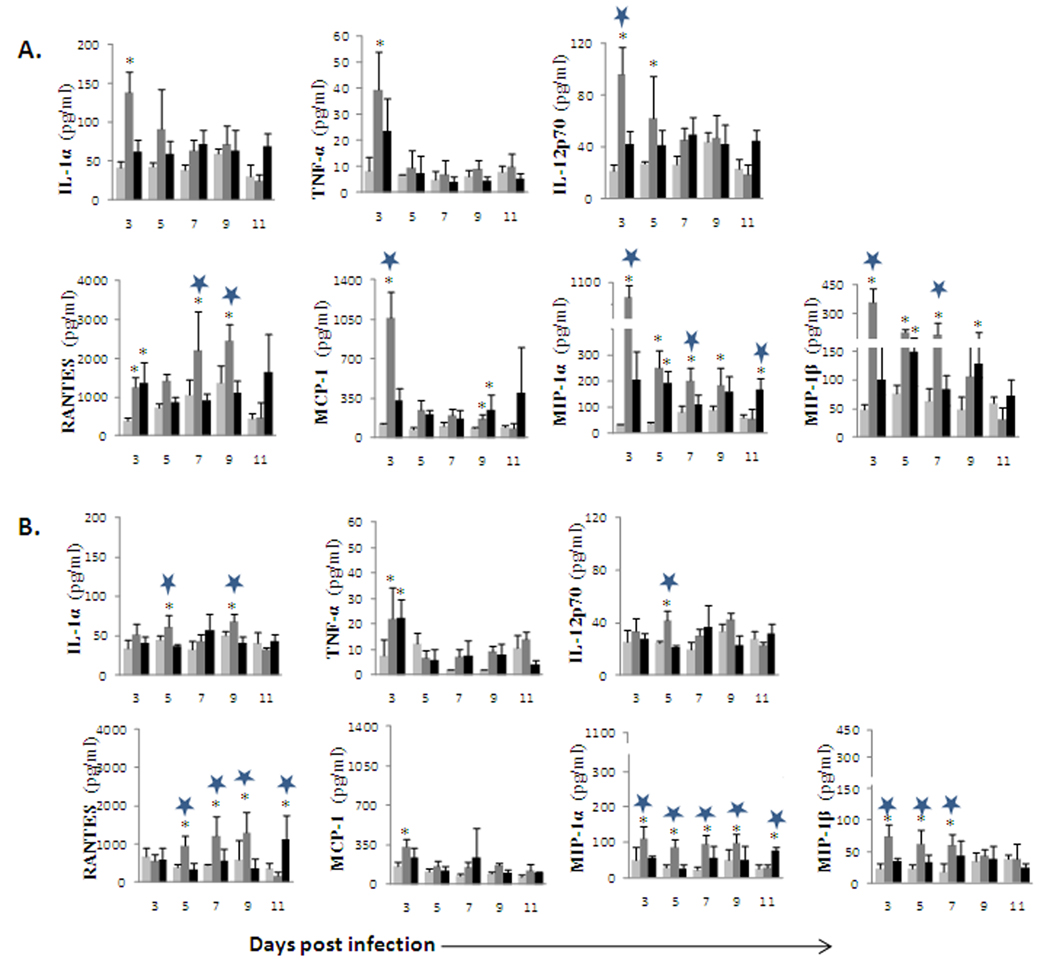
OHFV induced high concentration of proinflammatory mediators in the spleen
Previous studies showed that OHFV causes enlargement of the spleen with evidence of necrosis (Atrasheuskaya et al., 2003; Holbrook et al., 2005; Tigabu et al., 2009). We also found that the OHFV/RSSEV titer in the spleen ranges between 2–3 log10 pfu/ml. In the present study, we detected significantly higher levels of proinflammatory cytokines in the spleen from OHFV infected animals when compared with the mock or RSSEV infected mice. The concentration of inflammatory mediators peaked at 3 dpi in OHFV infected BALB/c mice (Fig 2A). TNF-α and IL-1α were elevated 2-fold at 3 dpi as compared to the RSSEV infected BALB/c. In addition, IL-12p70 was significantly (p<0.01) elevated in OHFV infected BALB/c mice as compared to RSSEV infected. Similarly, MCP-1, MIP-1α and MIP- 1β were significantly higher (p<0.001) at 3 dpi in OHFV infected BALB/c mice when compared with RSSEV infected mice. This elevation of proinflammatory cytokines and chemokines was at 3 dpi, and decreased over time. One exception was RANTES where the highest significant increase over RSSEV infection was on days 7 and 9 (p<0.01). In the case of RSSEV infection, cytokines remained low throughout the infection period. However, the chemokines were elevated at 11 dpi. IL-4, IL-5 and IL-6 were not detected in the spleen of uninfected or infected mice. In all cases, a higher cytokine release in the spleen corresponded to higher virus titer. There was no correlation between the virus titer in the brain and the cytokine concentration in the spleen (data not shown).
FIG. 2.
Inflammatory mediators in the spleen following ( ) mock, (
) mock, ( ) OHFV or (
) OHFV or ( ) RSSEV infection of (A) BALB/c and (B) C57BL/6 mice. Spleen was extracted at the indicated time and the level of the cytokine was measured from the clear homogenates. Data are the mean ± SD for 3–5 mice at each time point. *p<0.05 for the comparison between OHFV/RSSEV and mock,
) RSSEV infection of (A) BALB/c and (B) C57BL/6 mice. Spleen was extracted at the indicated time and the level of the cytokine was measured from the clear homogenates. Data are the mean ± SD for 3–5 mice at each time point. *p<0.05 for the comparison between OHFV/RSSEV and mock,  p<0.05 for comparison between RSSEV and OHFV.
p<0.05 for comparison between RSSEV and OHFV.
OHFV or RSSEV infected C57BL/6 mice had a similar pattern of cytokine release as that of OHFV infected BALB/c mice. However, the overall cytokine and chemokine release in the spleen of C57BL6 mice was lower than that of BALB/c mice (Fig 2B).
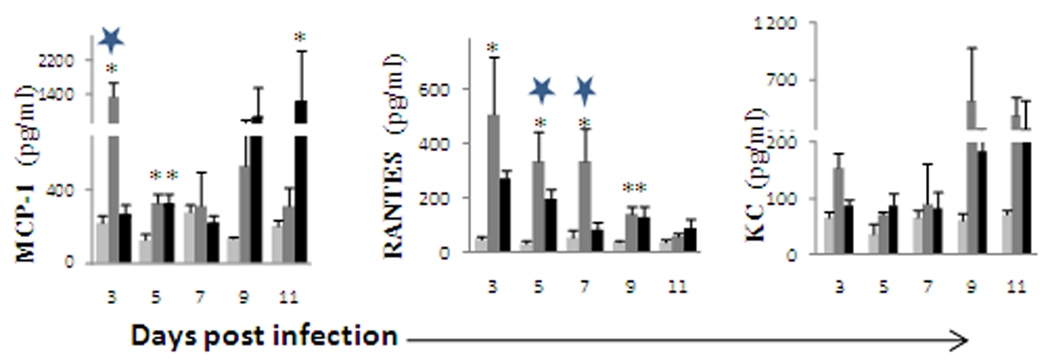
OHFV infection also induced chemokines in the lung and liver
Only a few mouse groups had positive OHFV titer in the lung, thus no correlation was established between the virus titer and the cytokine release. At 3 dpi, MCP-1 (p<0.05), RANTES (p<0.05) and KC were elevated in OHFV infected BALB/c mice as compared to that of mock, however, the elevation of RANTES was extended to day 7. In the case of MCP-1 and KC, the level went down and then increased again at 9–11 dpi (Fig 3). In RSSEV infected BALB/c mice, the level of MCP-1 and KC were increased at 9–11 dpi.
FIG. 3.
Chemokine expression in lung of balb/c mice following ( ) mock, (
) mock, ( ) OHFV or (
) OHFV or ( ) RSSEV infection of BALB/ c mice. Lung and liver were extracted at the indicated days and the supernatant was used to analyze the level of cytokines/chemokines. Data are shown as mean ± SD for 3–5 mice at each time point. *p<0.05 for the comparison between OHFV/RSSEV and mock,
) RSSEV infection of BALB/ c mice. Lung and liver were extracted at the indicated days and the supernatant was used to analyze the level of cytokines/chemokines. Data are shown as mean ± SD for 3–5 mice at each time point. *p<0.05 for the comparison between OHFV/RSSEV and mock,  p<0.05 for comparison between RSSEV and OHFV.
p<0.05 for comparison between RSSEV and OHFV.
OHFV infected mice had 2–2.8 log10 pfu/ml OHFV titer in the liver at 7 and 9 dpi, however, no RSSEV was detected in liver of infected mice using plaque assay (Tigabu et al., 2009). In terms of the hepatic cytokine level, there are few differences between infected and control BALB/c and C57BL6 mice (data not shown).
Cytokine levels in the brain and spleen were higher than those in serum
Previously, we reported that no virus was detected in the serum of either OHFV or RSSEV infected mice (Tigabu et al., 2009), thus a direct correlation between virus titer and cytokine release could not be established. In the current study, we found that the level of cytokines in the serum of OHFV or RSSEV infected mice increased above the level of the mock at 9 and 11 dpi; however, there was only slight variation in the cytokine levels between virus infected mice (data not shown). In addition, there was no significant correlation between either OHFV or RSSEV titer in the brain and the concentration of their corresponding cytokines in the serum (data not shown). All tested cytokines were higher in the brain of RSSEV infected mice compared with those of serum (Table 1). Similarly, the cytokines in the spleen of OHFV infected mice were higher as compared to the serum (data not shown). This higher cytokine concentration in the brain of RSSEV infected mice and visceral organs of OHFV infected mice as compared to their corresponding cytokines in the serum suggest localized inflammatory responses.
Table 1.
Comparison of the fold change of cytokine/chemokine levels in the brain and serum of RSSEV infected BALB/c mice over the mock
| 9 dpi | 11 dpi | |||
|---|---|---|---|---|
| Brain | Serum | Brain | Serum | |
| IL-1α | 23.5 ± 12.2 | 2.1 ±1.9 | 9.5 ± 4 | 116.0 ±133 |
| IL-1β | 3.9 ± 1.3 | 0.8 ±0.3 | 5.4 ± 1.7 | 5.1 ± 4.4 |
| TNF-α | 16.7 ± 9.8 | 0.3 ± 0.2 | 14.3 ± 8.5 | 2.7 ± 1.9 |
| IL-10 | 5.2 ± 2.6 | 0.4 ±0.3 | 15.4 ± 7.7 | 6.5 ± 6.6 |
| IL-12p40 | 271.6 ± 15.5* | 1.1 ±0.5 | 463.9 ± 207 | 4.7 ± 3.8 |
| IL-2 | 3.8 ±1.5 | 0.4 ± 0.2 | 6.1 ± 2.4 | 3.9 ± 3.4 |
| IFN-γ | 28.7 ± 20.9 | 0.5 ±0.4 | 186.5 ± 93.5 | 1.4 ± 1.5 |
| KC | 20.0 ± 10 | 1.4 ±0.9 | 46.8 ± 26.5 | 3.5 ± 2.4 |
| MCP-1 | 1315.1 ±1260 | 0.9 ±0.6 | 666.4 ± 600 | 9.6 ± 9.9 |
| MIP-1β | 661.7 ± 431 | 0.2 ±0.1 | 1389 ± 11.8* | 4.4 ± 3.9 |
| RANTES | 261.9 ±150 | 0.5 ±0.3 | 555 ± 293 | 2.5 ± 1.8 |
| G-CSF | 211.6 ± 122 | 3.7 ± 2.9 | 65.3 ± 33 | 121 ± 140 |
Data are shown as mean ± SE for 3–5 mice at each time point.
The cytokine level in the brain of RSSEV infected mice were significantly higher (p<0.05) when compared to that of that of the serum.
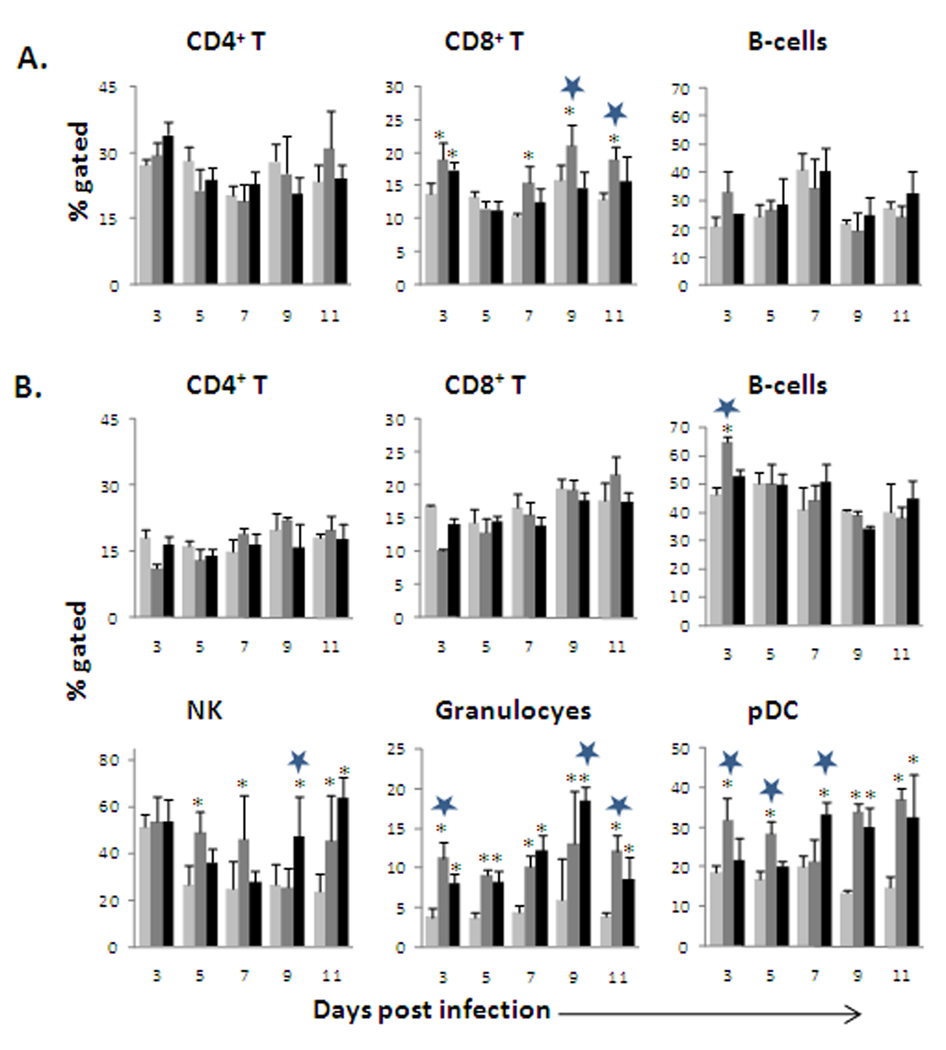
OHFV and RSSEV had limited effect on the proportion of splenic CD4+ and CD8+ cells
We have previously reported that OHFV infection leads to necrosis in the spleen (Holbrook et al., 2005). Thus, to better understand the effect of OHFV and RSSEV on the cell population of the spleen, we analyzed the proportion of total T cells, B-lymphocytes, CD4+ T cells, CD8+ T cells, granulocytes, NK, myeloid DC and plasmacytoid DC. The total number of splenocytes was not affected following RSSEV infection in either BALB/c or C57BL/6 mice. Fig 4 shows the results of flow cytometry analysis indicating the proportion of CD3+ T-cells, CD4+ and CD8+ T-cells, B-cells, plasmocytoid dendritic cells (pDCs), granulocytes and NK cells in the spleen. The proportion of splenic CD4+ T-cells was not significantly affected by OHFV or RSSEV infection in either BALB/c or C57BL/6 mice. However, both OHFV and RSSEV infections significantly (p<0.01) increased the proportion of CD8+ T-cells, as compared to mock, during the early phase of infection in BALB/c mice. This increase was enhanced significantly (p< 0.05) in the OHFV infected BALB/c mice on 7–11 dpi as compared to RSSEV.
FIG. 4.
The effect of OHFV and RSSEV infection on the proportion of the splenocyte subsets of BALB/c (A) and C57BL/6 (B) mice were infected with ( ) mock, (
) mock, ( ) OHFV or (
) OHFV or ( ) RSSEV. Dissociated splenocytes were isolated on the designated days post infection. The cells were stained with appropriate antibodies and analyzed using flow cytometry. Data are shown as mean ± SD for 5 mice at each time point. *p<0.05 for the comparison between OHFV/RSSEV and mock,
) RSSEV. Dissociated splenocytes were isolated on the designated days post infection. The cells were stained with appropriate antibodies and analyzed using flow cytometry. Data are shown as mean ± SD for 5 mice at each time point. *p<0.05 for the comparison between OHFV/RSSEV and mock,  p<0.05 for comparison between RSSEV and OHFV.
p<0.05 for comparison between RSSEV and OHFV.
In OHFV infected mice, the proportion of NK, granulocytes and pDC was higher at 3 dpi as compared to RSSEV. However, RSSEV infection increased the proportion of these cells at the later time of the infection period. The proportion of mDC did not vary appreciably between infected and control groups (data not shown).
Discussion
In this study, we examined the host immune response to OHFV and RSSEV infection in the mouse model with the objective of characterizing the response and correlating the response to disease progression and pathogenesis. As we have shown before (Holbrook et al., 2005; Tigabu et al., 2009), OHFV and RSSEV caused markedly different diseases in the animal model with viscerotropic disease associated with OHFV infection and neurologic disease characterizing RSSEV infection. Here we show that host immune response can be linked to differential disease manifestations.
The first important feature we found was that RSSEV infection, which causes encephalitis in humans and mice, results in an excessive release of proinflammatory cytokines in the brain. The peak concentration of the proinflammatory cytokines (IL-1α, IL-1β, IL-6 and TNF-α), and chemokines (MCP-1, MIP-1α, MIP-1β, KC and RANTES) positively correlated with the increase of the RSSEV titer in the brain. This release of proinflammatory cytokines was significantly higher when compared to OHFV. Similarly, TBE patients had the highest level of TNF-α, IL-1α and IL-6 during the first week of hospitalization, and treatment reducing these cytokines led to quicker improvement of symptoms and consequently faster recovery (Atrasheuskaya et al., 2003). IL-6 could be neurotoxic, has been associated with neurological disorders and disease (Okuda et al., 1998; Olson et al., 2001; Perrin et al., 2000) and induces other proinflammatory cytokines such as IL-1β and TNF-α. IL-1α and TNF-α act synergistically to initiate the cascade of inflammatory mediators by targeting the endothelium. Thus, increased brain production of IL-6, TNF-α and IL-1α/β may explain the observed inflammatory response, neuronal damage, meningitis, meningioencephalitis and meningioencephalomyelitis evident during RSSEV infection (Gunther et al., 1997; Toporkova et al., 2008).
RSSEV infection also caused a dramatic increase in the level of brain MCP-1, MIP-1β and RANTES compared to OHFV infection. Similar results were reported in human TBE patients where MCP-1 in CSF was significantly increased in comparison to the control group (Michalowska-Wender et al., 2006). Moreover, RANTES increased in the CSF, but not in serum of TBE patients (Grygorczuk et al., 2006; Lepej et al., 2007) suggesting its role in the recruitment of cells into CSF. Furthermore, chemokines alter the permeability of the BBB as well as stimulating macrophages and microglia in the brain leading to cellular migration and secretion of proinflammatory mediators (Ghoshal et al., 2007; Ishiguro et al., 1997).
In the present study, there was a modest increase of Th1 cytokines (IL-2, IFN-γ, IL-12p40 and IL-12p70) in the brain of RSSEV infected mice. In addition, the level of IL-4, IL-5 and IL-10 was either very low or undetectable in most RSSEV infected mice. Earlier studies indicated that TBE recovery was associated with decreased levels of proinflammatory cytokines accompanied by an elevation of IL-10 (Atrasheuskaya et al., 2003). Other studies also indicated that IL-2 was associated with recovery from TBE and had a strong negative correlation with IL-1β, TNF-α and IL-6 (Atrasheuskaya et al., 2003; Lepej et al., 2007). This diminished or absence of negative regulations in the brain of RSSEV infected mice may create perpetuation of a primarily proinflammatory environment in the brain that could result in death of the host through immunopathogenesis.
Contrary to RSSEV, OHFV infection caused a sharp decrease in the body weight of infected mice with pathology mainly in visceral organs. The main pathology of OHFV in human infections includes hemorrhages in the GI, urinary and reproductive tracts, and hyperplasia of spleen. In mice, OHFV was detected as early as 3 dpi in visceral organs and had higher virus titer compared to RSSEV (Holbrook et al., 2005; Tigabu et al., 2009). Our results indicated an early increase of proinflammatory mediators including IL-1α and TNF-α, together with chemokines (MCP-1, MIP-1α, MIP-1β and RANTES) in the spleens of OHFV infected mice. These cytokines and chemokines were elevated significantly at 3 dpi as compared to RSSEV or mock; and have a positive correlation with virus titer. The release of cytokines and chemokines in the spleen earlier than they are seen in the serum and brain suggests the amplification of the immune response to OHFV was generated peripherally. The high level of proinflammatory mediators with the absence of regulatory cytokines may induce tissue injury which can lead to organ dysfunction. A very limited amount of data regarding OHFV infection of humans and/or mice makes it difficult to correlate the cytokine level with disease stage or outcome. However, in several viral hemorrhagic fevers, overproduction of proinflammatory cytokines such as IL-1β and TNF-α contributes to vascular endothelial cell dysfunction and, consequently, shock (Geisbert and Jahrling, 2004; Marta et al., 1999).
Infection with other hemorrhagic fever viruses (e.g. Ebola) lead to depletion of peripheral and/or splenic NK, CD4+ and CD8+ T cells. The death of these cells was suggested to contribute to the disease progression (Reed et al., 2004 and Zaki et al., 1999). Previously we have shown that OHFV infected mice had their peripheral lymphocytes number dropped at 3 dpi and subsequently returned to the level of the control. However, in the current study, depletion of splenic subsets was absent. This may indicated that there might not be a strong correlation between the number of the splenocytes subsets and the pathology of OHFV infection. However, the activation state of the cells and their effect on the production and modulation of the cytokine and chemokine levels needs further study.
Serum cytokine levels of OHFV or RSSEV infected mice started to increase above mock late in the disease cycle. In addition, the level of cytokines in the serum was lower when compared to that of the brain and visceral organs. Activated macrophages in the spleen and brain macrophages/monocytes, astrocytes, microglial and endothelial cells in the CNS are known to release cytokines/chemokines when stimulated by viral infection (Chen et al., 2004; Chen et al., 2000; del Zoppo et al., 2000; Ghoshal et al., 2007) thereby releasing them to the circulation and contributing to the elevated cytokine level in serum. This further potentiates the exaggerated endogenous production of proinflammatory cytokines by brain and visceral organs which may be responsible for tissue injury and ultimately organ failure. Similarly, TBE patients demonstrated elevated IL-6, IL-10, and IFN-γ in the CSF relative to serum concentrations (Pietruczuk et al., 2006; Zajkowska et al., 2006) indicating that a serum cytokine profile is unreliable in determining the inflammation status in the brain.
In this report, we describe mouse strain-based differences in levels of cytokines and chemokines induced by infection with OHFV and RSSEV. Within each group of BALB/c or C57BL/6 mice the general trend of the cytokine response is similar where RSSEV infected mice have excess proinflammatory cytokines in the brain and OHFV infected mice have high cytokine response in the visceral organs. However, when the cytokine response was compared between the two groups of mice, the BALB/c mice exhibited a higher overall response. This could be due to other confounding factors, such as the genetic background of the mice and involvement of other aspects of the immune system (e.g. cell mediated immunity) in determining the survival time of infected mice. Differences in immune responses of BALB/c and C57BL/6 mice have been reported following infection with viruses (Culley et al., 2006; Weinberg et al., 2004). In general, C57BL/6 mice tend to mount a Th1 response, while BALB/c mice tend to mount a Th2 response (Spellberg and Edwards, 2001). In our previous work, we indicated that BALB/c mice have higher average survival time following RSSEV infection but shorter following OHFV infection when compared to infected C57BL/6 mice. In addition, the viral titer in CNS did not show significant difference between OHFV and RSSEV infected BALB/c and C57BL/6 mice (Tigabu et al., 2009).
Based on this and previous studies, we hypothesize that, although both OHFV and RSSEV replicate in the brain, they may have different cell tropism. RSSEV may lead to activation of inflammatory cells, including microglia, which results in release of exaggerated proinflammatory mediators that might play a major role in the mortality of infected mice due mainly to encephalitis. Excess proinflammatory mediators in the visceral organs due to OHFV infection may also be responsible for the visceral organ pathology. Deregulated or excess release of proinflammatory mediators by brain or visceral organs may induce tissue damage with different pathology and disease outcome. This suggests a major role of cytokines and chemokines in the immunopathogenesis of RSSEV and OHFV infection. The host immune response may therefore play a central role in determining the clinical outcome of OHFV/RSSEV infection. Further studies are needed to determine the target cells for each virus and address the specific contribution of the cytokines and chemokines in the pathogenesis of these diseases.
Materials and methods
Animals
Female C57BL/6 and BALB/c mice aged 8–10 weeks were purchased from Harlan Sprague-Dawley, and were kept in the ABSL-4 animal facilities of the UTMB, Galveston, TX. All procedures were performed in our ABSL-4 facility according to Institutional Animal Care and Use Committee guidelines, and were in accordance with the Principles of Laboratory Animal Care.
Virus and Challenge procedure
RSSEV (strain Sofjin) and OHFV (strain Guriev) were obtained from the World Reference Collection for Emerging Viruses and Arboviruses (WRCEVA), which is housed at UTMB, Galveston, TX. Animals were challenged with 1000 pfu (20 µl) in the left-rear footpad. Mock infected animals were inoculated with 20 µl of diluent (serum-free MEM).
Isolation of tissue
Five mice from each virus and mock infected group were sacrificed every other day for 15 days. Blood was drained by cardiac puncture and sera were separated by centrifugation. Half of each brain, spleen, liver, and lung were collected and homogenized in 0.5ml of PBS. The serum and tissue homogenates were immediately stored at −80°C until analyzed by cytokine Bioplex assay and virus titration. The viable virus titer in these tissue homogenates was determined using plaque assay and reported previously (Tigabu et al., 2009).
Cytokine analysis
Serum and clarified homogenate supernatant were used to determine the cytokine profile of the infected mice. Cytokine analysis was done in triplicate using a mouse-specific 23-plex Bioplex assay (Bio-Rad) according to the manufacturer’s instruction.
Preparation of spleen cell suspensions
Single cell suspensions were prepared from the spleens by forcing the spleen through 40-µm pore size screen. Splenocytes were suspended in cold complete RPMI 1640 and centrifuged to pellet the cells. The red blood cells were lysed using lysis buffer (eBiosciences). The cell suspension was washed twice with cold RMPI 1640 and the splenocyte count was performed using a hemocytometer. Cell viability was determined using the trypan blue (Gibco) exclusion method and was greater than 95%.
Flow cytometry
Flow cytometric analysis was performed on a FACSCanto (Becton Dickinson, San Jose, CA). All antibodies used in this experiment were purchased from eBiosciences. The splenocytes suspensions were incubated at 4°C for 30 minutes with antibodies. The splenocytes were then washed twice and fixed in 4% paraformaldeyde for 24 h. Cells were identified as T-cells (CD3+), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B lymphocytes (B220+, CD3−), mDC (CD11c+CD11b+B220−), pDC (CD11cloCD11b− B220+) and NK (CD3−DX5+B220−) using flow cytometry. Splenocytes were analyzed using FACS Canto, and FCS Express was used for data analysis.
Statistical analyses
Data are expressed as mean ± SD of 3–5 mice per group. Statistical analysis of the cytokines and chemokines was performed using two-way ANOVA followed by Tukey post Hoc or Kruskal-Wallis test. Relationships between variables w ere evaluated pair-wise by Pearson’s correlation. All data analyses were done using SigmaPlot®11 software and values of p<0.05 was considered to be statistically significant.
Acknowledgments
We thank N. Seth Linde, Allison Poussard, Colette Pietzsch and Melissa Worthy for their excellent technical assistance. This project was supported in part by a Western Regional Center of Excellence Career Development award (U54 AI057156) to MRH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atrasheuskaya AV, Fredeking TM, Ignatyev GM. Changes in immune parameters and their correction in human cases of tick-borne encephalitis. Clin. Exp. Immunol. 2003;131:148–154. doi: 10.1046/j.1365-2249.2003.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SM, Kar S, Singh R, Chakraborty D, Vipat V, Raut CG, Mishra AC, Gore MM, Ghosh D. Immunomodulatory cytokines determine the outcome of Japanese encephalitis virus infection in mice. J. Med. Virol. 2010;82:304–310. doi: 10.1002/jmv.21688. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Chen JH, Chen SY, Liao SL, Raung SL. Upregulation of RANTES gene expression in neuroglia by Japanese encephalitis virus infection. J. Virol. 2004;78:12107–12119. doi: 10.1128/JVI.78.22.12107-12119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Liao SL, Kuo MD, Wang YM. Astrocytic alteration induced by Japanese encephalitis virus infection. Neuroreport. 2000;11:1933–1937. doi: 10.1097/00001756-200006260-00025. [DOI] [PubMed] [Google Scholar]
- Culley FJ, Pennycook AM, Tregoning JS, Hussell T, Openshaw PJ. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J. Virol. 2006;80:4521–4527. doi: 10.1128/JVI.80.9.4521-4527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat. Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gelpi E, Preusser M, Garzuly F, Holzmann H, Heinz FX, Budka H. Visualization of Central European tick-borne encephalitis infection in fatal human cases. J. Neuropathol. Exp. Neurol. 2005;64:506–512. doi: 10.1093/jnen/64.6.506. [DOI] [PubMed] [Google Scholar]
- Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, Koli P, Sen E, Basu A. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia. 2007;55:483–496. doi: 10.1002/glia.20474. [DOI] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimaker M, Olcen P, Andersson B. Interferon-gamma in cerebrospinal fluid from patients with viral and bacterial meningitis. Scand. J. Infect. Dis. 1994;26:141–147. doi: 10.3109/00365549409011777. [DOI] [PubMed] [Google Scholar]
- Grygorczuk S, Zajkowska J, Swierzbinska R, Pancewicz S, Kondrusik M, Hermanowska-Szpakowicz T. [Concentration of the beta-chemokine CCL5 (RANTES) in cerebrospinal fluid in patients with tick-borne encephalitis] Neurol. Neurochir. Pol. 2006;40:106–111. [PubMed] [Google Scholar]
- Gunther G, Haglund M, Lindquist L, Skoldenberg B, Forsgren M. Intrathecal IgM, IgA and IgG antibody response in tick-borne encephalitis. Long-term follow-up related to clinical course and outcome. Clin. Diagn. Virol. 1997;8:17–29. doi: 10.1016/s0928-0197(97)00273-0. [DOI] [PubMed] [Google Scholar]
- Hayasaka D, Nagata N, Fujii Y, Hasegawa H, Sata T, Suzuki R, Gould EA, Takashima I, Koike S. Mortality following peripheral infection with tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology. 2009;390:139–150. doi: 10.1016/j.virol.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Holbrook MR, Aronson JF, Campbell GA, Jones S, Feldmann H, Barrett AD. An animal model for the tick-borne flavivirus--Omsk hemorrhagic fever virus. J. Infect. Dis. 2005;191:100–108. doi: 10.1086/426397. [DOI] [PubMed] [Google Scholar]
- Ishiguro A, Suzuki Y, Inaba Y, Fukushima K, Komiyama A, Koeffler HP, Shimbo T. The production of IL-8 in cerebrospinal fluid in aseptic meningitis of children. Clin. Exp. Immunol. 1997;109:426–430. doi: 10.1046/j.1365-2249.1997.4681366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrusik M, Pancewicz S, Zajkowska J, Hermanowska-Szpakowicz T. [Tumor necrosis factor alpha and interleukin 1-beta in serum of patients with tick-borne encephalitis] Pol. Merkur. Lekarski. 2001;11:26–28. [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Lepej SZ, Misic-Majerus L, Jeren T, Rode OD, Remenar A, Sporec V, Vince A. Chemokines CXCL10 and CXCL11 in the cerebrospinal fluid of patients with tick-borne encephalitis. Acta. Neurol. Scand. 2007;115:109–114. doi: 10.1111/j.1600-0404.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Lu Z, Broker M, Liang G. Tick-borne encephalitis in mainland China. Vector Borne Zoonotic Dis. 2008;8:713–720. doi: 10.1089/vbz.2008.0028. [DOI] [PubMed] [Google Scholar]
- Lvov DK, Vladimirtseva EA, Butenko AM, Karabatsos N, Trent DW, Calisher CH. Identity of Karelian fever and Ockelbo viruses determined by serum dilution-plaque reduction neutralization tests and oligonucleotide mapping. Am. J. Trop. Med. Hyg. 1988;39:607–610. doi: 10.4269/ajtmh.1988.39.607. [DOI] [PubMed] [Google Scholar]
- Marta RF, Montero VS, Hack CE, Sturk A, Maiztegui JI, Molinas FC. Proinflammatory cytokines and elastase-alpha-1-antitrypsin in Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 1999;60:85–89. doi: 10.4269/ajtmh.1999.60.85. [DOI] [PubMed] [Google Scholar]
- Michalowska-Wender G, Losy J, Kondrusik M, Zajkowska J, Pancewicz S, Grygorczuk S, Wender M. [Evaluation of soluble platelet cell adhesion molecule sPECAM-1 and chemokine MCP-1 (CCL2) concentration in CSF of patients with tick-borne encephalitis] Pol. Merkur. Lekarski. 2006;20:46–48. [PubMed] [Google Scholar]
- Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin PJ, Rumbley CA, Beswick RL, Lavi E, Phillips SM. Differential cytokine and chemokine production characterizes experimental autoimmune meningitis and experimental autoimmune encephalomyelitis. Clin. Immunol. 2000;94:114–124. doi: 10.1006/clim.1999.4825. [DOI] [PubMed] [Google Scholar]
- Pietruczuk M, Pietruczuk A, Pancewicz S, Zajkowska J, Swierzbinska R, Hermanowska-Szpakowicz T. [Intercellular adhesion molecules sICAM-1, sICAM-2, sICAM-3 and IFNgamma in neuroborreliosis and tick-borne encephalitis] Przegl. Epidemiol. 2006;60 Suppl 1:109–117. [PubMed] [Google Scholar]
- Reed DS, Hensley LE, Geisbert JB, Jahrling PB, Geisbert TW. Depletion of peripheral blood T lymphocytes and NK cells during the course of Ebola hemorrhagic fever in cynomolgus macaques. Viral Immunol. 2004;17:390–400. doi: 10.1089/vim.2004.17.390. [DOI] [PubMed] [Google Scholar]
- Ruzek D, Salat J, Palus M, Gritsun TS, Gould EA, Dykova I, Skallova A, Jelinek J, Kopecky J, Grubhoffer L. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology. 2009;384:1–6. doi: 10.1016/j.virol.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Spellberg B, Edwards JE. Type1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- Takashima I, Morita K, Chiba M, Hayasaka D, Sato T, Takezawa C, Igarashi A, Kariwa H, Yoshimatsu K, Arikawa J, Hashimoto N. A case of tick-borne encephalitis in Japan and isolation of the the virus. J. Clin. Microbiol. 1997;35:1943–1947. doi: 10.1128/jcm.35.8.1943-1947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigabu B, Juelich T, Bertrand J, Holbrook MR. Clinical evaluation of highly pathogenic tick-borne flavivirus infection in the mouse model. J. Med. Virol. 2009;81:1261–1269. doi: 10.1002/jmv.21524. [DOI] [PubMed] [Google Scholar]
- Timofeev AV, Kondrat'eva I, Orlovskii VG, Kurzhukov GP, Loktev VB, Karganova GG. [Connection between severity of the course of tick-borne encephalitis with the concentration of interleukin-2 and interleukin-6 in blood] Ter. Arkh. 2002;74:22–23. [PubMed] [Google Scholar]
- Tomazic J, Ihan A. Flow cytometric analysis of lymphocytes in cerebrospinal fluid in patients with tick-borne encephalitis. Acta. Neurol. Scand. 1997;95:29–33. doi: 10.1111/j.1600-0404.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Toporkova MG, Aleshin SE, Ozherelkov SV, Nadezhdina MV, Stephenson JR, Timofeev AV. Serum levels of interleukin 6 in recently hospitalized tick-borne encephalitis patients correlate with age, but not with disease outcome. Clin. Exp. Immunol. 2008;152:517–521. doi: 10.1111/j.1365-2249.2008.03617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Lutzke ML, Alfinito R, Rochford R. Mouse strain differences in the chemokine response to acute lung infection with a murine gammaherpesvirus. Viral Immunol. 2004;17:69–77. doi: 10.1089/088282404322875467. [DOI] [PubMed] [Google Scholar]
- Zajkowska J, Grygorczuk S, Pryszmont JM, Kondrusik M, Pancewicz S, Swierzbinska R, Hermanowska-Szpakowicz T, Klibingat M. [Concentration of interleukin 6 and 10 in tick-borne and purulend encephalomeningitis] Pol. Merkur. Lekarski. 2006;21:29–34. [PubMed] [Google Scholar]
- Zaki SR, Goldsmith CS. Pathologic features of filovirus infections in humans. Curr. Top. Microbiol. Immunol. 1999;235:97–116. doi: 10.1007/978-3-642-59949-1_7. [DOI] [PubMed] [Google Scholar]